Characteristics of the vaginal microbiota in pregnant women with preterm premature rupture of the membranes
Objective. To investigate the vaginal microbiota in women with preterm premature rupture of the membranes (PROM) at 22-36 weeks and 6 days’ gestation.Khodzhaeva Z.S., Guseinova G.E., Muravyeva V.V., Donnikov A.E., Mishina N.D., Priputnevich T.V.
Subjects and methods. Examinations were made in 150 women aged 18 to 40 years, who were divided into 3 groups: 1) 50 pregnant women with PROM; 2) 50 pregnant women with intact membranes, who delivered at 22-36 weeks and 6 days’ gestation; 3) 50 pregnant women who delivered spontaneously at term (at ≥ 37 weeks). The vaginal microbiota underwent comprehensive microbiological and molecular genetic studies. Microbial species identification was carried out by time-of-flight mass spectrometry.
Results. Pregnant women with PROM were somatically burdened with frequent respiratory and odontogenic inflammatory diseases; this pregnancy had a complicated course: threatened miscarriage due to protraction retroamniotic hematoma, the undulatory pattern of bloody vaginal discharge, and isthmic-cervical insufficiency that required surgical correction. Cesarean section was a statistically significantly more common childbirth delivery method in pregnant women. The women with PROM had a mixed, dysbiotic vaginal microflora. Unlike pregnant women who delivered at term, the women with PROM were deficient in lactobacillus and had larger relative number of opportunistic pathogens, mainly facultatively anaerobic bacteria.
Conclusion. The vaginal microbiota composition suggests that that there may be a risk for PROM. Monitoring the vaginal microbiota composition during pregnancy is a key step for the development of prognostic, prophylactic, and therapeutic strategies.
Keywords
Preterm birth (PB) is the leading cause of neonatal morbidity and mortality worldwide [1]. Consequences of preterm birth can include respiratory distress syndrome, bronchopulmonary dysplasia, retinopathy of prematurity, late-onset infection, developmental disability, and adverse neurological outcomes [2]. Preterm prelabor rupture of membranes (PPROM) is associated with 30% of all spontaneous preterm deliveries, thereby posing an important problem in obstetric practice [3]. The pathogenesis of the rupture of the membranes and subsequent maternal and neonatal diseases are associated with infection [4, 5]. In 80% of cases, childbirth occurs within nine days after the rupture of the membranes. During this period, the uterine cavity, placenta, and fetus are exposed to ascending infection resulting in high risk of chorioamnionitis with funisitis, which are associated with adverse maternal and neonatal outcomes [6–10]. The study of the vaginal microbiota and its characteristic features in pregnant women is important for pregnancy outcomes [11, 12]. The dysbiotic vaginal microbiota is a recognized risk factor for PPROM (22–36 weeks and 6 days) [13–15].
This study was aimed to characterize the changes in the composition of the vaginal microbiota of women with PPROM at 22–36 weeks and 6 days of gestation.
Materials and methods
This was a cross-sectional study comprising 150 women aged 18 to 40 years. All patients were divided into 3 groups. Group 1 consisted of 50 patients with PPROM at 36 weeks and 6 days’ gestation. Group 2 included 50 patients with intact fetal membranes at ≤ 36 weeks and 6 days’ gestation. Group 3 consisted of 50 somatically healthy pregnant women with uncomplicated obstetric and gynecological history and spontaneous full-term birth (≥37 weeks). Criteria for inclusion in groups 1 and 2 were as follows: a spontaneous singleton pregnancy which was complicated by PPROM at 22–36 weeks and 6 days’ gestation (group 1) and PB with intact fetal membranes (group 2). Criteria for inclusion in group 3 were spontaneous singleton pregnancy with a full-term (≥37 weeks) spontaneous delivery. All patients signed informed consent to take part in the study. Exclusion criteria were multiple-gestation pregnancy; pregnancy following assisted reproductive technologies (ART); structural and chromosomal abnormalities; severe somatic disorders of pregnancy; preeclampsia (PE during the index pregnancy; uterine malformations, and antibiotic therapy.
From each patient, samples of vaginal discharge from the posterior fornix were collected for culture, microscopy of gram-stained smears, and quantitative polymerase chain reaction (PCR) (Femoflor-16). In the group of pregnant women with PPROM, samples were collected at the time of the rupture of the membranes and before antibiotic therapy.
A comprehensive microbiological study included an assessment of the vaginal microbiocenosis according to microscopy data of vaginal gram-stained smears and culture by the medical technology “Integrated assessment of the vaginal microbiota. Diagnosis of opportunistic vaginitis.”. Vaginal discharge was cultured in a standard culture medium. To isolate facultative anaerobic microorganisms, we used Columbia Agar, Mannitol Salt Agar (Сonda, Spain), Endo Agar and Sabouraud Dextrose Agar (SRCAM&B. Obolensk, Russia). Lactobacilli were cultured on the Lactobacillus agar medium (SRCAM&B, Obolensk, Russia). Obligate anaerobes were cultured on pre-reduced Schaedler agar (Сonda, Spain) with the required additives. Cultures were incubated in a CO2 incubator (Jouan, France). Obligate anaerobes and lactobacilli were cultured in an anaerobic box (Jouan, France) in an atmosphere of a three-component gas mixture (N2-80%; CO2-10%; H2-10%). Species identification was conducted by the MALDI TOF MS method using an AutoFlex III Time-Of-Flight Mass Spectrometer with MALDI Biotyper software (Bruker Daltonik GmbH, Germany).
The molecular genetic study of vaginal microcenosis by quantitative PCR was performed using the Femoflor-16 test system (NPO DNA-Technology LLC, Russia) in a DT-96 detection amplifier (NPO DNA Technology LLC, Russia), according to manufacturer’s instructions.
Descriptive statistics included arithmetic mean and standard deviation M (SD) for continuous variables. Categorical variables were compared by the Chi-square test; the Fisher exact test was used if the expected count for a cell table was less than 5. Differences were considered statistically significant at p < 0.05.
Data on the samples are presented in the form of the content of microorganisms or their subgroups (hereinafter “microorganisms”). The proportion of each microorganism was determined by the degree of contamination in CFU/ml and GE. Descriptive statistics for microorganisms included medians (Me) and quartiles Q1 and Q3 for groups in the Me (Q1; Q3) format. The microorganisms were categorized as being in moderate/high (> 104 CFU/l) and low (<= 104 CFU/ml) concentrations. Therefore, to identify the statistical significance of intergroup differences, the Fisher exact test was used, which estimates the frequency of occurrence of microorganisms with a high (moderate) and low degree of colonization in the study groups.
Since the selected statistical test is used to compare two groups, but we had three study groups, the test was applied 6 times in the first block of tests:
- 3 times – for pairwise comparison of groups (groups 1 and 2, 1 and 3, and 2 and 3);
- 3 times - separately for each group (when dividing the entire sample in the “target group” and “rest,” respectively, comparing group 1 and (2, 3), group 2 and (1, 3), and group 3 and (1, 2); the symbol denotes pooling two groups that are included in the “rest”).
Some patients in groups 1 and 2 underwent surgical correction (SC) of ischemic-cervical insufficiency during pregnancy, which could affect vaginal microbiota composition at the time of sampling. To identify possible differences within or between study groups that could have SC consequences, the second set of statistical tests was also performed using Fisher’s exact test. At this stage, calculations were performed within groups: the frequency of the presence of microorganisms with high and low concentrations in the samples of patients of group 1 who had SC (group 1with SC) and did not have (group 1 without SC) was compared; a similar analysis was carried out within group 2. We also performed a pairwise comparison of the study groups only on samples of patients with SC (test for group 1 with SC and group 2 with SC) and on samples of patients without SC (respectively, tests for group 1 without SC and group 2 without SC, group2 without SC and group 3 without SC and group 3). For Fisher’s exact test, p <0.05 was considered statistically significant.
To visualize the most significant data, we used a heat map that displays the microorganism profiles in the samples. These microorganisms were selected after statistical tests (Fisher’s exact test), since their colonization rates significantly (p < 0.05) differed between the study groups, according to at least one of the tests.
Statistical analysis and visualization were performed using the Statistica 7.0 software package and additional Python 2.7 software developments and libraries, including Sklearn, Skipy, Pandas, and Matplotlib.
Results
Clinical and medical history characteristics of the study participants are presented in Table 1.
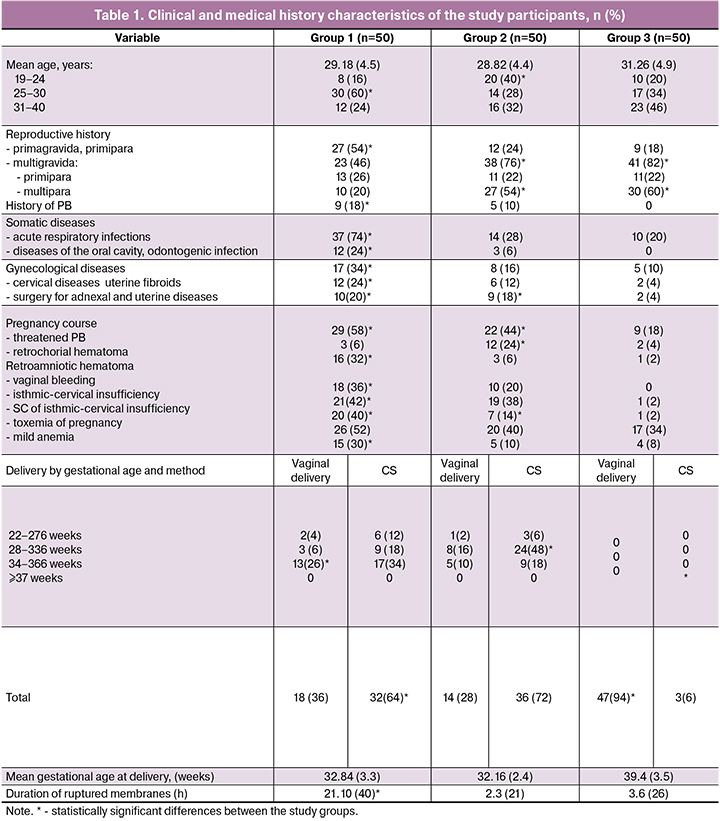
The age of the women ranged from 19 to 40 [mean 29.54 (5.0)] years, including 29.18 (4.5), 28.82 (4.4), and 31.26 (4.9) years in groups 1, 2, and control group, respectively. Women with PPROM were much more likely to be in the most active reproductive age of 25–30 years (p = 0.001). Primigravida women were statistically significantly more common in group 1 [27 (54%)], compared with groups 2 and 3 [12 and 9 (24% and 18%, respectively)], (p < 0.001).
There were no statistically significant differences between the study groups regarding multiparity. There were more multiparous women in groups 2 and 3 [27 and 30 (54% and 60%, respectively)] than in group 1 [10 (20%), (p <0.001)]. Respiratory and odontogenic inflammatory diseases were more common among women with PPROM; they also had a history of cervical diseases and uterine fibroids. Women with PPROM were notable for having a combination of several risk factors occurring during the index pregnancy, including a threatened miscarriage due to the presence of protractional retroamniotic hematomas, irregular bloody vaginal discharge, and isthmic-cervical insufficiency requiring surgical correction. The mean gestational age at delivery was 32.84 (3.3), 32.16 (2.4), and 39.4 (3.5) weeks in groups 1, 2, and 3, respectively. Women with PPROM had statistically significantly higher rates of a cesarean section (CS), but at 34–36 weeks and 6 days of pregnancy they had a vaginal delivery. The duration of ruptured membranes was statistically longer in patients with PPROM, ranging from 50 minutes to 105 hours 40 minutes [mean 21 hours 10 (40) minutes] compared with groups 2 and 3 [2.3 (21 minutes) and 3.6 (26 min), respectively (p <0.001)]. The most common indications for operative delivery in the group with PPROM were anhydramnios with more than 24-hour duration of ruptured membranes, irregular labor, impaired fetal status according to ultrasound and Doppler; in the group with intact fetal membranes - deterioration of the fetal condition, hypotonic uterine action, and the onset of acute fetal hypoxia. The mean birth weight was 2000.0 (605.0), 1917.0 (485.0), and 3308.0 (209.0) g in groups 1, 2, and 3, respectively (p = 0.01).
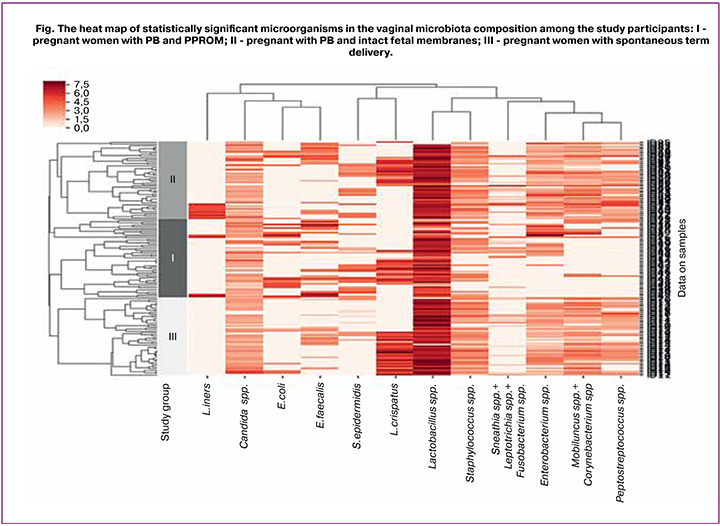
The study showed that in all three groups of women, the vaginal microbiota was characterized by a diversity of species composition. Forty-four groups of microorganisms were identified, including lactobacilli and opportunistic pathogens (OPs). Statistical analysis revealed 12 statistically significant species or groups of microorganisms (Figure). A microorganism was considered statistically significant if, when assessing the differences between groups and subgroups (with SC, without SC), at least one of the tests had a statistical significance of p < 0.05. The results of tests with high levels of significance of the selected microorganisms are given in Table 2.
The figure shows a heat map - a graphic image of selected microorganisms’ detection rates in the study groups and their quantitative characteristics. The dendrogram presents a graphical display of the results of preliminary cluster analysis with the selected distance metric - Spearman’s correlation coefficient. Cluster analysis was performed between microorganisms (columns) and within the study groups (the composition of the vaginal microflora in the samples is shown line by line).
Statistically significant microorganisms and their descriptive statistics, including median (Me) and quartiles Q1 and Q3, presented as Me (Q1; Q3) are given in Table. 2.
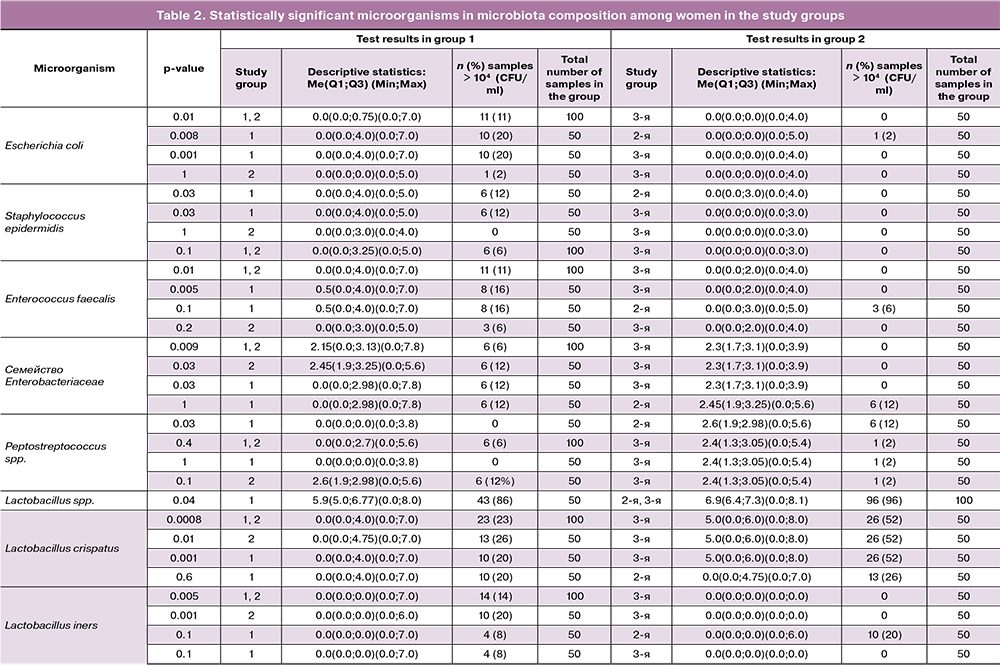
Results of culture tests and quantitative PCR (Table 2) identified the following statistically significant types of OPs and lactobacilli: Escherichiacoli (E. coli), Staphylococcus epidermidis (S.epidermidis), Enterococcus faecalis (E.faecalis), Enterobacteriaceae spp., Peptostreptococcus spp., Lactobacillus crispatus (L.crispatus), and Lactobacillus iners (L.iners) (p<0,05) (Fisher’s exact test).
E. coli in moderate or high concentration (104–107 CFU/ml) was found much more often (11/100; 11%) in pregnant women with PB (combined groups 1 and 2) (Me (Q1; Q3) - 0.0 (0.0; 0.75), while in most pregnant women of the control group the culture of this microorganism was negative or positive in rare cases with a low degree of colonization (less than 104 CFU/ml) (0.0 (0.0; 0.0), p = 0.01. Women with PPROM were found to have E. coli in moderate or high concentration 10 times more often (20 and 2%, respectively) than women with PB and intact fetal membranes; there was no statistically significantly difference between groups 1 and 2 [0.0 (0.0; 0.0; 4.0) and 0.0 (0.0; 0.0)], p = 0.008. At the same time, the detection rate and the degree of colonization of vaginal biotope with E.coli did not differ significantly (p = 1).
E.faecalis was isolated statistically more often (11/100; 11%) in moderate and high concentrations [104–107 CFU/ml - 0.0 (0.0; 4.0)] in patients with PB (combined groups 1 and 2) than in the control group (less than 104 CFU/ml – [0.0 (0.0; 2.0)], p = 0.01. The highest difference with the control group was noted in patients of group 1 (8/50 (16%)), p = 0.005, while group 2 (3/50; 6%) did not differ significantly difference from group 1 and the control group (p = 0.2 and p = 0.1, respectively).
S.epidermidis in group 1 was found in moderate concentrations [104–105 CFU/ml; 0.0 (0.0; 4.0)] in 6 (12%) women, while in groups 2 and 3 they were 0.0 (0.0 ; 3.0) and 0.0 (0.0; 0.0),i.e., this microorganism was either absent or the degree of colonization was low (less than 104 CFU/ml). A statistically significant difference was noted between groups 1 and 3 (p = 0.03) and between groups 1 and 2 (p = 0.03).
According to quantitative PCR, representatives of the Enterobacteriaceae family were found in 12 (12%) pregnant women with PB (combined groups 1 and 2) in moderate and high concentrations [104–107.8 GE –2.15 (0.0; 3.13)], while in group 3 most pregnant women had them in low concentrations [up to 103.9 HE - 2.3 (1.7; 3.1)], respectively, p = 0.009. In groups 1 and 2, OPs of the Enterobacteriaceae family were isolated in significant concentrations with similar detection rates [6/50 (12%) each], which had a statistically significant difference with the control group (p = 0.03). There were no statistically significant differences in this microorganism detection rates between groups 1 and 2 (p = 1).
Obligate anaerobes of the genus Peptostreptococcus, on the contrary, were found in moderate concentration [up to 105.6 GE - 2.6 (1.9; 2.98)] more often in group 2 (6/50; 12%), while in the majority of pregnant women in group they were absent or had low detection rates and had low concentrations [up to 103.8 GE - 0.0 (0.0; 0.0)], p = 0.03. Comparison of groups 1 and 3, combined groups 1 and 2 with group 3 and groups 2 and 3 showed no statistically significant differences.
According to direct protein profiling using the MALDI TOF MS method, 12 species of lactobacilli were identified. Four dominant species included L. crispatus, L. gasseri, L. jensenii, and L. iners. Statistically significant were L.crispatus and L.iners (Fisher’s exact test), p <0.05.
Species L. Crispatus in high concentration [105 - 108 CFU/ml - 5.0 (0.0; 6.0)] were most often isolated in the control group (26/50; 52%), but their detection rate among patients with PB (combined groups 1' and 2) was significantly lower (23/100; 23%) [≤107 CFU/ml - 0.0 (0.0; 4.0)], p = 0.0007. Seventy-seven women with PB had a significantly lower concentration of L. crispatus, not exceeding 104 CFU/ml. A separate comparison of this indicator in groups 1 and 3 and 2 and 3 also showed a significantly higher colonization level of the vaginal discharge with L. crispatus compared with the control group (p = 0.001 and p = 0.01, respectively). No statistically significant differences were found between groups 1 and 2.
In contrast, species L.iners were statistically more common in women with PB (combined groups 1 and 2) (14/100; 14%) with higher detection rate 1 group 2 (in 10 out of 14 cases); the colonization level varied within 104–107 CFU/ml (p = 0.001), while it was absent in the control group (p = 0.005). It should be noted that during this pregnancy, 20 (40%) women in group 1 and 7 (14%) women in group 2 underwent SC of ischemic-cervical insufficiency; however, analysis of vaginal microbiota showed no statistically significant differences.
Discussion
Our findings showed a diversity of the species composition of OPs in vaginal microbiota in women of all three groups. However, women with PB, including PPROM, were found to have more pronounced dysbiotic changes accompanied by lactobacilli deficiency and proliferation of OPs with a predominance of Enterobacteriaceae family (mainly E. coli), E. faecalis, S.epidermidis, and Peptostreptococcus spp. Similar data were obtained by Baldwin E.A. et al., and Brown RG. et al., who found that the colonization of the vaginal biotope by lactobacilli decreased markedly in pregnant women with PPROM, compared with women with an uncomplicated pregnancy and term delivery [16, 17]. Jeny P. Ghartey et al. studied the vaginal microbiota in pregnant women with PB at 18–32 weeks’ gestation and term delivery, and showed that the dominance of E. coli should be considered as a risk factor for adverse pregnancy outcomes and the cause of PPROM [18]. Dyatlova L.I. [19] analyzed vaginal microbiocenosis by PCR and reported that patients with PPROM at a gestational age of 22-34 weeks and low concentrations of lactobacilli, also showed a predominance of OPs including Enterobacteriaceae and Staphylococcus spp., Mobiluncus spp./Corynebacterium spp. Genove et al. [20] examined vaginal microbiota in 600 pregnant women. In 8 pregnant women with PPROM, the following microorganisms were isolated at 28 and 32 weeks: E. coli + Enterococcus spp. in 2 cases; E. coli + Peptococcus spp. in 1 case; E. coli + Enterobacter spp. in 1 case; Gardnerella vaginalis + Peptococcus spp. in 1 case in the absence of Lactobacillus.
Khodzhaeva Z.S. et al. [21] analyzed changes in vaginal microbiota in 6 women with PPROM throughout pregnancy. Analysis of vaginal microflora after rupture of the fetal membranes showed that four women had associations of two OPs (E. coli + E.faecalis and S. hominis + Candida albicans), four had (E. coli + S.lugdunensis + S. anginosus + Gardnerella vaginalis; S.epidermidis + S. agalactiae + E.faecalis + S.lugdunensis), and in one case E. Faecalis was isolated as a monoculture, indicating possible involvement of isolated OPs in PPROM. During the observation, all patients had at least one episode of nonspecific (aerobic) vaginitis caused by these microorganisms. These findings suggest that the detection of OPs in early pregnancy requires dynamic observation and timely correction of the vaginal microbiota.
The literature has been discussing the possible effect of various strains of lactobacilli on the stability of microbiota during pregnancy. Macintyre D. et al. [22] investigated changes in the vaginal microbiota during pregnancy and demonstrated that the highest stability of microbiota was observed when lactoflora was represented by species L. crispatus. Verstraelen H. et al. [23] noted that the highest stability of the vaginal ecosystem was determined by species L. crispatus and the lowest by L. gasseri and L. iners. It has been shown that the risk of dysbiotic disorders increases tenfold in patients who had vaginal colonization with lactobacilli of species L.gasseri/L. iners. In our study, L. crispatus was the dominant Lactobacillus strain among women who had term delivery, while in patients with PB, including with PPROM, the dominant strain was L.iners. The predominance of L. iners, according to several authors, is associated with an increased risk of developing severe dysbiotic disorders of the vaginal microbiota and is a risk factor for PB [24, 25]. Therefore, the identification of women with moderate dysbiosis of lactoflora, represented only by L. iners, is an unfavorable sign, and such variants of the microbiota may need correction.
Conclusion
Alterations of the vaginal microbiota composition in favor of OPs are associated with negative reproductive outcomes. Therefore, a comprehensive study of the vaginal microbiota composition in early pregnancy plays an important role in maintaining and preserving physiological pregnancy. A timely diagnosis of dysbiotic disorders is aimed to prevent PB, associated with PPROM, and improve maternal and neonatal outcomes.
References
- Liu L., Oza S., Hogan D., Perin J., Rudan I., Lawn J.E., et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015; 385(9966): 430–40. doi: 10.1016/S0140-6736(14)61698-6
- Iacovidou N., Varsami M., Syggellou A. Neonatal outcome of preterm delivery. Ann NY Acad Sci. 2010; 1205:130–4. doi: 10.1111/j.1749-6632.2010.05657.x
- Parry S., Strauss J.F. Premature rupture of the fetal membranes. N Engl J Med. 1998; 338(10): 663–70. doi: 10.1056/NEJM199803053381006
- Lamont R.F., Duncan S.L.B., Mandal D., Bassett P. Intravaginal clindamycin to reduce preterm birth in women with abnormal genital tract flora. Obstet Gynecol. 2003; 101(3): 516–22. doi: 10.1016/s0029-7844(02)03054-5
- Pappas A., Kendrick D.E., Shankaran S., et al. Chorioamnionitis and early childhood outcomes among extremely low-gestational-age neonates. JAMA Pediatr. 2014; 168(2):137–47. doi: 10.1001/jamapediatrics.2013.4248
- Rocha G., Proenca E., Quintas C., Rodrigues T., Guimaraes H. Chorioamnionitis and brain damage in the preterm newborn. J Matern Fetal Neonatal Med. 2007; 20(10): 745–9. doi: 10.1080/14767050701580515
- Lu H., Wang Q., Lu J., Zhang Q., Kumar P. Risk factors for intraventricular hemorrhage in preterm infants born at 34 weeks of gestation or less following preterm premature rupture of membranes. J Stroke Cerebrovasc Dis. 2016; 25(4): 807–12. doi: 10.1016/j.jstrokecerebrovasdis.2015.12.011
- Drassinower D., Friedman A.M., Obican S.G., Levin H., Gyamfi-Bannerman C. Prolonged latency of preterm premature rupture of membranes and risk of cerebral palsy. J Matern Fetal Neonatal Med. 2016; 29(17): 2748–52. doi: 10.3109/14767058.2015.1107539
- Van Dillen J., Zwart J., Schutte J., van Roosmalen J. Maternal sepsis: epidemiology, etiology and outcome. CurrOpin Infect Dis. 2010; 23(3): 249–54. doi: 10.1097/QCO.0b013e328339257c
- Puri K., Taft D.H., Ambalavanan N., Schibler K.R., Morrow A.L., Kallapur S.G. Association of chorioamnionitis with aberrant neonatal gut colonization and adverse clinical outcomes. PloS One. 2016; 11(9): e0162734. doi: 10.1371/journal.pone.0162734
- Romero R., Hassan S.S., Gajer P., Tarca A.L., Fadrosh D.W., Nikita L., Galuppi M., Lamont R.F., Chaemsaithong P., Miranda J., Chaiworapongsa T., Ravel J. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014; 2(1): 4. doi: 10.1186/2049-2618-2-4
- Romero R., Hassan S. Gajer P., Tarca A.L., Fadrosh D.W., Janine Bieda, Chaemsaithong P., Miranda J., Chaiworapongsa T., Rave J. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal deliveryat term. Microbiome. 2014; 2: 18, 1–15. https://doi.org/10.1186/2049-2618-2-18
- Fortunato S.J., Menon R., Lombardi S.J. Role of tumor necrosis factor-α in the premature rupture of membranes and preterm labor pathways. Am J Obstet Gynecol. 2002; 187(5): 1159–62. doi: 10.1067/mob.2002.127457
- Shobokshi A., Shaarawy M. Maternal serum and amniotic fluid cytokines in patients with preterm premature rupture of membranes with and without intrauterine infection. Int J Gynaecol Obstet. 2002; 79(3): 209–15. doi: 10.1016/s0020-7292(02)00238-2
- Helmig B.R., Romero R., Espinoza J., Chaiworapongsa T., Bujold E., Gomez R., et al. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J Matern Fetal Neonatal Med. 2002; 12(4): 237–46. doi: 10.1080/jmf.12.4.237.246
- Baldwin E.A., Walther-Antonio M., MacLean A.M., Gohl D.M., Beckman K.B., Chen J., White B., Creedon D.J., Chia N. Persistent microbial dysbiosis in preterm premature rupture of membranes from onset until delivery. Peer J. 2015; 3: e1398. doi: 10.7717/peerj.1398
- Brown R.G., Marchesi J.R., Lee Y.S., Smith A., Lehne B., Kindinger L.M., Terzidou V., Holmes E., Nicholson J.K., Bennett P.R., MacIntyre D.A. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018;16(1): 9. doi: 10.1186/s12916-017-0999-x.
- Ghartey J.P., Smith B.C., Chen Z., Buckley N., Lo Y., Ratner A.J., Herold B.C., Burk R.D. Lactobacillus crispatus Dominant Vaginal Microbiome Is Associated with Inhibitory Activity of Female Genital Tract Secretions against Escherichia coli. PLoSOne. 2014; 9(5): e96659. doi: 10.1371/journal.pone.0096659
- Дятлова Л.И. Особенности микробиоценоза влагалища при преждевременном разрыве околоплодных мембран при сроках гестации 22–34 недели. Международный журнал экспериментального образования. 2015; 3–4: 502–6. [Dyatlova L.I. Features vaginal microbiocenosis for rupture of membranes of 22-34 weeks. Mezhdunarodnyj zhurnal jeksperimental’nogo obrazovanija. 2015; 3–4: 502–6. (in Russian)]
- Genovese C., Corsello S., Nicolosi D., Aidala V., Falcidia E., Tempera G. Alterations of the vaginal microbiota in the third trimester of pregnancy and pPROM. European Review for Medical and Pharmacological Sciences. 2016; 20: 3336–43. PMID: 27608890
- Ходжаева З.С., Припутневич Т.В., Муравьева В.В., Гусейнова Г.Э., Горина К.А., Мишина Н.Д. Оценка состава и стабильности микробиоты влагалища у беременных в процессе динамического наблюдения. Акушерство и гинекология 2019; 7: 30–8. [Khodzhaeva Z.S., Priputnevich T.V., Murav’eva V.V., Guseinova G.E., Gorina K.A., Mishina N.D. The composition and stability of the vaginal microbiota in pregnant women during dynamic observation. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (7):30–8 (in Russian)]. doi:10.18565/aig.2019.7/30-38
- Macintyre D., Chandiramani M., Lee Y., Kindinger L., Smith A., Angelopoulos N., et al. The vaginal microbiome during pregnancy and the postpartum period in a European population. SciRep-Uk. 2015; 5: 8988. doi: 10.1038/srep08988
- Verstraelen H., Vilchez-Vargas R., Desimpel F., Jauregui R., Vankeirsbilck N., Weyers S., Verhelst R., De Sutter P., Pieper D.H., Van De Wiele T. Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1-2 region of the 16S rRNA gene. PeerJ. 2016; 4: e1602
- Kindinger L.M., Bennett P.R., Lee Y.S., Marchesi J.R., Smith A., Cacciatore S., et al. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome. 2017; 5(1): 6. doi: 10.1186/s40168-016-0223-9
- Petricevic L., Domig K.J., Nierscher F.J., Sandhofer M.J., Fidesser M., Krondorfer I., et al. Characterisation of the vaginal Lactobacillus microbiota associated with preterm delivery. Sci Rep. 2014; 4: 5136. doi: 10.1038/srep05136
Received 06.06.2019
Accepted 21.06.2019
About the Authors
Zulfiya S. Khodzhaeva, M.D., Professor, Head of High Risk Pregnancy Dept,National Medical Research Center for Obstetrics, Gynecology and Perinatology
named after Academician V.I. Kulakov Ministry of Healthcare of Russian Federation. Phone: +7 (916) 407-75-67. E-mail: zkhodjaeva@mail.ru
117997, Russia, Moscow, Akademika Oparina str. 4.
Gulnara E. Guseynova, graduate student in the Department of Pregnancy Pathology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Healthcare of Russian Federation. Phone: +7 (967) 153-18-81. E-mail: marysca666@rambler.ru
117997, Russia, Moscow, Akademika Oparina str. 4.
Vera V. Muravieva, senior researcher, PhD in the Laboratory of microbiology, Departament of microbiology and clinical pharmacology, National Medical Research Center
for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Healthcare of Russian Federation. Phone:+7(495)438-25-33;
Е-mail: v_muravieva@oparina4.ru
117997, Russia, Moscow, Akademika Oparina str. 4.
Andrew E. Donnikov, PhD, Leading Researcher of molecul-genetical, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Healthcare of Russian Federation, Phone:+7 (903) 684-52-47. Е-mail: donnikov@dna-technology.ru
117997, Russia, Moscow, Akademika Oparina str. 4.
Nataliia D. Mishina, junior researcher, Department of clinical and molecular genetics, FSBI «Research Center of Obstetrics, Gynecology and Perinatology of V.I. Kulakov» Ministry of Healthcare of the Russian Federation. Phone: +7(985)217-29-89. E-mail: mis7ha@gmail.com
119997, Russian Federation, Moscow, Akademika Oparina street, 4.
Tatyana V. Priputnevich, Doctor of science, Head of the Departament of microbiology and clinical pharmacology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Healthcare of Russian Federation. Phone: +7(903) 264-12-57. Е-mail: priputl@gmail.com
117997, Russia, Moscow, Akademika Oparina str. 4.
For citation: Khodzhaeva Z.S., Guseynova G.E., Muravjeva V.V., Donnikov A.E., Priputnevich T.V. Characteristics of the vaginal microbiota in pregnant women with preterm premature rupture of the membranes.
Akusherstvo i Ginekologiya /Obstetrics and Gynecology. 2019; (12):66-74.(in Russian)
http://dx.doi.org/10.18565/aig.2019.12.66-74



