Experience with surgery for adenomyosis and assessment of reproductive outcomes
Adenomyosis is a variety of the benign pathological process, in which tissue in endometriosis affects the muscular layer of the uterus. The main principle of surgical intervention is the removal of myometrial tissue with invasion of endometriosis. The prevalence of adenomyosis ranges from 5 to 70%. The disease affects 2 in 10 women under the age of 40 years, while the incidence rises to 8 in 10 women between the ages of 40 and 50 years. However, the incidence of adenomyosis is difficult to determine due to the lack of a unified definition and diagnostic criteria based on noninvasive diagnostic methods. Its detection rate in the population varies from 10 to 61% according to different authors.Bezhenar V.F., Krylov K.Yu., Makarenko T.A., Matukhin V.I., Rukhlyada N.N., Tskhay V.B.
This paper presents surgical procedures for adenomyosis excision in the patient to perform her further reproductive function. Since 2012, by using the procedure described by H. Osada, the researchers (Prof. V.B. Tskhay and T.A. Makarenko, MD) of the Department of Obstetrics, Krasnoyarsk State Medical University, have been performing surgical interventions for excision of adenomyosis in patients with its diffuse forms and uterine sizes at 10 to 22 weeks’ gestation. The authors have also shown an original approach to suturing the uterus during surgery. The researchers (Prof. N.N. Rukhlyada and K.Yu. Krylov, PhD) of the Department of Obstetrics and Gynecology, Saint Petersburg State Pediatric Medical University, did 242 operations, of which 172 were performed to restore fertility; moreover, the total number of pregnancies that had occurred was 74 (43.1% or 30.5% of all the interventions).
Conclusion: Considering our experience of surgical interventions by laparotomy, we can conclude that there is no unified technique for the surgical treatment of adenomyosis – the choice of incisions and the way of suturing the myometrium depend both on the size of the uterus and on the location of the affected myometrium, its symmetry and uniformity, as well as on the volume of remaining intact tissue and the form of the defect.
Authors’ contributions: Bezhenar V.F., Rukhlyada N.N., Tskhay V.B. – concept and design of the investigation; Bezhenar V.F., Makarenko T.A., Rukhlyada N.N., Tskhay V.B. – material collection and processing; Krylov K.Yu. – statistical data processing; Krylov K.Yu., Matukhin V.I. – writing the text; Bezhenar V.F., Rukhlyada N.N. – editing.
Conflicts of interest: The authors declare that there are no conflicts of interest.
Funding: This investigation has not been sponsored.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Bezhenar V.F., Krylov K.Yu., Makarenko T.A., Matukhin V.I., Rukhlyada N.N., Tskhay V.B. Experience with surgery for adenomyosis and assessment of reproductive outcomes.
usherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (2): 79-86 (in Russian)
https://dx.doi.org/10.18565/aig.2022.263
Keywords
Adenomyosis is a disease characterized by invasion of the endometrium into the myometrium, which results in an enlarged uterus, adenomyosis tumors, heavy menstrual and intermenstrual bleedings, and period pains [1–4]. Microscopically, there are ectopic non-tumor endometrial glands and stroma, which are surrounded by the hypertrophic and hyperplastic myometrium [5, 6]. Among all causes of female infertility, adenomyosis accounts for about 20% [7].
The prevalence of adenomyosis ranges from 5 to 70%. The disease affects 2 in 10 women under the age of 40 years, whereas the incidence rises to 8 in 10 women between the ages of 40 and 50 years. However, the incidence of adenomyosis is difficult to determine due to the lack of a unified definition and diagnostic criteria based on noninvasive diagnostic methods [8]. Its detection rate in the population varies from 10 to 61% according to various authors [9–12]. After analysis of hysterectomy specimens, the detection rate of adenomyosis amounts to as much as 46-70% in the population [13]. There are neither pathognomonic clinical signs of adenomyosis, nor laparoscopic criteria that could be used to diagnose this disease [14].
Adenomyosis was previously diagnosed in premenopausal women only at postmortem examination after hysterectomy [15, 16]. The diagnosis is currently based on imaging methods, such as transvaginal ultrasound and magnetic resonance imaging [17]. Adenomyosis is asymptomatic in a third of cases. The most common clinical symptoms are menorrhagia (as much as 50% of patients), dysmenorrhea (30%), and metrorrhagia (20%), as well as other manifestations, such as uterine enlargement and infertility [18].
Literature data
Surgical treatment for adenomyosis to preserve reproductive function was first proposed by Van Praagh I. in 1952 [19]. The affected myometrium was resected in 37 patients in 1991. After surgical treatment, pregnancy occurred in 6 patients who subsequently delivered infants [20]. In 1993, a number of surgical interventions were performed using the above technique in 28 patients, while 13 out of 18 patients planning pregnancy achieved the expected result, as reported by Fedele L. et al. [21].
There is frequently an entry into the uterine cavity during surgery; therefore, it is more difficult to repair the uterine defect after conservative organ-sparing surgery for adenomyosis than for uterine fibroids [22], which leads to poor healing of the uterine defect or uterine scar incompetence. After conservative organ-sparing surgery for adenomyosis, the uterine scars may be incompetent because the defect may contain adenomyosis foci.
Reduced uterine distention may also be insufficient for future pregnancy [23]; in this connection, we can expect the greater likelihood of a uterine rupture after conservative organ-sparing surgery for adenomyosis during pregnancy.
In their recent study, Saremi A.T. reported wedge resection of the uterine wall as far as the endometrium after a sagittal cut of the corpus uteri [24]. Reconstruction of the uterine wall involves suturing using the continuous horizontal mattress technique; a gray serosal suture is then placed inside to reduce the risk of adhesions. Of the 103 patients who had undergone surgery, 70 women attempted to conceive during the study period; of them, 21 (30%) patients achieved clinical pregnancies and 16 (22.8%) had successful pregnancies that ended in live births.
The options for radical removal of adenomyosis include the triple-flap method. This adenomyomectomy procedure is based on a radically new idea [24]. The method involves uterine wall defect reconstruction using the remaining intact uterine muscle. In 2017, Osada H. et al. conducted a study [25] that involved 113 women after surgery using this method and showed that within 6 months, blood supply to the area of surgery returned to normal in almost all cases (92/113, 81.4%). Of the 62 women who had planned pregnancy, 46 cases became pregnant and 32 gave birth to a healthy infant via planned caesarean section. There were no cases of uterine rupture. During the study period (27 years), there were only 4 (3.5%) cases of recurrences requiring repeated surgical treatment.
The laparoscopic surgical method includes a longitudinal [25] or transverse [25, 26] incision of the uterine wall along the adenomyoma. Adenomatosis is then resected with a monopolar needle or a laser knife [26]. Then the endometrial cavity is closed during perforation and the uterine wall is also sutured (which can be performed in the seromuscular layer, in two or more layers, or by the double-flap method [26].
In the laparoscopic adenomyomectomy report that presents cases of uterine rupture [27], 141 patients with focal adenomyosis underwent excision of the affected segments with laser energy. After removal of adenomyosis, the uterine muscular layer was sutured using the continuous technique (2-0 synthetic absorbable threads). A total of 102 women planned pregnancy, whereas the total clinical pregnancy rate was 31.4% (32/102). When the women were divided into age groups (those aged younger and older than 40 years), the clinical pregnancy rates were 41.3% and 3.7%, respectively. Kodama K. et al. [27] reported cases of 71 patients with focal adenomyosis who had undergone monopolar resection of the affected segments, including those of uterine rupture [28, 29]. Thirty-two (45.1%) of the patients who had undergone surgery, planned pregnancy. A clinical pregnancy occurred in 16 women, including three (18.7%) women who had spontaneous abortions and 13 who delivered live babies (40.6% birth rate) (Table).
The first report on uterine rupture during pregnancy after laparoscopic adenomyomectomy was presented by Wada S. et al. in 2006, which was more likely due to multiple pregnancy. Laparoscopic adenomyomectomy of focal adenomyosis was performed using monopolar energy, and the remaining myometrium was sutured with a 1-0 poliglecaprone thread in two layers [30–33]. The patient became pregnant at 10 months following adenomyomectomy, but there was a spontaneous uterine rupture along the scar at 30 weeks’ gestation. Despite this, two babies weighing 1585 g and 1545 g were delivered via caesarean section and had an Apgar of 5 out of the 9 scores. A uterine wall rupture 7 cm long was successfully repaired after a blood loss of 2600 ml was compensated [32, 33].
Removal of adenomyosis by laparoscopic via laparotomic access can lead to incomplete elimination of muscle defects, which may increase the risk of uterine rupture [34].
Researchers have found that this is a safe alternative to laparotomy myomectomy, is technically easier than laparoscopic myomectomy, enables complete reconstruction of the uterine wall, and moreover requires less time to perform the operation [35–37].
The authors’ surgical experience
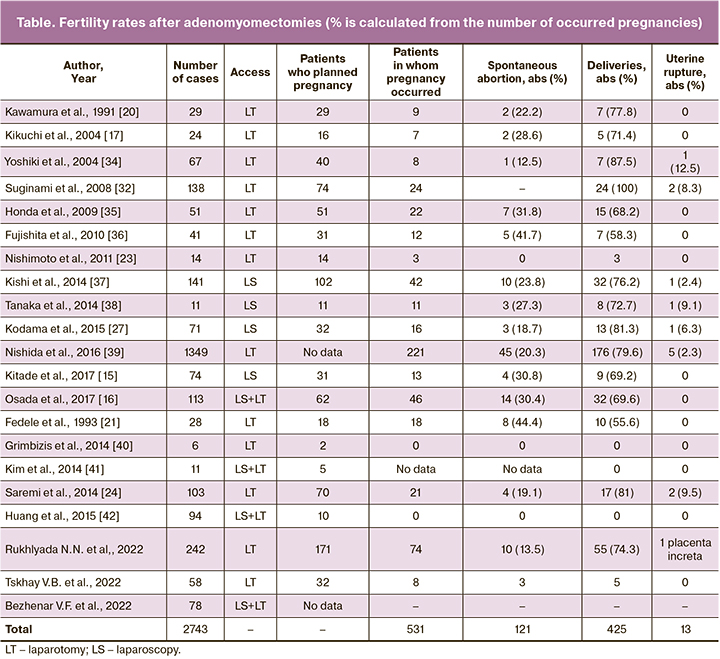
Since 2012, by using the procedure described by H. Osada, the researchers (Prof. V.B. Tskhay and T.A. Makarenko, MD) of the Department of Obstetrics, Krasnoyarsk State Medical University, have been performing surgical interventions for excision of adenomyosis in patients with its diffuse forms and uterine sizes at 10 to 22 weeks’ gestation (Fig. 1). Fifty-eight patients were operated on in the period of June 2012 to June 2022. Most women had clinical symptoms characteristic of severe forms of diffuse adenomyosis (heavy menstruation, chronic pelvic pains, dyspareunia, posthemorrhagic anemia, and poor quality of life).
There was a concurrence of diffuse adenomyosis and uterine fibroids in 48.1% (28/58) of cases. Infertility associated with this disease was observed in 40/58 patients (68.9%), At the same time, adenomyosis was removed in 31.03% (18/58) of patients in the older age group of 40 to 49 years old), who were uninterested in pregnancy and who had severe clinical symptoms of adenomyosis and poor quality of life; while all of them were interested in preserving the uterus.
To improve the results of postoperative surgical treatment, we proposed a hormone therapy protocol [38, 39] that is designed for 12 months of therapy and involves the administration of gonadotropin-releasing hormone agonists during the first 6 months and progestogens (dienogest) during the next 6 months. Since the first postoperative days, simultaneously with hormone therapy all the patients received epigenetic therapy with herbal remedies (indole-3-carbinol in combination with epigallate-catechin-3-gallate) for 12 months. The symptoms of abnormal uterine bleeding disappeared in all patients after discontinuation of drug treatment.
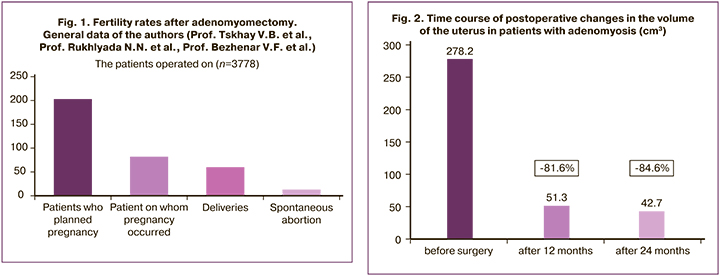
To evaluate the uterus and to identify possible adenomyosis recurrences, all the patients underwent ultrasound examination at least once a year. The sizes and volume of the uterus did not exceed normal values at 12 and 24 months after surgical adenomyosis excision in all the patients. We noted a statistically significant reduction in uterine volume by an average of 81.6% compared with baseline at 12 months and by 84.6% at 24 months (p<0.001) after surgery (Fig. 2).
Pregnancy occurred in 8/32 (25% or 20% of all interventions) women who were interested in restoring reproductive function. In 8 more women with infertility, less than 12 months have passed since the operation, which is highly undesirable for pregnancy after surgery.
The period of contraception after surgical treatment for adenomyosis varies with the opinion of an attending physician, but most institutions recommend a contraception period of at least 6–12 months [40–42].
In 5 patients, the pregnancy ended in the birth of full-term live babies (all by caesarean section), while all pregnancies occurred spontaneously. Two patients had a missed pregnancy (at 8 and 12 weeks), one had a late spontaneous abortion in twin pregnancy (at 18 weeks). In all these three cases, pregnancy results from IVF. In all the 5 patients, the pregnancy was relatively successful. In early pregnancy, all the women had sufficiently long-term progesterone support (for a minimum of up to 24 weeks, for a maximum of up to 32 weeks) [43, 44]. Four patients underwent a planned caesarean section at 38 weeks’ gestation; in one case, the patient was delivered at 39 weeks on an emergency basis (due to late hospitalization). Neonatal parameters, such as body weight and Apgar score, were normal in all cases.
The researchers (Prof. N.N. Rukhlyada and K.Yu. Krylov, PhD) of the Department of Obstetrics and Gynecology, Saint Petersburg State Pediatric Medical University, performed 242 operations, of which 172 were done to restore fertility; moreover, the total number of pregnancies that had occurred was 74/172 (43.1% or 30.5% of all the interventions) (Fig. 1). In other cases, the purpose of the operation was to normalize menstrual blood loss and to eliminate the pain syndrome when the proposed hysterectomy was refused. The reproductive outcomes were distributed as follows: 10 cases of spontaneous abortion at 22 weeks’ gestation; 55 deliveries, 23 of them were premature (13.5, 74.3, and 31.1%, respectively). Thus, the total proportion of successful pregnancies was 39.1% of those who were interested in giving birth. The efficiency of laparoscopically assisted myomectomy described by Nezhat et al. is reported to be quite high [44–47]. Only 5 pregnancies occurred spontaneously; all the rest resulted from assisted reproductive technologies.
In January 2018 to September 2022, seventy-eight patients with adenomyosis were operated on in the Clinic of Obstetrics and Gynecology, Academician I.P. Pavlov First Saint Petersburg State Medical University. At the same time, surgery was performed in 13/78 patients (16.7%) in 2018, 10/78 (12.8%) in 2019, 18/78 (23.1%) in 2020, 22/78 (28.2%) in 2021, and 15/78 (19.2%) in 2022. Adenomyosis was histologically confirmed in all the patients.
The age of the patients varied from 23 to 45 years; their mean age was 37.6±4.99 years. The analysis covered included patients of reproductive age (18–45 years).
The main clinical manifestations of the disease in this group of patients were distributed as follows: heavy uterine bleeding in 54 patients, infertility in 20, chronic pelvic pain syndrome in 49, algomenorrhea in 49, and intermenstrual bleeding in 5. The concurrence of different clinical manifestations was most common in 82% (64/78); the disease was manifested only by infertility in 6.4% (5/78) of women, by intermenstrual bleeding in 2.6% (2/78), by chronic pelvic pain syndrome in 3.9% (3/78), and by heavy uterine bleeding in 5,1% (4/78).
The surgical volume performed in the study group of patients during myometrectomy (Osada operation) in 10% (8/78) and excision of an adenomyosis node in 90% (70/78). At the same time, half (4/8) of the patients underwent myometrectomy by laparotomic access, the remaining 50% (4/8) had laparotomy in combination with laparoscopy. The adenomyosis node was excised mainly by laparoscopic access in 74.3% (52/70) of patients, by hysteroresectoscopy in 21.4% (15/70), and by laparoscopy in combination with hysteroscopy in 4.3% (3/70).
Analyzing anamnestic data, instrumental techniques, the scope of performed surgical treatment, and histological findings in the study group revealed that the diagnosis of adenomyosis was the only one in 18% (14/78) of patients, that concurrent with other gynecological diseases in 82% (64/78). Among the most common concomitant gynecological diseases, there were uterine fibroids in 43.7% (28/64) of patents, uterine fibroids concurrent with external genital endometriosis in 21.9% (14/64), the latter in 18.8 % (12/64), endometrial polyp in 7.8% (5/64), and ovarian cyst in 7.8% (5/64).
In the early postoperative period, 38.5% (30/78) of patients were prescribed hormone therapy: 16/30 took gonadotropin-releasing hormone agonists; of them 2/16 received dydrogesterone after taking agonists: dienogest was given to 7/30 patients, dydrogesterone was used in 1/30; 2/30 patients received levonorgestrel-containing intrauterine system; one patient was prescribed a selective progesterone receptor modulator; 3/30 had combined oral contraceptives. The remaining 48/78 patients were prescribed hormone therapy after receiving the results of histological examination.
Conclusion
Considering our experience of surgical interventions by laparotomy, we can conclude that there is no unified technique for the surgical treatment of adenomyosis. The choice of incisions and the way of suturing the myometrium depend on both the sizes of the uterus and the location of the affected myometrium, its symmetry and uniformity, as well as on the volume of remaining intact tissue and the form of the defect. In some cases, the thickness and density of the walls of the uterine muscular layer after excision of adenomyosis does not allow the uterus to be fully restored, which forces the surgeon to perform myometrial plastic surgery using the adjacent tissues.
References
- Стрижаков А.Н., Давыдов А.И., Пашков В.М. Аденомиоз: возможности и перспективы эндохирургического лечения с учетом морфологического строения миометрия, эндометрия и яичников. Журнал акушерства и женских болезней. 2002; 51(3): 28-31. [Strizhakov A.N., Davydov A.I., Pashkov V.M. Adenomyosis: possibilities and prospects of endosurgical treatment, taking into account the morphological structure of the myometrium, endometrium and ovaries. Journal of Obstetrics and Women's Diseases. 2002; 51(3): 28-31. (in Russian)]. https://dx.doi.org/10.17816/JOWD91084.
- Leyendecker G., Kunz G., Kissler S., Wildt L. Adenomyosis and reproduction. Best Pract. Res. Clin. Obstet. Gynaecol. 2006; 20(4): 523-46.https://dx.doi.org/10.1016/j.bpobgyn.2006.01.008.
- Vercellini P., Consonni D., Barbara G., Buggio L., Frattaruolo M.P., Somigliana E. Adenomyosis and reproductive performance after surgery for rectovaginal and colorectal endometriosis: a systematic review and meta-analysis. Reprod. Biomed. Online. 2014; 28(6): 704-13. https://dx.doi.org/10.1016/j.rbmo.2014.02.006.
- Pelage L., Fenomanana S., Brun J.L., Levaillant J.M., Fernandez H. Treatment of adenomyosis (excl uding pregnancy project). Gynecol. Obstet. Fertil. 2015; 43(5): 404-11. https://dx.doi.org/10.1016/j.gyobfe.2015.03.016.
- Deffieux X., Fernandez H. Physispathologic, diagnostic and therapeutic evolution in the management of adenomyosis: review of the literature. J. Gynecol. Obstet. Biol. Reprod. (Paris). 2004; 33(8): 703-12. https://dx.doi.org/10.1016/s0368-2315(04)96631-8.
- Somigliana E., Chiodini A., Odorizzi M.P., Pompei F., Viganò P. The therapy of endometriosis. New prospects. Minerva Gynecol. 2003; 55(1): 15-23.
- Дамиров М.М. Аденомиоз. М.: БИНОМ; 2004. 384с. [Damirov M.M. Adenomyosis. Moscow: BINOM; 2004. 384p. (in Russian)].
- Koch J., Rowan K., Rombauts L., Yazdani A., Chapman M., Johnson N. Endometriosis and Infertility - a consensus statement from ACCEPT. Aust. N. Z. J. Obstet. Gynaecol. 2012; 52(6): 513-22. https://dx.doi.org/10.1111/j.1479-828X.2012.01480.x.
- Баскаков В.П., Цвелев Ю.В., Кира Е.Ф. Эндометриоидная болезнь. СПб.: Изд-во Н-Л; 2002. 452с. [Baskakov V.P., Tsvelev Yu.V., Kira E.F. Endometrioid disease. St Petersburg: N-L Publ.; 2002. 452p. (in Russian)].
- Адамян Л.В., Кулаков В.И., Андреева Е.Н. Эндометриозы. М.: Медицина; 2006. 416с. [Adamyan L.V., Kulakov V.I., Andreeva E.N. Endometriosis. Moscow: Medicine; 2006. 416p. (in Russian)].
- Ищенко А.И., Кудрина Е.А. Эндометриоз: диагностика и лечение. М: ГЭОТАР-Мед; 2002.104с. [Ischenko A.I., Kudrina E.A. Endometriosis: diagnosis and treatment. Moscow: GEOTAR-Med; 2002.104p. (in Russian)].
- Korczynski J., Sobkiewicz S. [Adenomiosis. Diagnostic technique and treatment. Ginekol. Pol. 2001; 72(5): 317-21.
- Рухляда Н.Н. Диагностика и лечение манифестного аденомиоза.Цвелев Ю.В., ред. СПб.: ЭЛБИ; 2004. 205с. [Rukhlyada N.N. Diagnosis and treatment of manifest adenomyosis. Tsvelev Yu.V., ed. St Petersburg: ELBI; 2004. 205p. (in Russian)].
- Радзинский В.Е., Гус А.И., Семятов С.М., Бутарева Л.Б. Эндометриоз: учебно-методическое пособие. М.; 2001. 52с. [Radzinsky B.E., Gus A.I., Semuyatov S.M., Butareva L.B. Endometriosis: textbook. Мoscow; 2001. 52p. (in Russian)].
- Kitade M., Kumakiri K., Kuroda J., Jinushi M., Ujihira Y., Ikuma K., Ozaki R. et al. Shikyusenkinsho gappei-funin ni taishite fukukukyoka-shikyu-onzon-ryoho wa yukoka?–jutsugo-ninshinritsu to senko-shujutsu no umu ni yoru shusanki-yogo no kento. [Is laparoscopic uterine preservation surgery effective against infertility associated with uterine adenomyosis? A study of perinatal prognosis by postoperative pregnancy rate and the presence of prior surgery]. J. Jpn. Soc. Endometriosis. 2017; 38: 70. https://dx.doi.org/10.1016/j.fertnstert.2018.01.032.
- Osada H., Nagaishi M., Teramoto S. Shikyukin furappuho niyoru shikyu-senkinsho tekishutsujutsu: Rinshoteki choki-yogo oyobi shikyuharetsuyobokoka no kento. [Adenomyomectomy by uterine muscle flap method: clinical outcome and investigation of the preventive effect on uterine rupture]. Obstet. Gynecol. (Tokyo). 2017; 84: 1303-15.
- Kikuchi I., Takeuchi H., Aida T., Kitade M., Shimanuki H., Kinoshita K. To-in deno shikyusenkinsho ni okeru ninyo no onzon shujutsu no kento. [A study of fertility preservation surgery in uterine adenomyosis.]. Obstet. Gynecol. Surg, Medical View Tokyo. 2003; 14: 93-9.
- Ota Y., Hada T., Natsuura T., Kanao H., Takaki Y., Kojima N. et al. To-in deno fukukukyoka shikyusenkinsho-setsujojutsu: Byoso no keijo ni chumokushita jutsushiki no tsukaiwake. [Convex lens resection of adenomyosis with laparoscopic adenomyomectomy in our hospital.]. J. Jpn. Soc. Endometriosis. 2008; 29: 85-90.
- Van Praagh I. Conservative surgical treatment for adenomyosis uteri in young women: Local excision and metroplasty. Can. Med. Assoc. J. 1965; 93(22): 1174-5.
- Kawamura R., Mishima Y., Nakagome H., Iwaki A., Kanemaki Y. Shikyusenkinsho ni taisuru kenbikyo-ka-shujutsu. [Microsurgical treatment for uterine adenomyosis.]. J. Jpn. Soc. Gynec. Microsurgery. 1991; 4: 18-21.
- Fedele L., Bianchi S., Zanotti F., Marchini M., Candiani G.B. Fertility after conservative surgery for adenomyomas. Hum. Reprod. 1993; 8(10): 1708-10. https://dx.doi.org/10.1093/oxfordjournals.humrep.a137919.
- Nabeshima H., Murakami T., Terada Y., Noda T., Yaegashi N., Okamura K. Total laparoscopic surgery of cystic adenomyoma under hydroultrasonographic monitoring. J. Am. Assoc. Gynecol. Laparosc. 2003;10(2): 195-9.https://dx.doi.org/10.1016/s1074-3804(05)60298-8.
- Nishimoto M., Nabeshima H. Shikyusenkinkakushutsujutu. [Adenomyomectomy]. J. Obstet. Gynecol. Prac. (Tokyo). 2011; 60: 1001-7.
- Saremi A.T., Bahrami H., Salehian P., Hakak N., Poolad A. Treatment of adenomyomectomy in women with severe uterine adenomyosis using a novel technique. Reprod. Biomed. Online. 2014; 28(6): 753-60.https://dx.doi.org/10.1016/j.rbmo.2014.02.008.
- Osada H. Shikyusenkinsho. [Uterine adenomyosis]. In: Osada H. Jissen fujinka fukkukyoka-shujutsu. [Laparoscopy for gynecology: a comprehensive manual and procedure DVD]. Tokyo: Medical View; 2009: 118-53.
- Struble J., Reid S., Bedaiwy M.A. Adenomyosis: a clinical review of a challenging gynecologic condition. J. Minim. Invasive Gynecol. 2016; 23(2): 164-85. https://dx.doi.org/10.1016/j.jmig.2015.09.018.
- Kodama K., Shirane A., Yamanaka A., Yanai S., Nakajima S., Hukuda M. et al. Fukukukyoka shikyusenkinsho-tekishutsujutsu go ni shikyuharetsu, yuchakutaiban wo mitome shikyutekishutsu ni itatta ichi-shorei. [A case of hysterectomy due to uterine rupture and placenta accreta after laparoscopic adenomyomectomy]. J. Jpn. Soc. Endometriosis. 2015; 36: 189-92.
- Ofir K., Sheiner E., Levy A., Katz M., Mazor M. Uterine rupture: risk factors and pregnancy outcome. Am. J. Obstet. Gynecol. 2003; 189(4): 1042-6.https://dx.doi.org/10.1067/s0002-9378(03)01052-4.
- Рухляда Н.Н., Крылов К.Ю., Бирюкова Е.И. Возможности органосохраняющих операций при аденомиозе в аспекте репродуктивной функции. Акушерство и гинекология. 2018; 7: 120-4. [Rukhlyada N.N., Krylov K.Yu., Biryukova E.I. Possibilities of organ-sparing surgery for adenomyosis in the context of reproductive function preservation. Obstetrics and Gynecology. 2018; (7): 120-4. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.7.120-124.
- Morimatsu Y., Matsubara S., Higashiyama N., Kuwata T., Ohkuchi A., Izumi A. et al. Uterine rupture during pregnancy soon after a laroscopic adenomyomectomy. Reprod. Med. Biol. 2007; 6(3): 175-7. https://dx.doi.org/10.1111/j.1447-0578.2007.00182.x.
- Azziz R. Adenomyosis in pregnancy. A review. J. Reprod. Med. 1986; 31(4): 224-7.
- Suginami H., Taniguchi F., Tokushige M. Senkinsho no shujutsuryoho. [Surgical treatment of adenomyosis]. Obstet. Gynecol. (Tokyo). 2008; 75: 72-8.
- Wada S., Kudo M., Minakami H. Spontaneous uterine rupture of a twin pregnancy after a laparoscopic adenomyomectomy: a case report. J. Minim. Invasive Gynecol. 2006; 13(2): 166-8. https://dx.doi.org/10.1016/j.jmig.2005.12.002.
- Yoshiki H. Kaifuku ni yoru shikyusenkinsho tekishutsujutsu. [Adenomymectomy by laparotomy]. J. Jan. Soc. Reprod. Surg. 2004; 1: 14-8.
- Honda R., Katabuchi H. Shikyusenkinsho ni taisuru shujutsu-ryoho to ninyosei. [Surgical therapy and fertility for adenomyosis]. Obstet. Gynecol. (Tokyo). 2009; 76: 1554-8.
- Fujishita A., Hiraki K., Kitajima M., Matsumoto Y., Satoh H., Masuzaki H. et al. Shikyusenkinsho to shikyu no onzon-chiryo. [Uterine adenomyosis and uterine preservation treatment]. J. Obstet. Gynecol. Prac. (Tokyo). 2010; 59: 769-76.
- Kishi Y., Yabuta M., Taniguchi F. Who will benefit from uterus-sparing surgery in adenomyosis-associated subfertility? Fertil. Steril. 2014; 102(3): 802-7.e1. https://dx.doi.org/10.1016/j.fertnstert.2014.05.028.
- Tanaka Y., Tsuji S., Ono T., Ishikawa A., Kita N., Takahashi K. et al. Toin ni okeru shikyusenkinsho kakushutsujutsu go ninshin juichi-rei no kento. [A study of 11 cases of adenomyomectomy in our hospital]. J. Jpn. Soc. Perin. Neon. Med. 2014; 50: 905.
- Nishida M., Otsubo Y., Ichikawa R., Arai Y., Sakanaka S. Shikyusenkinshokakushutsujutsu- go ninshin-ji no shikyuharetsu-yobo ni tsuite [Prevention of uterine rupture during pregnancy after adenomyomectomy]. Obstet. Gynecol. Surg. 2016; 27: 69-76.
- Grimbizis G.F., Mikos T., Tarlatzis B. Uterus-sparing operative treatment for adenomyosis. Fertil. Steril. 2014; 101(2): 472-87. https://dx.doi.org/10.1016/j.fertnstert.2013.10.025.
- Kim J.K., Shin C.S., Ko Y.B., Nam S.Y., Yim H.S., Lee K.H. Laparoscopic assisted adenomyomectomy using double flap method. Obstet. Gynecol. Sci. 2014; 57(2): 128-35. https://dx.doi.org/10.5468/ogs.2014.57.2.128.
- Huang X., Huang Q., Chen S., Zhang J., Lin K., Zhang X. Efficacy of laparoscopic adenomyomectomy using double-flap method for diffuse uterine adenomyosis. BMC Women's Health. 2015; 15: 24. https://dx.doi.org/10.1186/s12905-015-0182-5.
- Макаренко Т.А., Цхай В.Б. Опыт органосохраняющего хирургического лечения больных с тяжелыми формами аденомиоза. Журнал акушерства и женских болезней. 2016; 65(5): 96-9. [Makarenko T.A., Tskhay V.B. Experience of organ-preserving surgical treatment of patients with severe forms of adenomyosis. Journal of Obstetrics and Women's Diseases. 2016; 65(5): 96-9 (in Russian)]. https://dx.doi.org/10.17816/JOWD65596-99.
- Nezhat C., Nezhat F., Bess O., Nezhat C.H., Mashiach R. Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int. J. Fertil. Menopausal Stud. 1994; 39(1): 39-44.
- Tskhay V.B., Schindler A.E., Mikailly G.T. Operation, hormone therapy and recovery of the patients with severy forms of adenomeosis. Gynecol. Endocrinol. 2018; 34(8): 647-50. https://dx.doi.org/10.1080/09513590.2017.1397116.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil. Steril. 2018; 109(3): 406-17. https://dx.doi.org/10.1016/j.fertnstert.2018.01.032.
- Цхай В.Б., Микаиллы Г.Т., Костарева О.В., Каплунов В.А., Руф Р.Р. Беременность и роды после радикальной аденомиомэктомии и метропластики по методу Хисао Осада у женщин с диффузным аденомиозом, ассоциированным с бесплодием. Российский вестник акушера-гинеколога. 2019; 19(2): 63-7. [Tskhaĭ V.B., Mikailly G.T., Kostareva O.V., Kaplunov V.A., Ruf R.R. Pregnancy and labor after radical adenomyomectomy and metroplasty using the procedure developedby Hisao Osada in women with diffuse adenomyosis associated with infertility. Russian Bulletin of Obstetrician-Gynecologist. 2019; 19(2): 63-7. (in Russian)]. https://dx.doi.org/10.17116/rosakush20191902163.
Received 07.11.2022
Accepted 16.01.2023
About the Authors
Vitaliy F. Bezhenar, Dr. Med. Sci., Professor, Head of the Department of Obstetrics, Gynecology and Neonatology/Obstetrics, Gynecology and Reproductology,Academician I.P. Pavlov First St. Petersburg State Medical University, Ministry of Health of Russia; Chief Freelance Specialist obstetrician-gynecologist of the Health Committee of the Government of St. Petersburg, +7(812)338-78-66, bez-vitaly@yandex.ru, https://orcid.org/0000-0002-7807-4929, 197022, Russia, St. Petersburg, Lev Tolstoy str., 6-8.
Kirill Yu. Krylov, PhD, obstetrician-gynecologist, Senior Researcher at the Department of Gynecology, Saint Petersburg I.I. Dzhanelidze Research Institute of Emergency Care, +7(911)168-70-73, drkrylov@mail.ru, https://orcid.org/0000-0003-2149-5957, 192242, Russia, St. Petersburg, Budapestskaya str., 3, lit. A.
Tatyana A. Makarenko, Dr. Med. Sci., Associate Professor, Head of the Department of Operative Gynecology, Institute of Postgraduate Education,
Prof. V.F. Voyno-Yasenetsky Krasnoyarsk State Medical University, Ministry of Health of Russia, +7(904)895-47-99, makarenko7777@yandex.ru,
660022, Russia, Krasnoyarsk, Partizan Zheleznyak str., 1.
Valeriy I. Matukhin, Assistant at the Department of Obstetrics and Gynecology, St. Petersburg State Pediatric Medical University, Ministry of Health of Russia,
+7(981)737-04-72, val-matukhin@mail.ru, https://orcid.org/0000-0002-8906-8356, 194100, Russia, St. Petersburg, Litovskaya str., 2.
Nikolai N. Rukhliada, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology, St. Petersburg State Pediatric Medical University,
Ministry of Health of Russia, +7(911)913-20-20, nickolasr@mail.ru, https://orcid.org/0000-0002-3548-0468, 194100, Russia, St. Petersburg, Litovskaya str., 2.
Vitaly B. Tskhay, Dr. Med. Sci., Professor, Head of the Department of Perinatology, Obstetrics and Gynecology, Prof. V.F. Voyno-Yasenetsky Krasnoyarsk State Medical University, Ministry of Health of Russia; Scientific Supervisor in Obstetrics and Gynecology, Federal Siberian Scientific and Clinical Center of the FMBA of Russia,
+7(923)287-21-34, tchai@yandex.ru, 660022, Russia, Krasnoyarsk, Partizan Zheleznyak str., 1.



