Risk factors for shoulder dystocia during labor in women with diabetes mellitus
Objective: Identify risk factors for shoulder dystocia in labor in women with diabetes mellitus (DM) and assess the contribution of the identified determinants of adverse obstetric and perinatal outcomes to this complication.Kapustin R.V., Kopteeva E.V., Alekseenkova E.N., Tsybuk E.M., Arzhanova O.N., Kogan I.Yu.
Materials and methods: This retrospective cohort study was conducted at the D.O. Ott Research Institute for OG&P. Pregnant women with different types of DM delivered over 10 years (2008–2017, n=3261) were divided into comparison groups. There were patients with T1DM receiving continuous subcutaneous insulin infusion (CSII) (n=60), patients with T1DM on multiple daily insulin injections (MDII) (n=446), patients with T2DM on diet (n=95), T2DM on insulin therapy (n=134), gestational DM on diet (n=1652), gestational DM on insulin therapy (n=735), and control group (n=139). The odds ratios (OR) were calculated to determine the risk and assess the contribution of determinants to the development of shoulder dystocia. Statistical analysis was performed using SPSS v 23.0 software.
Results: One of the most significant risk factors for shoulder dystocia in labor was fetal weight. In the presence of DM, the minimum fetal weight at which the risk of shoulder dystocia was significantly increased was 4250 g; at fetal weight greater than 4750 g, the odds ratio for shoulder dystocia increased 5-fold (OR 4.86; 95% CI 1.32–14.5). Other significant risk factors were maternal age (>30 years), prepregnancy body mass index (>30 kg/m2), glycated hemoglobin level in the first trimester (>6.5%), and gestational weight gain over 15 kg. At the same time, a history of DM was not found to be a predictor of fetal dystocia in women with DM.
Conclusion: Individual evaluation of the identified risk factors will optimize a rational pregnancy management algorithm and the choice of mode and timing of delivery, thereby reducing the incidence of shoulder dystocia in patients with DM.
Keywords
According to the International Diabetes Federation, the estimated prevalence of diabetes in the adult population has more than tripled, from 151 million (4.6%) in 2000 to 537 million (10.5%) in 2021. If trends continue, the number will jump to 783 million (12.2%) by 2045 [1]. It is estimated that 21.1 million (16.7%) of live births to women in 2021 had some form of hyperglycaemia in pregnancy. Of these, 80.3% were due to gestational diabetes mellitus (GDM), while 10.6% were the result of diabetes detected prior to pregnancy, and 9.1% due to diabetes (including type 1 and type 2) first detected in pregnancy [1]. Recent advances in obstetric diabetology have allowed one to prolong pregnancy in women with DM and reduce the incidence of maternal and newborn complications [2]. However, the risks of adverse outcomes in women with diabetes in pregnancy are still higher than in the general population.
The disorder of maternal carbohydrate metabolism has been shown to lead to excessive fetal growth resulting in macrosomia [3]. Macrosomia is associated with the risk of maternal trauma (deep perineal tears, hypotonic bleeding, and complicated postpartum period) and injuries to the newborn (shoulder dystocia, brachial plexus injuries, cervical spine displacement due to excessive extraction during delivery, neurological pathology). Fetal macrosomia can develop with a proportional increase in all fetometric parameters (constitutional type) or with an increase in breast and abdominal size relative to the size of the fetal head (asymmetric type) [3].
One of the most common complications of vaginal delivery in fetal macrosomia is shoulder dystocia. Shoulder dystocia happens when one of the baby’s shoulders gets stuck behind the mother’s pubic bone or sacrum during birth, and further advancement of the baby is halted. This complication is characterized by a high risk of intrapartum morbidity and mortality, and to overcome it there is a need for additional labor maneuvers for successful delivery [2]. The authors rate fetal macrosomia, DM, and maternal obesity as the most significant risk factors for fetal dystocia [4-8].
Fetal macrosomia is an important risk factor for fetal shoulder dystocia during delivery. Fetal macrosomia is considered a fetal anthropometric index exceeding the 90th percentile for gestational age or a birth weight of more than 4000 g.
Overland E.A. et al. (2012) demonstrated a direct correlation between fetal weight and the odds of birth dystocia: 3500–3999 g (OR 3.2), 4000-4499 g (OR 17.2), 4500–4999 g (OR 60.3), over 5000 g (OR 176.52) [4]. However, there is evidence that the majority of children born with a birth weight greater than 4500 g did not have this complication during delivery, and in 48% of cases shoulder dystocia occurred even when the fetal weight was less than 4000 g [5]. An equally important risk factor for shoulder dystocia is the history of fetal shoulder dystocia in previous deliveries. If the mother has a history of this complication, the incidence of shoulder dystocia is 10 times higher than in the general population (5, 6).
Not only fetal macrosomia but also maternal DM was found to be an independent risk factor for shoulder dystocia: OR, 2.23 (95% CI 2.00–2.48) [7, 8]. An increase in this risk in DM is associated with the development of a symptomatic fetal diabetic fetopathy (DF). DF is characterized by a disproportional distribution of subcutaneous adipose tissue in the fetus with predominant localization in the upper half of the trunk.
The growth of adipose tissue naturally leads to an increase in fetal weight and, combined with the accumulation of fat mainly in the shoulder girdle, increases the risk of shoulder dystocia in labor.
Given the risk of adverse pregnancy outcomes associated with shoulder dystocia, models for predicting this complication of labor in women with DM are needed.
This study aimed to identify risk factors for shoulder dystocia in labor in women with DM and assess the contribution of the identified determinants of adverse obstetric and perinatal outcomes to this complication.
Materials and methods
A retrospective cohort study was conducted at the Ott Research Institute for Obstetrics, Gynecology, and Reproductology, St. Petersburg (Diabetes In Pregnancy StudyY, DIPSY). The study followed the STROBE reporting guideline for the observational studies [9]. The study was approved by the Research Ethics Committee of the D.O. Ott Research Institute for OG&R (Ref. No: # 83 of 2017-04-21).
Pregnant women with different types of DM who delivered over 10 years (2008–2017, n=3261) constituted the following comparison groups:
1 – DM type 1 (n=506);
1a – Received continuous subcutaneous insulin infusion (CSII) – 60 (11.86%);
1b – Received multiple daily insulin injections (MDII) – 446 (88.14%);
2 – DM type 2 (n=229);
2a – Received diet – 95 (41.5%);
2b – Received insulin therapy – 134 (58.5%);
3 – GDM (n=2387);
3a – Received diet – 1,652 (69.2%);
3b – Received insulin therapy – 735 (30.8%);
4 – Control group (n=139).
Inclusion criteria (study groups) were presence of DM type 1, 2, GDM; singleton pregnancy; patient's consent to participate in the study.
The study inclusion criteria (control group) were the absence of carbohydrate metabolism disorders; absence of obesity; singleton pregnancy; patient's consent of the patient to participate in the study.
Exclusion criteria for the study:
— Diseases associated with symptomatic diabetes: thyrotoxicosis, hyperadrenocorticism, pituitary adenoma, and pheochromocytoma;
— severe comorbidities;
— malignancy;
— multiple pregnancies;
— patient's refusal to participate in the study.
Fetal weight, age of the patient, pregestational body mass index, gestational weight gain, duration of DM (length of pregnancy), and glycated hemoglobin level in the first trimester in women with pregestational types of DM were identified as determinants of risk in women with DM.
Statistical analysis
Statistical analysis was performed using SPSSV.23.0 (USA). The distribution of continuous variables was tested for normality using the Kolmogorov–Smirnov test. Quantitative variables showing a normal distribution were expressed as means (M) and standard deviation (SD). Comparisons among the three groups were performed with one-way analysis of variance (ANOVA) using Tukey's test for post hoc comparisons. Variables not meeting normality assumptions were reported as the median (Me) and interquartile range (Q1; Q3). Categorical variables were reported as counts and proportions (%) and compared using the χ² test. The odds ratio (OR) with a 95% confidence interval (95% CI) was calculated to identify the effect size. The critical level of significance when testing statistical hypotheses was considered at p<0.05.
Results
Clinical characteristics of the study groups
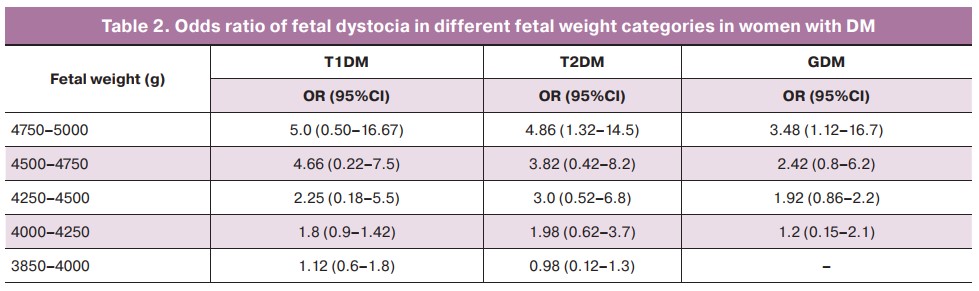
The women in the study groups differed in age. Patients with DM type 2 were older than patients in other groups. The highest age, 34.0 (5.2) years, was observed in pregnant women with type 2 DM on insulin therapy. The lowest age was observed in pregnant women with DM type 1 receiving MDII. Body mass index was highest in women with type 2 DM: 33.8 (6.6) kg/m2 on diet, 33.8 (6.2) kg/m2 on insulin therapy, and lowest in the control subjects, 24.0 (3.1) kg/m2 (p<0.0001). Furthermore, BMI was significantly higher in pregnant women with GDM than in the control group (p<0.05). In pregnant women with type 1 DM, obesity was reported in 16.7% (10/60) of cases with CSII and 20.8% (93/446) with MDII, with more severe obesity (II, III) extremely rare in 3.5% (16/446) of MDII patients. The prevalence of obesity in pregnant women with type 2 DM was highest: 62.1% (59/95) for diet correction and 70.9% (95/134) for insulin therapy, with a higher class of obesity (II, III) prevailing in these subgroups, respectively. Pregnant women with GDM had twice lower obesity rate than those with type 2 DM, and the incidence of severe obesity did not exceed 10% with both diet therapy and insulin therapy (p<0.0001). In 82.7% (115/134) of women in the control group, the body mass index did not exceed 25 kg/m2 (Table 1).
The development of fetal macrosomia complicated a significant proportion of DM pregnancies. One in four pregnancies in women with type 1 DM [CSII – 26.7% (16/60); MDII – 24.9% (111/446)], one in five in patients with GDM – 19.7% (326/1652 ) diet therapy; insulin therapy – 21.4% (157/735), and one in six women with type 2 DM – 17.9% (17/95) diet therapy; insulin therapy – 15.7% (21/134) ended with the birth of a large fetus (Table 1).
Antenatal ultrasound signs of DF were found in 15.2–16.6% of women with type 1 DM. In patients with type 2 DM and GDM, the frequency of DF diagnosis by echography was observed in no more than 10% of cases. At the same time, postnatal manifestations of DF symptoms were observed 4-fold more frequently, with the highest incidence in women with type 1 DM (61%), and type 2 DM on insulin therapy (37.3%, 50/134) (Table 1).
Shoulder dystocia was more common in women with type 2 DM: 5.3% (5/95) on diet therapy and 3.0% (4/134) on insulin therapy. In patients with type 1 DM, the incidence of shoulder dystocia was higher for MDII hyperglycemia correction at 2.5% (11/446) than for CSII at 1.7% (1/60). In women with GDM, fetal shoulder dystocia was observed in 1.4% (10/735) of women who received insulin therapy and 0.6% (10/1652) on diet (Table 1).
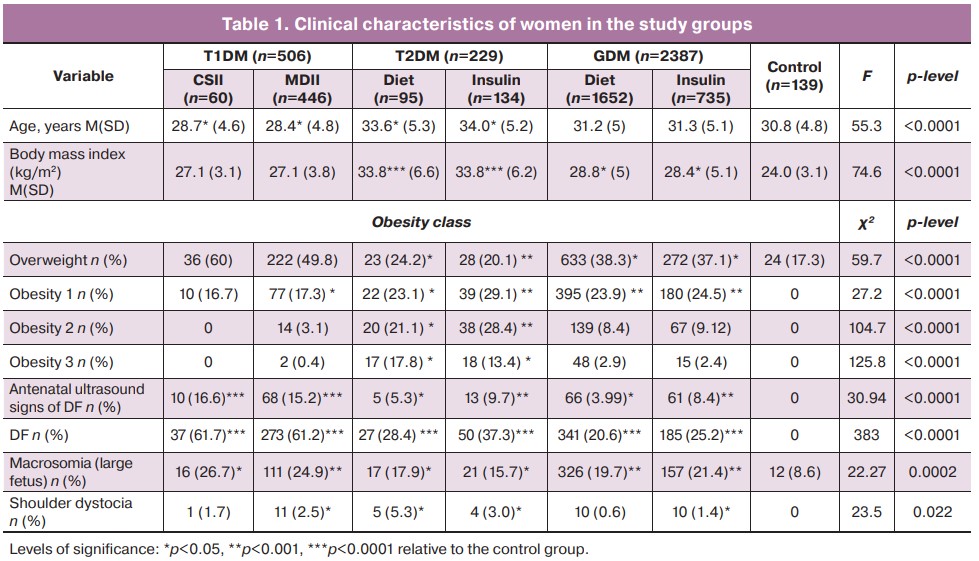
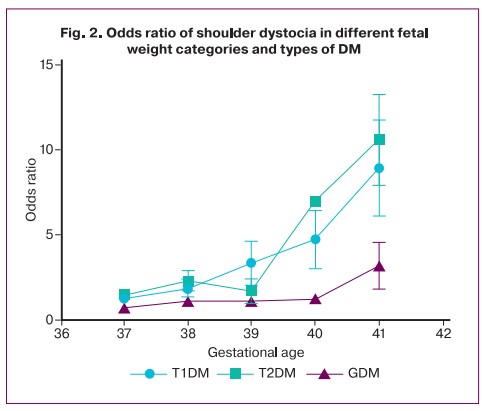
According to our study, with any type of DM in a pregnant woman, the minimum fetal weight at which there is a significant increase in the risk of fetal dystocia is 4250 g. Interestingly, in patients with type 1 DM, the risk score for shoulder dystocia increased sharply with a fetal weight greater than 4,500 g, and in patients with type 2 DM and GDM, with a fetal weight greater than 4,750 g (Fig. 1). At the same time, the odds for this complication in women with any type of DM increased fivefold for fetal weights greater than 4750 g (OR 4.86; 95% CI 1.32–14.5) (Table 2).
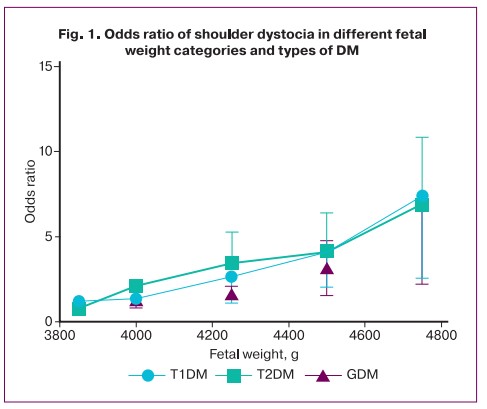
The risk of shoulder dystocia increases with gestational age, independently of other factors. Our study showed that with increasing gestational age over 40 weeks in patients with pregestational types of DM, and over 41 weeks in women with GDM, the risk for shoulder dystocia dramatically increased several-fold, reaching the highest values in women with DM types 1 and 2 (Fig. 2).
The next step of the study was to determine the contribution for different risk factors of shoulder dystocia. The following were identified as the main determinants: age of the woman, pregestational body mass index, weight gain during pregnancy, duration of DM disease (length of pregnancy) and glycated hemoglobin level in the first trimester of pregnancy in women with pregestational types of DM.
The study results demonstrate that the risk for fetal dystocia in women with type 1 DM and GDM increases with maternal age, reaching the highest values after age 35 years (Table 3). Interestingly, this pattern was less pronounced for patients with type 2 DM. For women with type 2 DM and GDM (OR, 1.88; 95% CI, 0.83–9.5; OR, 3.66; 95% CI, 1.68–6.4), class II and III obesity were significant risk factors. Gestational weight gain greater than 15 kg in all women with DM increased the risk of shoulder dystocia by 1.5-fold. Furthermore, in pregnant women with type 2 DM, total weight gain of more than 20 kg increased the risk of shoulder dystocia by 6.2-fold (OR, 6.2; 95% CI 1.12–29.6), and in GDM even total weight gain of more than 25 kg increased the risk for this complication by 3.5 times (OR, 3.47; 95% CI 1.9–12.4) (Table 3).
Elevated glycated hemoglobin in the first trimester in women with pregestational types of DM was an equally significant risk factor for dystocia. There was a direct correlation between the increased level of glycated hemoglobin and the OR of the risk of this complication. The highest ORs were observed for glycated hemoglobin values of 7.1–7.5%: the OR for type 1 DM was 4.45 (95% CI 1.34–9.2), the OR for type 2 DM was 3.42 (95% CI 1.57–8.9) (Table 3).
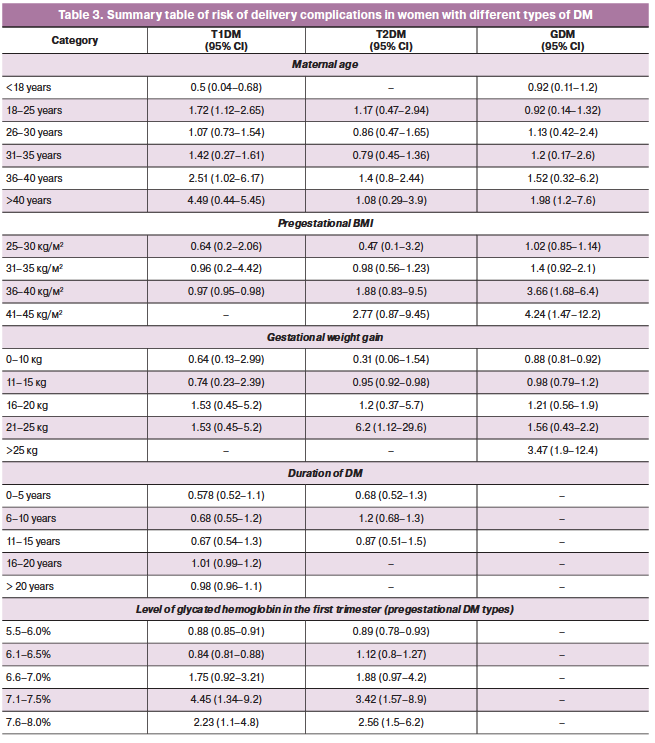
The duration of DM did not show significance as a valid predictor for the risk of fetal dystocia, which may suggest a greater role of carbohydrate metabolism compensation in the present pregnancy.
Discussion
Shoulder dystocia is one of the most severe obstetric complications that can lead to fetal death. Published in 2021, the results of a survey of Spanish residents and practitioners showed that 87.1 and 60.4% of respondents, respectively, were unsure of their skills and felt a lack of accurate criteria for evaluation, diagnosis, and recommendations for the management of patients with shoulder dystocia [10]. Clearly, the increasing prevalence of obesity and DM among pregnant women will naturally increase the incidence of this complication in childbirth. Thus, it is important for the practicing obstetrician not only to know exactly how to deliver shoulder dystocia, but also to be able to predict this complication.
In a meta-analysis by Beta J. et al. (2019) that included 17 studies, the authors analyzed data on maternal and/or newborn complications in cases of fetal macrosomia using a random-effects model [11]. Fetal weight >4000 g increased the risk of maternal complications: the OR of emergency cesarean section was 1.98 (95% CI 1.80–2.18), postpartum hemorrhage was 2.05 (95% CI 1.90–2.22), and deep perineal tears was 1.91 (95% CI 1.56–2.33). The ORs for fetal weight >4500 g were: emergency cesarean section, 2.55 (95% CI 2.33–2.78); postpartum hemorrhage, 3.15 (95% CI 2.14–4.63); and deep perineal tears, 2.56 (95% CI 1.97-3.32). Similarly, the risk of neonatal complications was compared: at fetal weight greater than 4000 g, the OR of shoulder dystocia was 9.54 (95% CI 6.76–13.46), brachial plexus injury 11.03 (95% CI 7.06–17.23) and bone fractures 6.43 (95% CI 3.67–11.28). For fetal weights >4500 g, the ORs for these categories were: 15.64 (95% CI 11.31-21.64), 19.87 (95% CI 12.19–32.40), and 8.16 (95% CI 2.75–24.23), respectively [11]. Similar results were obtained in our study.
This study found that the presence of any type of DM in a woman during pregnancy is an independent risk factor for shoulder dystocia during delivery. The highest incidence of shoulder dystocia was observed in women with type 2 DM (3.0-5.3%), which is associated not only with hyperglycemia but also with the high obesity rate among these patients, which is an independent risk factor for shoulder dystocia in labor [2, 5]. Slightly lower rates of shoulder dystocia were found in patients with type T1DM (1.7–3.5%) and GDM (0.6–1.4%). Fetal weight greater than 4250 g was found to be a significant risk factor for shoulder dystocia in women with carbohydrate metabolism disorders.
Maternal DM during pregnancy as an important risk factor for shoulder dystocia in labor was first demonstrated by Acker D.B. et al. in 1985. [12]. They found a fivefold risk of shoulder dystocia in women with impaired carbohydrate metabolism, even when the fetal weight was less than 4000 g [13]. An extensive population study by Nesbitt TS et al. (1998) found that maternal DM significantly increased the likelihood of developing shoulder dystocia (OR 1.7), with this pattern persisting with increasing fetal weight and obstetric delivery (OR 1.7–10.1) [7]. The authors have shown that children with a birth weight of 3750–4000 g, born to mothers with DM, have an equal risk of developing dystocia compared to children with a weight of 4250–4500 g from mothers without carbohydrate metabolism disorders [7]. Langer et al. (1991), having analyzed 75979 natural deliveries (1589 deliveries in women with DM), found that women with DM had a 10-fold increased risk of shoulder dystocia (3.2% versus 0.3% in the population) [13]. Casey B.M. et al. (1997) showed that mothers with GDM have a 3-fold higher risk of shoulder dystocia compared to the general population [14]. Another study showed that the incidence of shoulder dystocia in mothers with DM was 8.4–16.7%, compared to 1.4% in patients without carbohydrate metabolism disorders. In fetuses weighing more than 4,500 g, shoulder dystocia develops in 20–50% of cases with maternal DM and 9.2–24% of cases without it [15].
Among the adverse effects of shoulder dystocia in the newborn, brachial plexus palsy (BPP) is the most common fetal complication. The risk of BPP is directly proportional to the risk of shoulder dystocia [16]. Ecker J.L. et al. (1997) studied risk factors for BPP, which was observed in 80 cases in the study population (1.03‰) based on an analysis of 77616 deliveries [16]. Of the 77616 pregnant women in the study, 3526 (4.5%) had GDM. The results suggest that GDM (OR, 3.19; 95% CI, 1.62–6.27) and large fetal size at natural delivery (OR, 9.56–17.9) are independent risk factors for dystocia in labor and the development of BPP [16]. Bryant et al. (1998) showed that the incidence of BPP increases directly with increasing fetal weight: from 0.8% (3500–3999 g); 3.3% (4000–4499 g); 8% (4500–4999 g); and >20% at fetal weight greater than 5000 g [17].
In addition to fetal weight, another factor that increases the risk of shoulder dystocia is the mode of delivery. A study by Kolderup L.B. et al (1997) showed that surgical delivery increased the risk of shoulder dystocia and BPP by a factor of 6.7: forceps by 3.8 (95% CI 3.5–5.9) and vacuum extractor by 2.9 (95% CI 1.1–8.0) [18]. On the other hand, the presence of fetal macrosomia was also an independent risk factor for vaginal delivery (OR, 6.8) [19]. Unreasonable and aggressive labor management including early pushing, untimely correction of uterine contractions, and oxytocin labor stimulation are no less significant factors contributing to the increased risk of dystocia in labor [19–23].
Predicting this complication may play an important role in the prevention of fetal dystocia and associated birth trauma. Our study demonstrated that the use of simple parameters (fetal weight, female age, pregestational body mass index, weight gain during pregnancy, glycated hemoglobin levels in women with pregestational types of DM) could help identify women with DM type 1 and 2 and GDM with different risk for shoulder dystocia. This is also supported by other studies. A retrospective study was conducted in 2006 to develop a prognostic model for predicting the risk of shoulder dystocia [24]. The authors reported that the best combination of variables for predicting neonatal injury associated with shoulder dystocia were maternal height and weight, gestational age, parity, and newborn weight [24]. With a score greater than 0.5, 50.7% of cases of shoulder dystocia with BBP were detected, and the false positive rate was 2.7%. However, the data used for this prediction model were based on actual birth weight rather than estimated fetal weight. It is worth noting that clinical assessment of estimated fetal weight is unreliable; third-trimester ultrasound scans have at least a 10% error to actual birth weight, and only a 60% sensitivity for predicting fetal macrosomia (over 4500 g) [25–27].
Kleitman V. et al. (2016) demonstrated that women with a history of fetal shoulder dystocia were more likely to be older, have GDM, fetal macrosomia, polyhydramnios, and prolonged second period of labor [28]. The presence of fetal dystocia in a previous delivery was found to be an independent risk factor for recurrence of this complication (OR, 6.1; 95% CI, 3.2–11.8) in a multivariate regression analysis [27].
According to Mochalova M.N. et al. (2016), prognostically favorable factors in the management of large fetus vaginal delivery are a history of labor, gestational age not more than 39 weeks, symmetrical fetal macrosomia, the absence of maternal obesity, and period of hyperglycemia during pregnancy [29]. The ratio of fetal head size to transverse shoulder size plays a significant role in the development of shoulder dystocia [28].
On the contrary, other studies have shown that shoulder dystocia is difficult to predict [25, 26]. Ouzounian S.G. et al. (2016) studied the most significant prognostic risk factors for this complication, including fetal macrosomia or suspected macrosomia, prolonged second period of labor, presence of maternal DM, and use of oxytocin in labor [26]. The authors found that none of the models had high sensitivity or high specificity, a positive predictive value for predicting fetal dystocia [26].
A systematic review by Al-Hawash S. et al. (2019) on recurrent shoulder dystocia demonstrated that many risk factors are common in women who have no history of this complication [30]. Furthermore, women with recurrent shoulder dystocia rarely had identifiable risk factors except for a prior history of shoulder dystocia (30). The sample sizes of many studies that address this issue are single-center retrospective cohorts with low rates of subsequent pregnancies and vaginal deliveries because many women opt for the cesarean section in subsequent pregnancies. Therefore, the high level of study bias and heterogeneity prevented qualitative meta-analysis [30]. Thus, fetal dystocia remains a largely unpredictable clinical phenomenon.
In addition to prognosis, the prevention of shoulder dystocia in women with DM is an important issue. It is clear that induction of labor at 37–39 weeks «almost full term' can help prevent the development of fetal overgrowth and naturally reduce the risk of shoulder dystocia in this situation [31]. This is supported by our study showing that delivering at less than 39–40 weeks with pregestational types of DM and less than 40–41 weeks with GDM is associated with a lower risk of fetal shoulder dystocia (Fig. 2). Other authors have been reported by other authors. A randomized controlled trial by Boulvain M. et al. (2015) showed that induction of labor with suspected fetal macrosomia leads to lower fetal birth weight, lower incidence of shoulder dystocia and birth trauma, and cesarean section [32]. However, the results of the studies included in the review show that to prevent one clavicle fracture it is necessary to perform about 60 inductions of labor [32]. Therefore, more studies are needed on labor induction for suspected fetal macrosomia are needed. In these studies, particular emphasis should be placed on clarifying the optimal time for labor induction of labor and improving the precision of the diagnosis of fetal macrosomia.
We found that the duration of DM is not a predictor of the risk of fetal dystocia in women with DM. This fact may be due to the presence of diabetic vasculopathy, which is common in patients with a long DM history and is associated with a high risk of preeclampsia and early delivery (often by cesarean section), as well as fetoplacental insufficiency and fetal growth restriction [33].
Conclusion
Our study identified a number of criteria that allow clinicians to improve the prediction of the risk of shoulder dystocia during labor in women with different types of DM. The most significant risk factors for shoulder dystocia were fetal weight greater than 4250 g, maternal age (>30 years), pregestational body mass index (>30 kg/m2), and glycated hemoglobin level in the first trimester (>6.5%).
However, labor management should be tailored for individual patients and a decision should be made based on the history, clinical observations, and specific situation to assess the risk of fetal dystocia in women with DM.
References
- Wang H., Li N., Chivese T., Werfalli M., Sun H., Yuen L. et al.; IDF Diabetes Atlas Committee Hyperglycaemia in Pregnancy Special Interest Group. IDF Diabetes Atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group's Criteria. Diabetes Res. Clin. Pract. 2022; 183: 109050. https://dx.doi.org/10.1016/j.diabres.2021.109050.
- Feig D.S., Berger H., Donovan L., Godbout A., Kader T., Keely E., Sanghera R.; Diabetes Canada Clinical Practice Guidelines Expert Committee. Diabetes and pregnancy. Can. J. Diabetes. 2018; 42(Suppl. 1): S255-S282. https://dx.doi.org/10.1016/j.jcjd.2017.10.038.
- Alekseenkova E.N., Selkov S.A., Kapustin R.V. Fetal growth regulation via insulin-like growth factor axis in normal and diabetic pregnancy. J. Perinat. Med. 2022; 50(7): 947-60. https://dx.doi.org/10.1515/jpm-2021-0510.
- Overland E.A., Vatten L.J., Eskild A. Risk of shoulder dystocia: associations with parity and offspring birthweight. A population study of 1 914 544 deliveries. Acta Obstet. Gynecol. Scand. 2012; 91(4): 483-8. https://dx.doi.org/10.1111/1600-0412.2011.01354.x.
- Usta I.M., Hayek S., Yahya F., Abu-Musa A., Nassar A.H. Shoulder dystocia: what is the risk of recurrence? Acta Obstet. Gynecol. Scand. 2008; 87(10): 992-7. https://dx.doi.org/10.1080/00016340802415614.
- Mehta S.H., Blackwell S.C., Chadha R., Sokol R.J. Shoulder dystocia and the next delivery: outcomes and management. J. Matern. Fetal Neonatal Med. 2007; 20(10): 729-33. https://dx.doi.org/10.1080/14767050701563826.
- Nesbitt T.S., Gilbert W.M., Herrchen B. Shoulder dystocia and associated risk factors with macrosomic infants born in California. Am. J. Obstet. Gynecol. 1998; 179(2): 476-80. https://dx.doi.org/10.1016/s0002-9378(98)70382-5.
- Practice Bulletin No 178: Shoulder dystocia. Obstet. Gynecol. 2017; 129(5): e123-e133. https://dx.doi.org/10.1097/AOG.0000000000002043.
- Von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P.; STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007; 4(10): e296. https://dx.doi.org/10.1371/pmed.0040296.
- Salvador López R., Cruz Melguizo S., Sanz Lorenzana A., Encinas Pardilla B., Serrano Palacios C., Nieto Jiménez Y. et al. Evaluación del conocimiento en la resolución de la distocia de hombros en profesionales españoles [Knowledge assessment of shoulder dystocia management by Spanish professionals.]. Rev. Esp. Salud Publica. 2021; 95: e202106090. [In Spanish].
- Beta J., Khan N., Khalil A., Fiolna M., Ramadan G., Akolekar R. Maternal and neonatal complications of fetal macrosomia: systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2019; 54(3): 308-18. https://dx.doi.org/10.1002/uog.20279.
- Acker D.B., Sachs B.P., Friedman E.A. Risk factors for shoulder dystocia. Obstet. Gynecol. 1985; 66(6): 762-8.
- Langer O., Berkus M.D., Huff R.W., Samueloff A. Shoulder dystocia: should the fetus weighing greater than or equal to 4000 grams be delivered by cesarean section? Am. J. Obstet. Gynecol. 1991; 165(4, Pt 1): 831-7. https://dx.doi.org/10.1016/0002-9378(91)90424-p.
- Casey B.M., Lucas M.J., Mcintire D.D., Leveno K.J. Pregnancy outcomes in women with gestational diabetes compared with the general obstetric population. Obstet. Gynecol. 1997; 90(6): 869-73. https://dx.doi.org/10.1016/s0029-7844(97)00542-5.
- Sentilhes L., Sénat M.V., Boulogne A.I., Deneux-Tharaux C., Fuchs F., Legendre G. et al. Dystocie des épaules: recommandations pour la pratique Clinique -Texte court [Shoulder dystocia: guidelines for clinical practice - short text]. J. Gynecol. Obstet. Biol. Reprod. (Paris). 2015; 44(10): 1303-10. [In French]. https://dx.doi.org/10.1016/j.jgyn.2015.09.053.
- Ecker J.L., Greenberg J.A., Norwitz E.R., Nadel A.S., Repke J.T. Birth weight as a predictor of brachial plexus injury. Obstet. Gynecol. 1997; 89(5, Pt 1): 643-7. https://dx.doi.org/10.1016/s0029-7844(97)00007-0.
- Bryant D.R., Leonardi M.R., Landwehr J.B., Bottoms S.F. Limited usefulness of fetal weight in predicting neonatal brachial plexus injury. Am. J. Obstet. Gynecol. 1998; 179(3 Pt 1): 686-9. https://dx.doi.org/10.1016/s0002-9378(98)70065-1.
- Kolderup L.B., Laros R.K. Jr., Musci T.J. Incidence of persistent birth injury in macrosomic infants: association with mode of delivery. Am. J. Obstet. Gynecol. 1997; 177(1): 37-41. https://dx.doi.org/10.1016/s0002-9378(97)70435-6.
- Bjørstad A.R., Irgens-Hansen K., Daltveit A.K., Irgens L.M. Macrosomia: mode of delivery and pregnancy outcome. Acta Obstet. Gynecol. Scand. 2010; 89(5): 664-9. https://dx.doi.org/10.3109/00016341003686099.
- Davis D.D., Roshan A., Canela C.D., Varacallo M. Shoulder dystocia. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. 2022 Jun 21.
- Draycott T., Sanders C., Crofts J., Lloyd J. A template for reviewing the strength of evidence for obstetric brachial plexus injury in clinical negligence claims. Clin. Risk. 2008; 14(3): 96-100. https://dx.doi.org/10.1258/cr.2008.080020.
- Laughon S.K., Berghella V., Reddy U.M., Sundaram R., Lu Z., Hoffman M.K. Neonatal and maternal outcomes with prolonged second stage of labor. Obstet. Gynecol. 2014; 124(1): 57-67. https://dx.doi.org/10.1097/AOG.0000000000000278.
- Allen R.H., Gurewitsch E.D. Temporary Erb-Duchenne palsy without shoulder dystocia or traction to the fetal head. Obstet. Gynecol. 2005; 105(5, Pt 2): 1210-2. https://dx.doi.org/10.1097/01.AOG.0000141635.94905.21.
- Dyachenko A., Ciampi A., Fahey J., Mighty H., Oppenheimer L., Hamilton E.F. Prediction of risk for shoulder dystocia with neonatal injury. Am. J. Obstet. Gynecol. 2006; 195(6): 1544-9. https://dx.doi.org/10.1016/j.ajog.2006.05.013.
- Gupta M., Hockley C., Quigley M.A., Yeh P., Impey L. Antenatal and intrapartum prediction of shoulder dystocia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010; 151(2): 134-9. https://dx.doi.org/10.1016/j.ejogrb.2010.03.025.
- Ouzounian J.G., Korst L.M., Sanchez M., Chauhan S., Gherman R.B., Opper N., Wilson M.L. Clinical risk factors do not predict shoulder dystocia. J. Reprod. Med. 2016; 61(11-12): 575-80.
- Coomarasamy A., Connock M., Thornton J., Khan K.S. Accuracy of ultrasound biometry in the prediction of macrosomia: a systematic quantitative review. BJOG. 2005; 112(11): 1461-6. https://dx.doi.org/10.1111/j.1471-0528.2005.00702.x.
- Kleitman V., Feldman R., Walfisch A., Toledano R., Sheiner E. Recurrent shoulder dystocia: is it predictable? Arch. Gynecol. Obstet. 2016; 294(6): 1161-6. https://dx.doi.org/10.1007/s00404-016-4139-1.
- Mochalova M.N., Ponomareva Yu.N., Mudrov V.A. Prediction of birth trauma during childbirth with a large fetus. Modern problems of science and education. 2015; (2-1): 105. (in Russian).
- Al-Hawash S., Whitehead C.L., Farine D. Risk of recurrent shoulder dystocia: are we any closer to prediction? J. Matern. Fetal Neonatal Med. 2019; 32(17): 2928-34. https://dx.doi.org/10.1080/14767058.2018.1450382.
- Schmitz T. Modalités de l'accouchement dans la prévention de la dystocie des épaules en cas de facteurs de risqué identifiés [Delivery management for the prevention of shoulder dystocia in case of identified risk factors]. J. Gynecol. Obstet. Biol. Reprod. (Paris). 2015; 44(10): 1261-71. [In French]. https://dx.doi.org/10.1016/j.jgyn.2015.09.051.
- Boulvain M., Senat M.V., Perrotin F., Winer N., Beucher G., Subtil D. et al.; Groupe de Recherche en Obstétrique et Gynécologie (GROG). Induction of labour versus expectant management for large-for-date fetuses: a randomised controlled trial. Lancet. 2015; 385(9987): 2600-5. https://dx.doi.org/10.1016/S0140-6736(14)61904-8.
- Kapustin R.V., Kopteeva E.V., Alekseenkova E.N., Korenevsky A.V., Smirnov I.V., Arzhanova O.N. Prediction of preeclampsia based on maternal serum endoglin level in women with pregestational diabetes mellitus. Hypertens. Pregnancy. 2022; Apr. 27: 1-8. https://dx.doi.org/10.1080/10641955.2022.2068574.
Received 14.06.2022
Accepted 11.08.2022
About the Authors
Roman V. Kapustin, MD, PhD, Academic Secretary, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductive Medicine, St. Petersburg, Russia;Associate Professor, Department of Obstetrics, Gynecology and Reproductive Sciences, Medical Faculty, Saint Petersburg State University, St. Petersburg, Russia,
+7(911)089-07-69, kapustin.roman@gmail.com, SPIN: 7300-6260, https://orcid.org/0000-0002-2783-3032
Ekaterina V. Kopteeva, MD, Junior Researcher, Department of Obstetrics, Division of Maternal-Fetal Medicine, D.O. Ott Research Institute of Obstetrics,
Gynecology and Reproductive Medicine, St. Petersburg, Russia, +7(911)218-86-69, ekaterina_kopteeva@bk.ru, SPIN: 9421-6407, https://orcid.org/0000-0002-9328-8909
Elena N. Alekseenkova, MD, Junior Researcher, Department of Obstetrics, Division of Maternal-Fetal Medicine, D.O. Ott Research Institute of Obstetrics,
Gynecology and Reproductive Medicine, St. Petersburg, Russia, ealekseva@gmail.com, SPIN: 3976-2540, https://orcid.org/0000-0002-0642-7924
Elizaveta M. Tsybuk, student, Faculty of Medicine, St. Petersburg State University, St. Petersburg, Russia, elizavetatcybuk@gmail.com, SPIN: 3466-7910,
https://orcid.org/0000-0001-5803-1668
Olga N. Arzhanova, Dr. Med. Sci., Principal Researcher at the Department of Obstetrics, Division of Maternal-Fetal Medicine, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductive Medicine, St. Petersburg, Russia; Professor at the Department of Obstetrics, Gynecology and Reproduction, Faculty of Medicine, St. Petersburg State University, St. Petersburg, Russia, arjanova_olga@mail.ru, SPIN: 7910-6039, https://orcid.org/0000-0003-3059-9811
Igor Yu. Kogan, Dr. Med. Sci., Director, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductive Medicine, St. Petersburg, Russia; Professor at the Department of Obstetrics, Gynecology and Reproduction, Faculty of Medicine, St. Petersburg State University, St. Petersburg, Russia, ikogan@mail.ru, SPIN: 6572-6450,
https://orcid.org/0000-0002-7351-6900
Corresponding author: Roman V. Kapustin, kapustin.roman@gmail.com
Authors' contributions: Kapustin R.V., Arzhanova O.N., Kogan I.Yu. – conception and design of the study; Arzhanova O.N., Kogan I.Yu. – critical content check, manuscript approval for publication; Kapustin R.V. – material collection and analysis; Kapustin R.V., Alekseenkova E.N. – statistical analysis; Kapustin R.V., Kopteeva E.V., Alekseenkova E.N., Tsybuk E.M. –
data analysis and interpretation, review of relevant publications, manuscript drafting; Kopteeva E.V., Alekseenkova E.N.,
Tsybuk E.M. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: This work was conducted as part of a basic scientific research, state registration number 1021062812133-0.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the D.O. Ott Research Institute for OG&P (Ref. No: # 83 of 2017-04-21).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Kapustin R.V., Kopteeva E.V., Alekseenkova E.N., Tsybuk E.M., Arzhanova O.N., Kogan I.Yu. Risk factors for shoulder dystocia during labor in women with diabetes mellitus.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 9: 54-63 (in Russian)
https://dx.doi.org/10.18565/aig.2022.9.54-63



