Characteristics of dynamic changes in proteomic composition of cervicovaginal fluid in cervical diseases associated with HPV infection
Objective. To develop a non-invasive approach to the differential diagnosis of HPV-associated cervical diseases in patients of reproductive age with minor lesions of the cervical epithelium based on a proteomic analysis of cervicovaginal fluid (CVF).Starodubtseva N.L., Brzhozovskiy A.G., Bugrova A.E., Kononikhin A.S., Gusakov K.I., Nazarova N.M., Frankevich V.E.
Materials and methods. Samples of CVF were obtained from seven patients aged 26–36 years (31 ± 4) with minor HPV-associated cervical lesions (ASCUS, LSIL) during the dynamic observation (0, 6 and 12 months). Semi-quantitative analysis of proteomic data (HPLC-MS/MS), including protein identification and annotation, was carried out using the MaxQuant and STRING software.
Results. A significant change in the level of seven proteins SOD1, CSTA, FGB, ENO1, BPI, BPIFB1, SERPINB13 was revealed during the dynamic observation. The analysis of enriching functional groups based on GeneOntology data showed that these proteins are most associated with the activation of immune (innate immunity) and antimicrobial processes, and are also involved in the regulation of apoptosis and epithelial-mesenchymal transformation. The change in the level of the proteins is likely to be mediated by the elimination of the HPV virus and associated neoplastic transformation of the cervical epithelium.
Conclusion. Proteomic analysis of CVF revealed a panel of proteins that can be used to assess the dynamics of the pathological process and the effectiveness of the chosen treatment strategy for patients with neoplastic changes in the cervix.
Keywords
Nowadays, the number of cases of cervical diseases associated with the long-term persistence of human papillomavirus (HPV) in reproductive-aged women is increasing [1]. According to the World Health Organization (WHO), more than 490,000 new cases of HPV-associated cervical cancer are diagnosed every year. As a rule, the oncological process is preceded by cervical intraepithelial neoplasia of various degrees of severity. In low-grade squamous intraepithelial lesions (LSIL), the process often spontaneously regresses within a few months. However, long-term persistence of high-risk HPV leads to high-grade squamous intraepithelial lesions (HSIL) in 27% of cases, which develop into cervical cancer in 40–45% of cases [2, 3]. The transformation of the cervical epithelium from negative for intraepithelial lesion or malignancy (NILM) to HSIL takes from 1 to 10 years in patients with papillomavirus infection. At the molecular level, there is a change in cell differentiation, imbalance in the mechanisms of cell proliferation and apoptosis.
The specialists in different countries use various diagnostic schemes for HSIL and cervical cancer, including morphological examination of biopsy material (which is a gold standard), cytological screening of epithelial smear, extended colposcopy, liquid-based cytology, HPV typing and determining viral load, and other methods. The most common method for diagnosing abnormal cervical epithelium is colposcopy examination with biopsy [4]. The structure of the tissue can be evaluated on histological examination; however, it is impossible to assess the risk of progression of the pathological process. The choice of patient management strategy, including the extent of invasive interventions, is a key point for patients of reproductive age with cervical intraepithelial neoplasia. Dynamic observation is the primary tactic for managing patients with LSIL, but in some cases it is necessary to administer a therapy, namely in case of a high risk for disease progression. In this regard, the diagnosis of HPV-associated diseases at the early stages of their development is an important timely help for patients at risk.
Modern research methods which are applied in the analysisofthemolecularcompositionofvariousbiological fluids have potential for the diagnosis of HPV-associated cervical diseases. In such studies, biomarker molecules have been frequently detected using postgenomic methods, namely proteomic ones, which can determine the protein composition of blood plasma, urine, cervicovaginal fluid (CVF), liquor, etc. [5–7].
Identification of potential marker proteins in CVF in various pathological conditions (bacterial vaginosis, vaginitis, cervicitis, premature rupture of membranes) allows specialists to make an early non-invasive diagnosis and monitor gynecological and obstetric pathologies [8–10]. Changes in the CVF proteome can be suggestive of the pathogenesis, cause and stage of the disease.
The purpose of this study is to identify the dynamic changes in the proteomic composition of CVF for the validation of a non-invasive differential approach to the diagnosis of HPV-associated cervical diseases in patients of reproductive age with minor lesions of the cervical epithelium.
Materials and Methods
Study design
Characteristics of the protein composition of CVF in patients with HPV-associated minor cervical lesions (atypical squamous cells of undetermined significance (ASCUS) and LSIL) were determined on the basis of a comprehensive assessment of the dynamics of the pathological process. The study included samples of seven reproductive-aged patients (mean age is 31 years) who were monitored in the National Medical Research Centre for Obstetrics, Gynecology and Perinatology, Moscow. The samples were obtained three times during the dynamic observation within a year. The exclusion criteria were pregnancy/lactation, hormone replacement therapy, inflammatory diseases in the stage of exacerbation, impaired functions of the kidneys, liver, or lungs in the decompensation stage, and neuropsychiatric diseases. All patients underwent extended colposcopy with the collection of material for cytological, molecular genetic (HPV genotyping type 21) and proteomic studies three times: on their first admission, 6 and 12 months later. The study was approved by the Local Ethics Committee of the National Medical Research Centre for Obstetrics, Gynecology and Perinatology; informed consent was obtained from all the patients.
Sample preparation and proteomic analysis of CVF samples
In order to minimize the risk of blood contamination of the sample, the vagina and cervix were irrigated with saline before performing any manipulations. Then, centrifugation was performed to remove epithelial cells and supernatant fluid was frozen and stored at -80º C. After quick defrosting, the proteins were restored in 100 mM dithiothreitol followed by alkylation with 50 mM iodoacetamide, precipitation of the protein mixture with 0.1% trifluoroacetic acid in acetone and trypsinolysis according to the existing protocol [7, 11].
Tryptic peptides were separated by reversed-phase high-performance liquid chromatography (Agilent 1100, USA) with identification by tandem mass spectrometry (7-Tesla LTQ-FT Ultra mass spectrometer, Thermo Electron, Germany) with an electrospray ion source in the DDA mode [7, 11]. Each sample was analyzed in three consecutive repetitions.
Bioinformatic and statistical analysis
Processing of mass spectrometric data was performed in the MaxQuant software package (version 1.5) [12] with identification of peptides and their corresponding proteins from the SwissProt database with the following parameters: the minimum length of the amino acid sequence is seven amino acid residues, FDR<0.01, the minimum number of tryptic peptides per protein is two, and at least one is unique [11].
Semi-quantitative data obtained in the MaxQuant program were further processed in the Perseus program (1.5.5.3). After loading the matrix, proteins that were only identified by site and by Gene Ontology (Reverse) base were excluded from the analysis. ANOVA test (p>0.01) and Welch’s t-test were performed to detect statistically significantly changing proteins in comparison with the control group. The analysis of overrepresentation of biological processes by Gene Ontology (GO) base was performed using the STRING resource [13]. Experimental data is available on the ProteomeXchange resource using ID PXD011472.
Results and Discussion
In this work, the changes in the proteomic composition of 24 CVF samples obtained from 4 patients with ASCUS and 3 patients with LSIL were analyzed during dynamic observation. Samples were collected every 6 months after the first admission (each patient had only 3 periods). High-risk HPV was revealed in 90% of patients with ASCUS. In the LSIL group, all patients had high-risk HPV. During the observation there was an elimination of HPV virus in all cases, the cytological analysis did not deteriorate.
As a result of proteomic analysis, more than 400 different proteins were detected. Statistical analysis showed a significant change in the level of 44 CVF proteins. After 6 months, the level of 17 different proteins changed, including ANXA2, CSTA, and SERPINB13 which were suggested as potential markers of neoplastic transformation in the previous study [7]. CSTA is an important mucosal protein with significant immunomodulatory properties, inhibiting cysteine proteases that block the action of endogenous and bacterial proteases [14]. CSTA also participates in desmosomal cell adhesion at the lower levels of the epidermis [15]. There was a decrease in the level of SERPINB13, which belongs to the serine protease inhibitor family. Elevated levels of SERPINB13 were previously observed in inflammatory and neoplastic skin diseases, including squamous cell carcinoma [16].
The level of SOD1, CSTA, FGB, ENO1, BPI, BPIFB1, and SERPINB13 proteins significantly changed during the entire dynamic observation (Fig. 1). Analysis of the enrichment of functional groups using GeneOntology (GO) base revealed that the most significant issue about these proteins is their participation in the processes of humoral antimicrobial response, negative regulation of the cellular process, response to external stimulus, negative regulation of the apoptotic process, multiorgan processes, response to an exogenous organism, negative regulation of the apoptotic process of epithelial cells and regulation of the apoptotic signaling pathway (Fig. 2).
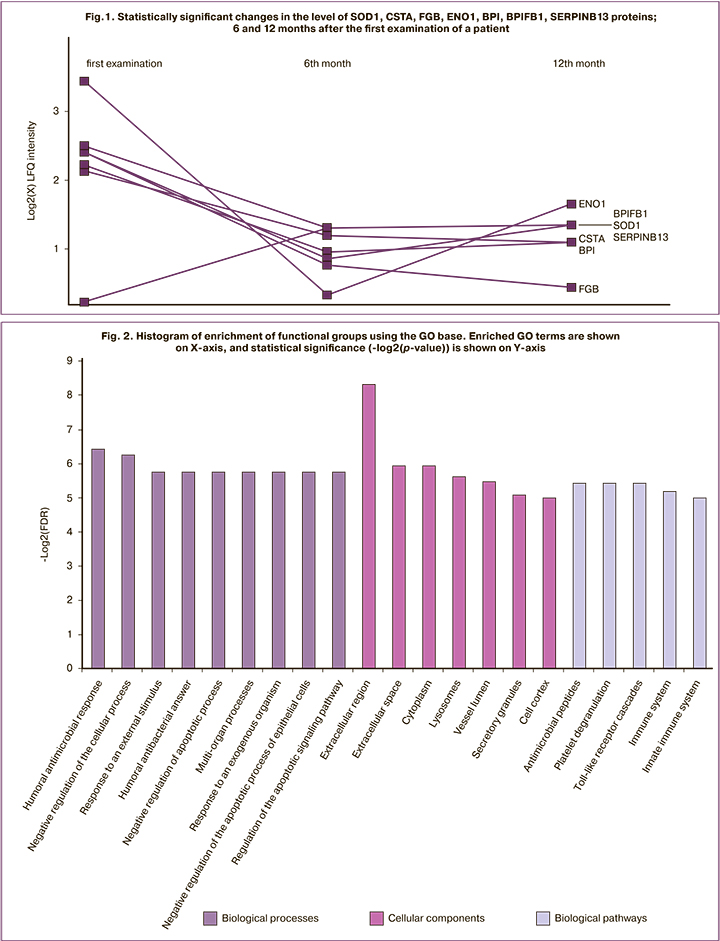
Activation of immune processes may be due to the changes in the vaginal and endocervical micr obial composition during the pathological process [17], as well as the persistence of HPV virus. This is indicated by the participation of these proteins in the biological pathways of innate immunity, as well as in antimicrobial processes. Reduced levels of BPI, BPIFB1 and CSTA proteins may result from patients’ recovery. BPI proteins are important cytotoxic proteins of polymorphonuclear leukocytes; BPI proteins often have an affinity for gram-negative bacteria [18] that may not be typical for the microbiotic composition of the cervical canal, colonized by Lactobacillus sp. [17]. In turn, these proteins have a high affinity for Sneathia spp., which is a microbiological marker of papillomavirus infection [19]. Another important contributor to innate immunity is FGB. This protein participates in the immune response by transmitting a signal via Toll-like receptors [20]. Moreover, FGB participates in the apoptotic process, namely, in the regulation of epithelial cell apoptosis and the regulation of the apoptotic cascade [21].
The levels of 34 CVF proteins significantly changed 12 months after the first treatment. Among them, protein KLK6 is worth noting, as it performs a protective function in the development of the malignant process, and participates in epithelial-mesenchymal transformation [22], as well as the protein SBSN, which is a significant predictive protein, a marker of epidermal cells. The changes in the levels of these proteins are likely to be mediated by the elimination of the pathological process in patients.
Conclusion
The panel of CVF proteins specific to HPV-associated neoplastic process was tested during the dynamic observation of patients with minor lesions of the cervical epithelium who were assessed three times within a year (once in every 6 months). These proteins reflect the dynamics of the pathological process, and their assessment can be used in the evaluation of the effectiveness of the chosen treatment strategy for patients with neoplastic changes in the cervix in terms of preserving reproductive function.
References
- Torre L.A., Bray F., Siegel R.L., Ferlay J. Global Cancer Statistics, 2012. CA Cancer J. Clin. 2015; 65: 87-108. https://dx.doi.org/10.3322/caac.21262.
- Wheeler C.M., Hunt W.C., Schiffman M., Castle P.E., Squamous A. Human papillomavirus genotypes and the cumulative 2-year risk of cervical precancer. J. Infect. Dis. 2006; 194(9): 1291-9. https://dx.doi.org/10.1086/507909.
- Doeberitz M.V.K. New markers for cervical dysplasia to visualise the genomic chaos created by aberrant oncogenic papillomavirus infections. Eur. J. Cancer. 2002; 38(17): 2229-42. https://dx.doi.org/10.1016/s0959-8049(02)00462-8.
- Ullal A.J., Litaker R.W., Noga E.J. Antimicrobial peptides derived from hemoglobin are expressed in epithelium of channel catfish. Dev. Comp. Immunol. 2008; 32(11): 1301-12. https://dx.doi.org/10.1016/j.dci.2008.04.005.
- Van Ostade X., Dom M., Tjalma W., Van Raemdonck G. Candidate biomarkers in the cervical vaginal fluid for the ( self‑) diagnosis of cervical precancer. Arch. Gynecol. Obstet. 2018; 297: 295-311. https://dx.doi.org/10.1007/s00404-017-4587-2.
- Siciliano R.A., Mazzeo M.F., Spada V., Facchiano A., Acierno A., Stocchero M. et al. Rapid peptidomic profiling of peritoneal fluid by MALDI-TOF mass spectrometry for the identification of biomarkers of endometriosis. Gynecol. Endocrinol. 2014; 30(12): 872-6. https://dx.doi.org/10.3109/09513590.2014.943718.
- Starodubtseva N.L., Brzhozovzkiy A.G., Kononikhin A.S., Bugrova A.E., Nazarova N.M., Gusakov K.I., Indeykina M.I., Chagovets V.V., Frankevich V.E., Nikolaev E.N., Sukhikh G.T. Label‐free cervicovaginal fluid proteome profiling reflects the cervix neoplastic transformation. J. Mass Spectrom. 2019; 54(8): 693-703. https://dx.doi.org/10.1002/jms.4374.
- Zegels G., Van Raemdonck G.A.A., Tjalma W.A.A., Van Ostade X.W.M. Use of cervicovaginal fluid for the identification of biomarkers for pathologies of the female genital tract. Proteome Sci. 2010; 8: 63. https://dx.doi.org/10.1186/1477-5956-8-63.
- Novak R.M., Donoval B.A., Graham P.J., Boksa L.A., Spear G., Hershow R.C. et al. Cervicovaginal levels of lactoferrin , secretory leukocyte protease inhibitor and RANTES and the effects of coexisting vaginoses in human immunodeficiency virus ( HIV ) -seronegative women with a high risk of heterosexual acquisition of HIV infection. Clin. Vaccine Immunol. 2007; 14(9): 1102-7. https://dx.doi.org/10.1128/CVI.00386-06.
- Shaw J.L.V., Smith C.R., Diamandis E.P. Proteomic analysis of human cervico-vaginal fluid. J. Proteome Res. 2007; 6(7): 2859-65.
- Starodubtseva N.L., Kononikhin A.S., Bugrova A.E., Chagovets V., Indeykina M., Krokhina K.N., Nikitina I.V., Kostyukevich Y.I., Popov I.A., Larina I.M., Timofeeva L.A., Frankevich V.E., Ionov O.V., Nikolaev E.N., Sukhikh G.T. Investigation of urine proteome of preterm newborns with respiratory pathologies. J. Proteomics. 2016; 149: 31-7. https://dx.doi.org/10.1016/j.jprot.2016.06.012.
- Tyanova S., Temu T., Cox J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016; 11(12): 2301-19. https://dx.doi.org/10.1038/nprot.2016.136.
- von Mering C., Huynen M., Jaeggi D., Schmidt S., Bork P., Snel B. STRING: a database of predicted functional associations between proteins. Nucleic Acids Res. 2003; 31: 258-61. https://dx.doi.org/10.1093/nar/gkg034.
- Fábián T.K., Hermann P., Beck A., Fejérdy P., Fábián G. Salivary defense proteins: Their network and role in innate and acquired oral immunity. Int. J. Mol. Sci. 2012; 13(4): 4295-320. https://dx.doi.org/10.3390/ijms13044295.
- Blaydon D.C., Nitoiu D., Eckl K.M., Cabral R.M., Bland P., Hausser I. et al. Mutations in CSTA, encoding cystatin A, underlie exfoliative ichthyosis and reveal a role for this protease inhibitor in cell-cell adhesion. Am. J. Hum. Genet. 2011; 89(4): 564-71. https://dx.doi.org/10.1016/j.ajhg.2011.09.001.
- Bylaite M., Moussali H., Marciukaitiene I., Ruzicka T., Walz M. Expression of cathepsin L and its inhibitor hurpin in inflammatory and neoplastic skin diseases. Exp. Dermatol. 2006; 15(2): 110-8. https://dx.doi.org/10.1111/j.1600-0625.2005.00389.x.
- Lehne B., Smith A., Bennett P.R., Mitra A., Lee Y.S., Li J.V. et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci. Rep. 2015; 5: 1-11. https://dx.doi.org/10.1038/srep16865.
- Gray P.W., Flaggs G., Leong S.R., Gumina R.J., Weiss J., Ooi C.E., Elsbach P. Cloning of the cDNA of a human neutrophil bactericidal protein. Structural and functional correlations. J. Biol. Chem. 1989; 264(16): 9505-9.
- Mitra A., MacIntyre D.A., Marchesi J.R., Lee Y.S., Bennett P.R., Kyrgiou M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: What do we know and where are we going next? Microbiome. 2016; 4(1): 58. https://dx.doi.org/10.1186/s40168-016-0203-0.
- Boyd J.M., Schlievert P.M., Rosenthal C.B., Crosby H.A., Salgado-Pabón W., Walker J.N. et al. The Staphylococcus aureus ArlRS Two-Component System Is a Novel Regulator of Agglutination and Pathogenesis. PLoS Pathog. 2013; 9: e1003819. https://dx.doi.org/10.1371/journal.ppat.1003819.
- Pluskota E., D’Souza S.E. Fibrinogen interactions with ICAM-1 (CD54) regulate endothelial cell survival. Eur. J. Biochem. 2000; 267(15): 4693-704. https://dx.doi.org/10.1046/j.1432-1327.2000.01520.x.
- Pampalakis G., Sotiropoulou G., Vlahou A., Agalioti T., Prosnikli E., Zoumpourlis V. A tumor-protective role for human kallikrein-related peptidase 6 in breast cancer mediated by inhibition of epithelial-to-mesenchymal transition. Cancer Res. 2009; 69(9): 3779-87. https://dx.doi.org/10.1158/0008-5472.can-08-1976.
Received 24.04.2020
Accepted 17.06.2020
About the Authors
Natalia L. Starodubtseva, Ph.D., Head of the Laboratory of Proteomics of Human Reproduction, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Healthcare of Russian Federation. Tel.:+7(916)463-98-67. E-mail: n_starodubtseva@oparina4.ru.4, Oparina str., Moscow, 117997, Russian Federation.
Alexander G. Brzhozovskiy, Researcher, Laboratory of Proteomics of Human Reproduction, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Healthcare of Russian Federation. Tel.: +7(495)531-44-44. E-mail: agb.imbp@gmail.com.
4, Oparina str., Moscow, 117997, Russian Federation.
Anna E. Bugrova, Ph.D., Senior Researcher, Laboratory of Proteomics of Human Reproduction, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Healthcare of Russian Federation. Tel.: +7(926)562-65-90. E-mail: a_bugrova@oparina4.ru.
4, Oparina str., Moscow, 117997, Russian Federation.
Aleksey S. Kononikhin, Ph.D. Researcher, Laboratory of Proteomics of Human Reproduction, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I.Kulakov of the Ministry of Healthcare of Russian Federation. Tel.: +7(926)562-65-90. E-mail: konoleha@yandex.ru.
4, Oparina str., Moscow, 117997, Russian Federation.
Kirill I. Gusakov, PhD Student, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Healthcare of Russian Federation. Tel.: +7(495)438-14-03. E-mail: kigusakov@gmail.com. 4, Oparina str., Moscow, 117997, Russian Federation.
Niso M. Nazarova, MD, Leading Researcher, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I.Kulakov of the Ministry of Healthcare of Russian Federation. Tel.: +7(495)438-14-03. E-mail: grab2@yandex.ru. 4, Oparina str., Moscow, 117997, Russian Federation.
Vladimir E. Frankevich, Ph.D., Head of the Department of system biology in reproduction, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Healthcare of Russian Federation. Tel.: +7(495)438-07-88 (2198). E-mail: v_frankevich@oparina4.ru.
4, Oparina str., Moscow, 117997, Russian Federation.
For citation: Starodubtseva N.L., Brzhozovskiy A.G., Bugrova A.E., Kononikhin A.S., Gusakov K.I., Nazarova N.M., Frankevich V.E. Characteristics of dynamic changes in proteomic composition of cervicovaginal fluid in cervical diseases associated with HPV infection.
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2020; 7: 111-116 (in Russian)
https://dx.doi.org/10.18565/aig.2020.7.111-116



