Risk factors and protective factors for refractory postpartum hemorrhage
Artymuk N.V., Marochko T.Yu., Artymuk D.A., Apresyan S.V., Kolesnikova N.B., Atalyan A.V., Shibelgut N.M., Batina N.A.
Objective: To study the incidence of refractory postpartum hemorrhage (PPH) at a tertiary-level hospital and identify its risk factors and protective factors.
Materials and methods: In the first stage, a cross-sectional study was conducted to assess the incidence of PPH and refractory PPH in a tertiary-level hospital. This study included all patients who delivered between 2019 and 2022 (n=15,480). In the second stage, a retrospective case-control study was conducted with 220 patients to assess the protective and risk factors for refractory PPH. Using univariate binary logistic regression analysis, 178 clinical, anamnestic, and laboratory factors were evaluated.
Results: The incidence of PPH in tertiary-level hospital was 0.67%, while the incidence of refractory PPH was 0.36%. Significant risk factors for refractory PPH were placenta accreta – OR 23.77 (95% CI 2.85–198.01), p=0.003 and augmentation of labor – OR 17.09 (95% CI 1.43–204.16), p=0.02. In addition, significant risk factors for refractory PPH were placental abruption – OR 13.87 (95% CI 2.85–67.56), p<0.001; uterine hypotony during cesarean section – OR 10.0 (95% CI 3.04–32.94), p<0.001; placenta adhaerens – OR 9.48 (95% CI 2.36–38.04), p=0.002; placenta previa – OR 4.81 (95% CI 1.9–12.16), p<0.001; cesarean delivery – OR 4.61 (95% CI 2.3–9.23), p<0.001; Uterine scar after Cesarean section – OR 4.48 (95% CI 2.32–8.65), p<0.001; cesarean delivery due to severe preeclampsia – OR 4.03 (1.04–15.57), p=0.04. The protective factors for refractory PPH included vaginal delivery – OR 0.22 (95% CI 0.11–0.43; p<0.001), fibrinogen level – OR 0.65 (95% CI 0.47–0.91; p=0.01), and gestational age – OR 0.85 (95% CI 0.74–0.98; p=0.02).
Conclusion: Risk factors for refractory postpartum hemorrhage (PPH) include abnormal placentation (such as placenta accreta, placenta increta, placenta percreta, and placenta previa), augmentation of labor, cesarean delivery, cesarean delivery due to severe preeclampsia, placental abruption, the presence of a post-cesarean uterine scar, and uterine hypotony during cesarean section. Protective factors include gestational age, vaginal delivery, and fibrinogen level.
Authors' contributions: Artymuk N.V., Apresyan S.V., Atalyan A.V. – conception and design of the study; Artymuk D.A., Marochko T.Yu., Kolesnikova N.B., Shibelgut N.M., Batina N.A. – data collection; Atalyan A.V. – analysis, management and visualization of data; Artymuk D.A., Marochko T.Yu. – drafting of the manuscript; Artymuk N.V., Apresyan S.V., Atalyan A.V. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Kemerovo State Medical University (Ref. No: 309/k of 14.06.2023).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Artymuk N.V., Marochko T.Yu., Artymuk D.A., Apresyan S.V., Kolesnikova N.B., Atalyan A.V.,
Shibelgut N.M., Batina N.A. Risk factors and protective factors for refractory postpartum hemorrhage.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (10): 82-90 (in Russian)
https://dx.doi.org/10.18565/aig.2024.169
Keywords
Postpartum hemorrhage (PPH) remains a major cause of maternal morbidity and mortality worldwide; however, most maternal deaths associated with PPH are preventable [1, 2]. The prevalence of PPH, according to various authors, varies from 1.2% to 18.2% [3–5]. Most PPH cases are believed to be treatable with first-line interventions, such as uterine massage, uterotonic therapy, and tranexamic acid, with only 10–20% being refractory [6]. Refractory cases require second-line interventions, which include uterine balloon tamponade [7–9], recombinant human factor VIIa [10], or uterine artery embolization [11, 12]. Refractory PPH is often the reason for emergency peripartum hysterectomy, leading to the loss of menstrual and reproductive functions [6]. The frequency of peripartum hysterectomy varies across countries; for example, in high-resource nations, it occurs in less than one case per 1,000 births, whereas in Nigeria and Pakistan, the rates are 4 and 11 cases per 1,000 births, respectively [13].
Currently, it is believed that the main challenges in obstetrics are accurate assessment of blood loss, identification of risk factors, and timely recognition of PPH [1]. Although the risk factors for PPH are well described, there is conflicting data regarding the influence of some of these factors on the risk of PPH [14–16]. Furthermore, the ability to identify women at risk of obstetric complications is limited [17].
Abnormal placentation and operative delivery are generally accepted as significant risk factors for PPH [14]. A meta-analysis conducted by Nigussie J. et al. [18] in Ethiopia identified several significant risk factors for PPH, including women aged ≥35 years, prolonged labor, parity, lack of antenatal care, and a history of PPH. A proven relationship exists between PPH and severe anemia [15]. Several studies have demonstrated that low fibrinogen levels before delivery are a risk factor for PPH [19–21]. Moreover, fibrinogen levels were included in a predictive model for PPH prognosis in multiple pregnancies as developed by Qi S. et al. (2023) [22]. Additionally, a meta-analysis by de Moreuil C. et al., which included 81 studies, showed that women with severe PPH had lower platelet counts before delivery, despite no statistically significant differences in fibrinogen levels or other hemostatic parameters [23].
The characteristics of the PPH risk factors in various populations and ethnic groups have also been documented. There is a higher frequency of PPH among African Americans and descendants of Africans [24], as well as in Asians and Hispanics [14]. A relationship has been established between PPH and the socioeconomic development level of the region [18]. According to a systematic review by Okunlola et al., which included seven studies, individuals of Latin American, Asian, Native Hawaiian, and other Pacific Islander descent have a higher risk of developing PPH due to uterine atony. Caucasians have a higher rate of blood transfusions, while Africans show a lower risk of atonic PPH but a higher risk of atonic PPH requiring surgical intervention. Native Americans have a higher risk of uterine atony, whereas Caucasians have the lowest risk of PPH [24].
Conflicting data exist regarding women's age, body mass index, and hematological parameters, among others [14, 25, 26]. Furthermore, to date, no studies have focused on assessing the protective and risk factors for refractory PPH.
This study aimed to investigate the frequency of refractory PPH in a tertiary-level hospital and to identify its risk and protective factors.
Materials and methods
The study was conducted at the Perinatal Center (PC) of the Kuzbass Regional Clinical Hospital named after S.V. Belyaev, which is a clinical affiliate of the Kemerovo State Medical University. The PC is the "anchor" medical organization of the Kemerovo region (Kuzbass) in the profile of obstetrics and gynecology, to which high-risk PPH patients are referred according to the local order. The study was reviewed and approved by the Research Ethics Committee of the Kemerovo State Medical University (Ref. No: 309 / k of 14.06.2023).
In the first phase, a cross-sectional study was conducted to assess the incidence of PPH and refractory PPH in a tertiary hospital, including all patients who gave birth in PC between 2019 and 2022 (n=15,480). In the second phase, a retrospective case-control study was conducted to assess protective and risk factors for refractory PPH.
The study design is illustrated in Figure 1.
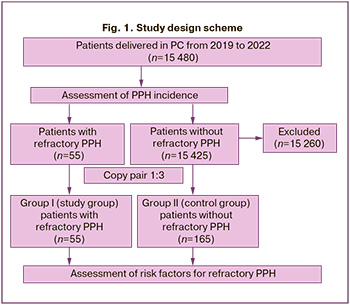
The first (study) group of the trial included 55 patients with refractory PPH who gave birth in the PC between 2019 and 2022. Inclusion criteria for group I were the presence of PPH, blood loss of 1000 ml or more, and no response to first-line therapy. The exclusion criteria were as follows: blood loss during childbirth and a postpartum period of less than 1000 ml and complete resolution of PPH with first-line therapy. The first-line therapy was intravenous oxytocin solution at a dose of 10 IU and tranexamic acid at a dose of 1000 mg.
In the second (control) group, 165 women were selected on a 1:3 copy-pair basis. The inclusion criteria for group II were blood loss during childbirth and a postpartum period of less than 800 ml and complete resolution of PPH with first-line therapy. The exclusion criteria were the presence of PPH with blood loss greater than 800 ml and no effect of first-line therapy. Patients in group II were selected by simple random sampling from the total number of numbered patient cards without PPH that met the inclusion/exclusion criteria by creating a table of random numbers (the sample() function in R was used). Anamnestic information, information on the characteristics of the course of pregnancy and delivery, and laboratory parameters were obtained by copying birth records. Hemoglobin, hematocrit, and platelet counts were measured using a Mindray BC-6800 Plus hematology analyzer based on SF Cube technology. Fibrinogen and activated partial thromboplastin time were measured using a SYSMEX CS-1600 automated hemostasis analyzer based on multiwave analysis technology in the third trimester of pregnancy before delivery. Blood loss was estimated gravimetrically by weighing the dressing material in graduated containers.
Statistical analysis
The results are presented as M (SD), where M is the mean and SD is the standard deviation, or Me (Q1; Q3), where Me is the median and Q1 and Q3 are the first and third quartiles, respectively. The distribution of continuous variables was tested for normality using the Kolmogorov–Smirnov test. The equality of the measures of central tendency in the two independent groups was analyzed using the parametric Student's t-test or nonparametric Mann–Whitney U test.
Categorical variables are described using frequency and percentage (n/N), where n is the absolute value of the frequency, N is the number of observations in the study group, and % is the relative value of the frequency. Contingency tables were analyzed using the χ2 test or two-tailed Fisher’s exact test when the expected numbers were small. The level of statistical significance for type I error was set at p<0.05. The obtained data were analyzed using Python programming language version 3 and the pandas, scipy.stats, and matplotlib.pyplot libraries.
To ascertain the strength of the relationship between the target variable and the studied characteristics, univariate regression analysis was employed to construct a series of univariate binary logistic regression models, and odds ratios (OR) and 95% confidence intervals (CI) were calculated. The variable containing information on belonging to groups I and II (the presence and absence of refractory PPH, respectively) was taken as the target variable (response variable), and the parameters of the clinical and anamnestic characteristics of the examined subjects and the features of the course of pregnancy and childbirth (Table 1) were used as predictor variables. A forest plot was used to visualize the significant results. Statistical analysis was performed using the basic functions glm0 and ggplot() of the tidyverse library in R, version 4.3.3.
Results
The incidence of postpartum hemorrhage (PPH) in tertiary-level hospital was 0.67% (104/15,480). Furthermore, in over half of the cases (52.9%, 55/104), initial treatment was unsuccessful. Consequently, alternative measures, including intravenous oxytocin, tranexamic acid, uterine massage, and other methods for stopping PPH, were required. Consequently, the incidence of refractory PPH with a blood loss of ≥ 1,000 ml or more in a high-risk hospital was 0.36% (55/15,480).
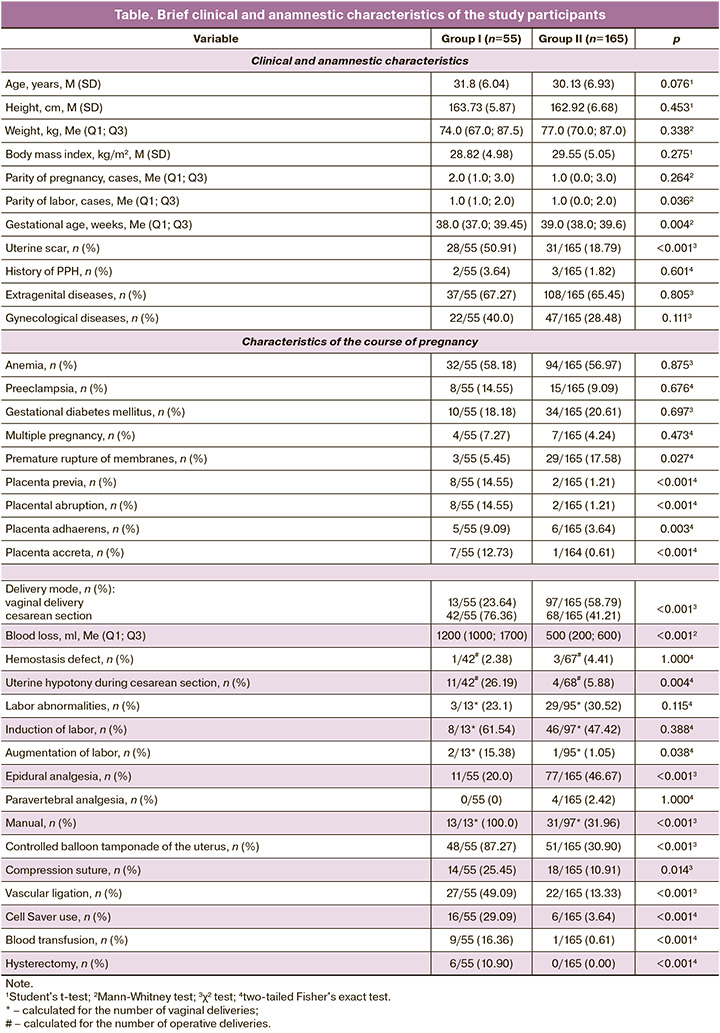
A brief description of the participants is provided in table.
The clinical and anamnestic characteristics of patients in group I (study group) compared with those in group II included higher parity, shorter gestational age at delivery, and a higher incidence of post-cesarean uterine scars (50.91% of women; p<0.001).
Among the gestational complications in group I, there was a significantly higher incidence of placenta previa (p<0.001), pathological placentation, and placental abruption (p<0.001); however, premature rupture of membranes occurred less frequently (p=0.027).
Cesarean section was performed in 42 of 55 (76.36%) women in group I and in 68 of 165 (41.21%) women in group II (p<0.001).
In group I patients, labor intensification (p=0.034), manual examination of the uterine cavity during vaginal births, and controlled balloon tamponade of the uterus (p<0.001) were performed significantly more often. Additionally, uterine hypotony was observed during cesarean section (p<0.001).
Using univariate binary logistic regression, 12 of 178 clinical, anamnestic, and laboratory factors were identified to have a statistically significant relationship with refractory postpartum hemorrhage (Fig. 2).
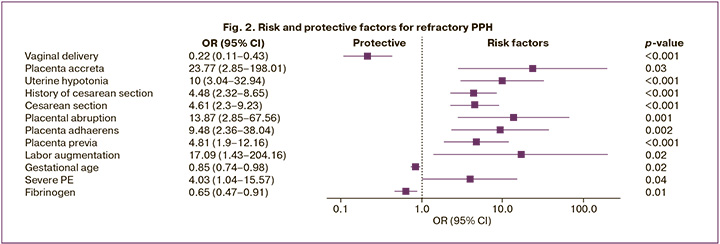
Significant risk factors for refractory postpartum hemorrhage included placenta accreta – OR 23.77 (95% CI 2.85–198.01), p=0.003, and augmentation of labor – OR 17.09 (95% CI 1.43–204.16), p=0.02. Other statistically significant risk factors included placental abruption – OR 13.87 (95% CI 2.85–67.56), p<0.001; uterine hypotonia during cesarean section – OR 10.0 (95% CI 3.04–32.94), p<0.001; placenta adhaerens – OR 9.48 (95% CI 2.36–38.04), p=0.002; placenta previa – OR 4.81 (95% CI 1.9–12.16), p<0.001; delivery by cesarean section – OR 4.61 (95% CI 2.3–9.23), p<0.001; post-cesarean uterine scar – OR 4.48 (95% CI 2.32–8.65), p<0.001; cesarean delivery due to severe preeclampsia – OR 4.03 (95% CI 1.04–15.57), p=0.04.
Protective factors for refractory postpartum hemorrhage included vaginal delivery – OR 0.22 (95% CI 0.11–0.43); p<0.001), fibrinogen level – OR 0.65 (95% CI 0.47–0.91), p=0.01; and gestational age – OR 0.85 (95% CI 0.74–0.98), p=0.02.
The results of the study did not reveal any influence of other anamnestic, clinical, or laboratory parameters, as well as characteristics of the course of pregnancy and drug treatment for complications of pregnancy and childbirth, on the risk of refractory postpartum hemorrhage.
Discussion
Our study showed that the incidence of PPH in tertiary-level hospital was 0.67%. Approximately half of the patients did not respond to first-line therapy (intravenous oxytocin, tranexamic acid, and uterine massage) and required additional methods to control the hemorrhage. Epidemiological studies assessing the prevalence of PPH have been conducted in various countries and have shown wide fluctuations in frequency, which is significantly higher than our findings. For instance, a study by Feduniw S. et al. reported a PPH frequency of 1.2–12.5% [4]. According to Doherty S. et al., the frequency of PPH among rural women ranges from 5.0 to 18.2% [3]. In a multicenter study conducted in 80 Level II hospitals in Kenya, Nigeria, South Africa, and Tanzania, the frequency of PPH during vaginal births was 4.3% [5].
In contrast, the proportion of patients with refractory bleeding in our study was higher than that reported in the literature. This may be attributed to the nature of our institution (tertiary-level hospital), where high-risk obstetric cases are more commonly managed. Refractory PPH occurred more frequently (76.36%) after cesarean delivery. To manage refractory PPH, controlled balloon tamponade was used in 87.27% of the cases, uterine vessel ligation in 49.09%, and compression sutures in 25.45%. Blood-saving technologies were employed in one-third of the patients with refractory PPH, and blood transfusions were performed in 16.36% of the patients. The incidence rate of peripartum hysterectomy was 10.9%.
In comparison, the multicenter World Maternal Antifibrinolytic (WOMAN) study, conducted in 193 hospitals across 21 countries, reported a peripartum hysterectomy rate of 5%, with placenta previa and accreta being the main causes. Other risk factors for hysterectomy include maternal age, cesarean delivery, and Asian ethnicity [13]. Our study demonstrated that abnormal placental attachment was a significant risk factor for refractory PPH, with odds ratios (OR) of 23.77 for placenta accreta (95% CI 2.85–198.01), 9.48 for placenta adhaerens (95% CI 2.36–38.04), and 4.81 for placenta previa (95% CI 1.9–12.16). Labor augmentation also increased risk (odds ratio [OR] 17.09, 95% CI 1.43–204.16).
Other statistically significant risk factors for refractory PPH in our study included placental abruption, cesarean delivery, uterine hypotension during surgery, history of uterine scar, and cesarean delivery due to severe preeclampsia. Huque S. et al. found that cesarean section was associated with a fourfold increase in the risk of hysterectomy (OR 4.3, 95% CI 3.6–5.0) [13]. Surgical delivery for severe preeclampsia is often performed as an emergency procedure, which increases the risk of complications and intraoperative blood loss. Additionally, preeclampsia is associated with coagulation disorders, such as thrombocytopenia and, in some cases, hypofibrinogenemia [27]. Prolonged use of magnesium sulfate in such cases may also increase the risk of PPH. Crowther C.A. et al. reported that massive PPH occurred more frequently in patients receiving intravenous magnesium sulfate—3.4% (25/729) versus 1.7% (12/704) in the placebo group, with an adjusted risk ratio of 1.98 (95% CI 1.01–3.91) [28].
A meta-analysis by Ende et al. found that placental abruption/previa increased the risk of PPH by 2.74 times, and labor induction by 1.23 times [14]. Labor induction is widely recognized as a risk factor for PPH [29, 30]. Kumar B. et al. reported that 14% of induced labors were complicated by PPH with blood loss exceeding 1000 ml [30]. Similarly, a cohort study by Bernitz S. et al. showed that oxytocin doses ≥20 mU/min were associated with an increased risk of PPH regardless of the duration of administration [31].
It is generally accepted that late reproductive age and prior uterine surgeries, particularly cesarean sections, increase the risk of placental disorders in subsequent pregnancies [13]. Ende H.B. et al., in a systematic review and meta-analysis of 13 studies, found that risk factors for PPH include extragenital diseases (arterial hypertension, diabetes mellitus), pregnancy characteristics (polyhydramnios, multiple gestations, macrosomia), and labor complications (prolonged labor, especially during the first stage; chorioamnionitis, and trauma) [14]. However, our study did not find a statistically significant impact of these factors on the risk of refractory PPH.
Omotayo et al. [15] demonstrated that severe anemia increased the risk of PPH (OR 3.54, 95% CI 1.20–10.4, p=0.020). Similarly, Jung J. et al. [16] reported an association between hemoglobin levels and adverse maternal outcomes, including maternal mortality from PPH. Lower initial hemoglobin levels predicted worse PPH outcomes. Nevertheless, our study did not find a statistically significant effect of anemia on the risk of refractory PPH (p=0.111), even though anemia was present in more than half of the women in both the groups. A systematic review by de Moreuil C. et al. also found no statistically significant differences in anemia between women with and without severe PPH [23].
The protective factors against refractory PPH identified in our study were vaginal delivery, gestational age, and fibrinogen levels. Increased gestational age and higher fibrinogen levels were associated with a reduced risk of PPH.
In a systematic review by de Moreuil et al. [23], which included 81 studies, the mean values of biomarkers (platelets, fibrinogen, hemoglobin, D-dimer, activated partial thromboplastin time, and prothrombin time) were not significantly different between women with and without PPH. However, prenatal platelet counts were lower in women with severe PPH than those in the control group. Salomon C. et al. [26] identified three hematological parameters as independent risk factors for PPH: platelets <150 g/L (OR 2.98, 95% CI 1.63–5.46), fibrinogen <4.5 g/L (OR 1.86, 95% CI 1.21–2.87), and activated partial thromboplastin time ≥1.1 (OR 2.16, 95% CI 1.31–3.57). However, the platelet count was not associated with PPH in our study.
Conclusion
The incidence of postpartum hemorrhage in a tertiary-level hospital was 0.67%, while refractory PPH occurred in 0.36% of the cases. Risk factors for refractory PPH included abnormal placentation (placenta accreta, placenta adhaerens, placenta previa), labor augmentation, cesarean section, cesarean section for severe preeclampsia, placental abruption, uterine scar, and uterine hypotension during cesarean section. Protective factors included gestational age, vaginal delivery, and fibrinogen level.
References
- Andrikopoulou M., D'Alton M.E. Postpartum hemorrhage: early identification challenges. Semin. Perinatol. 2019; 43(1): 11-7. https://dx.doi.org/10.1053/j.semperi.2018.11.003.
- Артымук Н.В., Марочко Т.Ю., Апресян С.В., Артымук Д.А. Методы компрессионного гемостаза в управлении рефрактерными послеродовыми кровотечениями. Акушерство и гинекология. 2023; 12: 16-24. [Artymuk N.V., Marochko T.Yu., Apresyan S.V., Artymuk D.A. Methods of compression hemostasis in the management of postpartum hemorrhage. Obstetrics and Gynecology. 2023; (12): 16-24. (in Russian)]. https://dx.doi.org/10.18565/aig.2023.203.
- Doherty S., Asghari S., Heeley T., House-Denine M., Hall A., Swab M. Postpartum haemorrhage in rural Indigenous women: scoping review of a global obstetrical challenge. Int. J. Circumpolar. Health. 2022; 81(1): 2090066. https://dx.doi.org/10.1080/22423982.2022.2090066.
- Feduniw S., Warzecha D., Szymusik I., Wielgos M. Epidemiology, prevention and management of early postpartum hemorrhage - a systematic review. Ginekol. Pol. 2020; 91(1): 38-44. https://dx.doi.org/10.5603/GP.2020.0009.
- Gallos I., Devall A., Martin J., Middleton L., Beeson L., Galadanci H. et al. Randomized trial of early detection and treatment of postpartum hemorrhage. N. Engl. J. Med. 2023; 389(1): 11-21. https://dx.doi.org/10.1056/NEJMoa2303966.
- Liu L.Y., Nathan L., Sheen J.J., Goffman D. Review of current insights and therapeutic approaches for the treatment of refractory postpartum hemorrhage. Int. J. Womens Health. 2023; 15: 905-26. https://dx.doi.org/10.2147/IJWH.S366675.
- Anger H.A., Dabash R., Durocher J., Hassanein N., Ononge S., Frye L.J. et al. The effectiveness and safety of introducing condom-catheter uterine balloon tamponade for postpartum haemorrhage at secondary level hospitals in Uganda, Egypt and Senegal: a stepped wedge, cluster-randomised trial. BJOG. 2019; 126(13): 1612-21. https://dx.doi.org/10.1111/1471-0528.15903.
- Rozenberg P., Sentilhes L., Goffinet F., Vayssiere C., Senat M.V., Haddad B. et al; Groupe de Recherche en Obstétrique et Gynécologie. Efficacy of early intrauterine balloon tamponade for immediate postpartum hemorrhage after vaginal delivery: a randomized clinical trial. Am. J. Obstet. Gynecol. 2023; 229(5): 542.e1- 2.e14. https://dx.doi.org/10.1016/j.ajog.2023.05.014.
- Cebekhulu S.N., Abdul H., Batting J., Chauke L., Dlakavu F., Fawcus S. et al. "Suction Tube Uterine Tamponade" for treatment of refractory postpartum hemorrhage: Internal feasibility and acceptability pilot of a randomized clinical trial. Int. J. Gynaecol. Obstet. 2022; 158(1): 79-85. https://dx.doi.org/10.1002/ijgo.13963.
- Lavigne-Lissalde G., Aya A.G., Mercier F.J., Roger-Christoph S., Chauleur C., Morau E. et al. Recombinant human FVIIa for reducing the need for invasive second-line therapies in severe refractory postpartum hemorrhage: a multicenter, randomized, open controlled trial. J. Thromb. Haemost. 2015; 13(4): 520-9. https://dx.doi.org/10.1111/jth.12844.
- Liu Z., Wang Y., Yan J., Li J., Liu X., Zhang L. et al. Uterine artery embolization versus hysterectomy in the treatment of refractory postpartum hemorrhage: a systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 2020; 33(4): 693-705. https://dx.doi.org/10.1080/14767058.2018.1497599.
- Бреслав И.Ю., Колотилова М.Л., Григорьян А.М., Барыкина О.П., Скрябин Н.В., Гапоненко Е.А., Нигматуллина Э.Р. Роль эмболизации маточных артерий в лечении поздних послеродовых кровотечений. Акушерство и гинекология. 2022; 12: 100-6. [Breslav I.Yu., Kolotilova M.L., Grigoryan A.M., Barykina O.P., Skryabin N.V., Gaponenko E.A., Nigmatullina E.R. The role of uterine artery embolization in the treatment of late postpartum hemorrhage. Obstetrics and Gynecology. 2022; (12): 100-6. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.239.
- Huque S., Roberts I., Fawole B., Chaudhri R., Arulkumaran S., Shakur-Still H. Risk factors for peripartum hysterectomy among women with postpartum haemorrhage: analysis of data from the WOMAN trial. BMC Pregnancy Childbirth. 2018; 18(1): 186. https://dx.doi.org/10.1186/s12884-018-1829-7.
- Ende H.B., Lozada M.J., Chestnut D.H., Osmundson S.S., Walden R.L., Shotwell M.S. et al. Risk factors for atonic postpartum hemorrhage: a systematic review and meta-analysis. Obstet. Gynecol. 2021; 137(2): 305-23. https://dx.doi.org/10.1097/AOG.0000000000004228.
- Omotayo M.O., Abioye A.I., Kuyebi M., Eke A.C. Prenatal anemia and postpartum hemorrhage risk: A systematic review and meta-analysis. J. Obstet. Gynaecol. Res. 2021; 47(8): 2565-76. https://dx.doi.org/10.1111/jog.14834.
- Jung J., Rahman M.M., Rahman M.S., Swe K.T., Islam M.R., Rahman M.O. et al. Effects of hemoglobin levels during pregnancy on adverse maternal and infant outcomes: a systematic review and meta-analysis. Ann. N. Y. Acad. Sci. 2019; 1450(1): 69-82. https://dx.doi.org/10.1111/nyas.14112.
- Goad L., Rockhill K., Schwarz J., Heyborne K., Fabbri S. Development and validation of a prediction model for postpartum hemorrhage at a single safety net tertiary care center. Am. J. Obstet. Gynecol. MFM. 2021; 3(5): https://dx.doi.org/100404. 10.1016/j.ajogmf.2021.100404.
- Nigussie J., Girma B., Molla A., Tamir T., Tilahun R. Magnitude of postpartum hemorrhage and its associated factors in Ethiopia: a systematic review and meta-analysis. Reprod. Health. 2022; 19(1): 63. https://dx.doi.org/10.1186/s12978-022-01360-7.
- Gayat E., Resche-Rigon M., Morel O., Rossignol M., Mantz J., Nicolas-Robin A. et al. Predictive factors of advanced interventional procedures in a multicentre severe postpartum haemorrhage study. Intensive Care Med. 2011; 37(11): 1816-25. https://dx.doi.org/10.1007/s00134-011-2315-0.
- Yilmaz E.P.T., Celik Y., Topdagi Y.E., Guzel A.I., Al R.A. New approach to the risk variables for administration of fibrinogen in patients with postpartum hemorrhage by using cluster analysis. Int. J. Gynaecol. Obstet. 2021; 152(2): 256-61. https://dx.doi.org/10.1002/ijgo.13386.
- Cortet M., Deneux-Tharaux C., Dupont C., Colin C., Rudigoz R.C., Bouvier-Colle M.H. et al. Association between fibrinogen level and severity of postpartum haemorrhage: secondary analysis of a prospective trial. Br. J. Anaesth. 2012; 108(6): 984-9. https://dx.doi.org/10.1093/bja/aes096.
- Qi S., Fu X. Establishment of a predictive model for postpartum hemorrhage in twins: a retrospective study. BMC Pregnancy Childbirth. 2023; 23(1): 644. https://dx.doi.org/10.1186/s12884-023-05933-7.
- de Moreuil C., Mehic D., Nopp S., Kraemmer D., Gebhart J., Schramm T. et al. Hemostatic biomarkers associated with postpartum hemorrhage: a systematic review and meta-analysis. Blood Adv. 2023; 7(19): 5954-67. https://dx.doi.org/10.1182/bloodadvances.2023010143.
- Okunlola O., Raza S., Osasan S., Sethia S., Batool T., Bambhroliya Z. et al. Race/Ethnicity as a risk factor in the development of postpartum hemorrhage: a thorough systematic review of disparity in the relationship between pregnancy and the rate of postpartum hemorrhage. Cureus. 2022; 14(6): e26460. https://dx.doi.org/10.7759/cureus.26460.
- Wang C., Zhang C. Meta-analysis to assess the role of maternal characteristics and risk factors on postpartum hemorrhage. Adv. Clin. Exp. Med. 2023; 32(7): 723-31. https://dx.doi.org/10.17219/acem/158474.
- Salomon C., de Moreuil C., Hannigsberg J., Trémouilhac C., Drugmanne G., Gatineau F. et al. Haematological parameters associated with postpartum haemorrhage after vaginal delivery: Results from a French cohort study. J. Gynecol. Obstet. Hum. Reprod. 2021; 50(9): 102168. https://dx.doi.org/10.1016/j.jogoh.2021.102168.
- Li S., Jin Y., Gong Y., Luo X. Preeclampsia complicated with hypofibrinogenemia: 2 case reports and review of the literature. BMC Pregnancy Childbirth. 2023; 23(1): 631. https://dx.doi.org/10.1186/s12884-023-05965-z.
- Crowther C.A., Ashwood P., Middleton P.F., McPhee A., Tran T., Harding J.E.; MAGENTA Study Group. Prenatal intravenous magnesium at 30-34 weeks' gestation and neurodevelopmental outcomes in offspring: the MAGENTA randomized clinical trial. JAMA. 2023; 330(7): 603-14. https://dx.doi.org/10.1001/jama.2023.12357.
- Артымук Н.В., Марочко Т.Ю., Апресян С.В., Артымук Д.А., Шибельгут Н.М., Батина Н.А., Хлуденцова А.А. Частота встречаемости, основные факторы риска и эффективность лечения пациенток с послеродовыми кровотечениями. Доктор.Ру. 2023; 22(5): 14-9. [Artymuk N.V., Marochko T.Yu., Apresyan S.V., Artymuk D.A., Shibelgut N.M., Batina N.A., Khludentsova A.A. Frequency of occurrence, main risk factors and effectiveness of treatment of patients with postpartum hemorrage. Doctor.Ru. 2023; 22(5): 14-9. (in Russian)]. https://dx.doi.org/10.31550/1727-2378-2023-22-5-14-19.
- Kumar B., Kumari S., Hughes S., Savill S. Prospective cohort study of induction of labor: Indications, outcome and postpartum hemorrhage. Eur. J. Midwifery. 2021; 5: 53. https://dx.doi.org/10.18332/ejm/142782.
- Bernitz S., Betran A.P., Gunnes N., Zhang J., Blix E., Øian P. et al. Association of oxytocin augmentation and duration of labour with postpartum haemorrhage: A cohort study of nulliparous women. Midwifery. 2023; 123: 103705. https://dx.doi.org/10.1016/j.midw.2023.103705.
Received 17.07.2024
Accepted 08.10.2024
About the Authors
Natalya V. Artymuk, Dr. Med. Sci., Professor, Head of the Prof. G.A. Ushakova Department of Obstetrics and Gynecology, Kemerovo State Medical University,Ministry of Health of Russia, 650056, Russia, Kemerovo, Voroshilova str., 22a, +7(3842)73-48-56, artymuk@gmail.com, https://orcid.org/0000-0001-7014-6492
Tatyana Yu. Marochko, PhD, Associate Professor at the Prof. G.A. Ushakova Department of Obstetrics and Gynecology, Kemerovo State Medical University,
Ministry of Health of Russia, 650060, Russia, Kemerovo, Voroshilova str., 22a, marochko.2006.68@mail.ru, https://orcid.org/0000-0001-5641-5246
Dmitry A. Artymuk, Clinical Resident at the Department of Obstetrics and Gynecology, Peoples’ Friendship University of Russia named after Patrice Lumumba,
117198, Russia, Moscow, Miklukho-Maklay str., 6, martynych98@mail.ru, https://orcid.org/0000-0002-7099-4405
Sergey V. Apresyan, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology, Peoples’ Friendship University of Russia named after Patrice Lumumba,
117198, Russia, Moscow, Miklukho-Maklay str., 6, sapresyan@mail.ru, https://orcid.org/0000-0002-7310-974X
Natalia B. Kolesnikova, PhD, Associate Professor at the Prof. G.A. Ushakova Department of Obstetrics and Gynecology, Kemerovo State Medical University,
Ministry of Health of Russia, 650060, Russia, Kemerovo, Voroshilova str., 22a, marochko.2006.68@mail.ru, https://orcid.org/0000-0001-6563-5507
Alina V. Atalyan, PhD (Bio), Senior Researcher, Head of the Functional Group of Information Systems and Biostatistics, Scientific Center for Family Health and Human Reproduction, 664003, Russia, Irkutsk, Timiryazeva str., 16, atalyan@sbamsr.irk.ru, https://orcid.org/0000-0002-3407-9365
Nonna M. Shibelgut, PhD, Deputy Chief Physician for Obstetric Care, Kuzbass Regional Clinical Hospital named after S.V. Belyaev,
650066, Russia, Kemerovo, Oktyabrsky Ave., 22, nonna.shibelgut@mail.ru
Natalya A. Batina, Head of the Maternity Department, Kuzbass Regional Clinical Hospital named after S.V. Belyaev, 650066, Russia, Kemerovo, Oktyabrsky Ave., 22,
batinan@inbox.ru
Corresponding author: Natalya V. Artymuk, artymuk@gmail.com



