The role of fetal gender in the pathogenesis of gestational diabetes mellitus and obstetric complications
Objective: To reveal the mechanisms leading to the development of gestational diabetes mellitus and associated obstetric complications depending on the fetal gender.Botasheva T.L., Andreeva V.O., Rymashevskiy A.N., Tezikov Yu.V., Lipatov I.S., Fabrikant A.D., Lebedenko E.Yu., Zheleznyakova E.V.
Materials and methods: The study included 642 women aged from 18 to 28 years with a spontaneous singleton pregnancy. The main group consisted of 328 patients with gestational diabetes mellitus: 152 women carried male fetuses and 176 women carried female fetuses. The control group included 314 patients with normal pregnancy: 158 women carried male fetuses and 156 women carried female fetuses. The levels of hormones were evaluated in the dynamics of gestation.
Results: During the first trimester, pregnant women carrying male fetuses were found to have a reduced integration between stress liberating and steroid hormone subsystems, an increase in the production of contra-insular hormones, and thus more predisposed to the development of obstetric complications.
Conclusion: The male gender of the fetus is a factor that contributes to the increased incidence of gestational diabetes mellitus, placental insufficiency, and the risk of preterm birth; therefore, it is necessary to develop individual support programs in gestational diabetes.
Keywords
Metabolic disease which includes gestational diabetes mellitus (GDM) occupies one of the leading places in the WHO classifications [1–4]. For the last 20 years, there has been a significant increase in its incidence from 3–4% to 8–22% in all countries of the world [5–9]; therefore, much attention is paid to the study of GDM pathogenesis. GDM is characterized by hypoglycemia which is detected for the first time during gestation. Among all cases of pregnancies accompanied by diabetes mellitus, GDM is detected in 90% of cases [5, 10–13].
Severe hormone-producing function of syncytiotrophoblast, decidual structures and placenta is known to contribute to the formation of disorders of carbohydrate metabolism [14, 15]. One of the leading functions of these structures is the synthesis of contrinsular hormones which leads to insulin resistance [15, 16]. This resistance together with hyperinsulinism contributes to the formation of disorders of almost all types of metabolism and hormonal abnormalities which may cause systemic inflammation, endotheliopathy, disorders in the balance of pro- and anticoagulant links of hemostasis, etc. [17, 18]. In this regard, GDM is considered by many authors to be a genetically determined manifestation of the maladaptation of the maternal organism to gestational restructuring [2, 5, 11, 19, 20], and GDM is seen as a predictor of a number of obstetric complications, perinatal morbidity and mortality [10, 14, 21–23]. The maladaptation of the maternal organism, in turn, can be potentiated by the specific relationship between the maternal and fetal organisms due to a number of their phenotypic and genotypic features [4].
Traditionally, when studying gestational complications, it is accepted to consider the mechanisms of fetal-maternal interaction within the framework of a systematic approach that is the functional system «mother-placenta-fetus» (FSMPF) [20, 24]. However, this concept does not take into account an important characteristic of the «fetus» subsystem, its gender. There are some studies showing the modulating role of fetal gender in the formation of fetal-maternal «signaling» which determines the nature of obstetric complications [24–26]. According to FIGO, the male gender of the fetus is a risk factor for premature birth [4, 27]. The same variant of gender dimorphism affects the increased risk of placental dysfunction due to the impaired processes of trophoblast invasion [28–30]. As for the female gender of the fetus, it is characteristic of a high frequency of preeclampsia, but its severe forms are more often observed in the male gender of the fetus [24, 29]. According to T.L. Botasheva et al. (2020), a high frequency of GDM was noted in the male gender of the fetus [24], however, there is practically no data in the literature explaining the relationship between the frequency of GDM and obstetric complications accompanying GDM and fetal gender.
The aim of the study is to reveal the mechanisms leading to the development of gestational diabetes mellitus and associated obstetric complications depending on the fetal gender.
Materials and methods
The study included retrospective and prospective approaches in the formation of the clinical groups. In order to study the incidence of GDM and obstetric complications accompanying GDM, 2048 patients (1114 pregnant women with male fetuses and 943 pregnant women with female fetuses) were selected retrospectively from 3780 primiparous women with a singleton pregnancy who were examined in the Department of Pregnancy Pathology in the Research Centre of Obstetric and Pediatrics, Rostov-on-Don, Russia, in the period from 2018 to 2021. The patients formed group 0 and were selected randomly using the EXCEL program of the MS OFFICE software.
In the prospective part of the study, 642 women aged from 18 to 28 years with a spontaneous singleton pregnancy were selected from the patients of the outpatient department using the criteria of inclusion and exclusion. The patients were divided into two clinical groups. The first (main) group consisted of 328 patients with GDM: 152 women carried male fetuses (group Ia) and 176 women carried female fetuses (group Ib). The second (control) group included 314 patients with normal pregnancy: 158 women carried male fetuses (group IIa) and 156 women carried female fetuses (group IIb).
There were the following criteria for inclusion in clinical group I: new-onset hyperglycemia on an empty stomach and after a glucose–tolerant test at any stage of pregnancy (WHO special criteria, 2013) (5.1-7.1 mmol/L), glycated hemoglobin index (HbA1c) (≥6.5%) (certified in accordance with the National Glycohemoglobin Standardization Program (NGSP) and standardized in accordance with the reference values of the Diabetes Controland Complications Study (DCCT)), the absence of any types of insulin therapy and hormone therapy. Pregnant women of group I with GDM did not have any severe obstetric pathology.
There were the following criteria for inclusion in clinical group II: the first singleton spontaneous pregnancy; the absence of obstetric complications confirmed by the results of clinical, hormonal, biochemical, ultrasound and dopplerometry studies.
There were the following exclusion criteria: type 1 and type 2 diabetes mellitus; repeated pregnancy and childbirth; congenital malformations and chromosomal abnormalities in the fetus; assisted reproductive technology treatment; decompensated forms of extragenital pathology; congenital malformations, including abnormalities of the structure of the female genitourinary system; refusal of a woman to participate in the study, administration of insulin therapy.
Hormone levels (cortisol, adrenocorticotropic hormone (ACTH), progesterone; unconjugated estriol; placental lactogen) were determined using enzyme immunoassay (ELISA). The results were calculated using a TECAN SUNRISE photometer (Austria). Melatonin production was determined by the level of excretion of 6-sulfatoxymelatonin (6-SOMT) (its main metabolite) in the morning urine using the ELISA kit (BUHLMANN 6–Sulfatoxymelatonin ELISA, Germany).
During the research, we clearly observed the conditions of multiplicity, sequence and time of conducting the studies; blood sampling in pregnant women was carried out before the therapy measures. All pregnant women gave the informed consent to participate in the study. This study was approved by the local ethics committee of Rostov State Medical University, Ministry of Health of Russia.
Statistical analysis
The values of median (Me) and interquartile range (25%, 75%) were estimated during data processing; the statistical significance of the results was calculated with a confidence interval of 95%; the nonparametric Mann–Whitney U test (with a significance level of 0.05) was used to compare the intergroup differences, the nonparametric Friedman method was used to compare the three dependent groups (Tables 1, 2) in the absence of a normal distribution. A posteriori analysis was carried out for the revealed statistically significant differences using the Wilcoxon test with Bonferroni correction; the degree of the correlation between the analyzed factors was determined using a nonparametric Spearman correlation test (correlation coefficients were considered at a significance level of 0.05); to determine the hierarchy of significance of the analyzed parameters, a multivariate analysis «Decision Trees» was used. Relative parameters (frequencies, fractions, percentages) between groups were compared using chi-squared (χ2) or Fisher’s exact test. Statistical data processing was carried out using Statistica software packages version 10.01, EXCEL 2010, IBM SPSS 24.0.
Results
The hormonal system undergoes significant transformations during gestational restructuring, therefore, the greatest attention has been paid to this system in this research. Progesterone, unconjugated estriol, placental lactogen, ACTH, cortisol and 6-sulfatoxymelatonin (6-SOMT) as well as the gender of the fetus were included in the study of the hormonal profile of pregnant women (Table 1).
The analysis of 6-SOMT parameters in the morning urine sample in women with normal pregnancy did not reveal statistically significant differences in boy’s mothers (BM) and girl’s mothers (GM). Its higher level in trimesters II and III was observed in GM (17.0 compared to 13.6 in BM, p=0.03 in trimester II and 15.0 compared to 12.0, p=0.04 in trimester III).
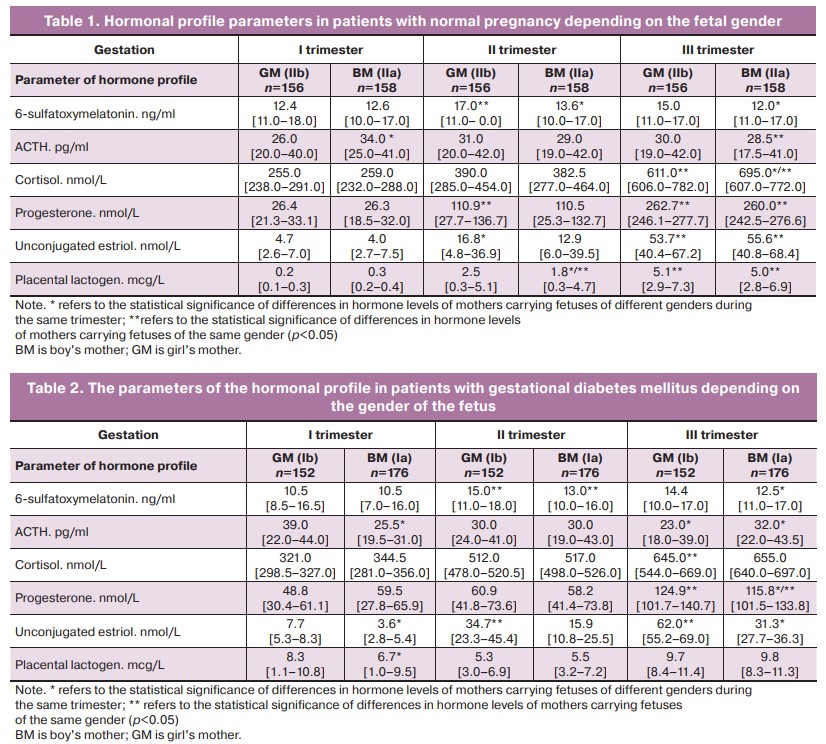
It is known that during pregnancy stress hormones are produced by the adrenal glands of a woman together with the placenta, fetal liver and its adrenal glands, which are necessary for ensuring the optimal metabolism in the maternal body. Therefore, the levels of ACTH and cortisol were analyzed in women with normal pregnancy and gestational diabetes depending on the gender of the fetus. The absolute levels of ACTH in women with normal pregnancy in the first trimester were significantly higher in BM (34.0 compared to 26.0 in GM, p=0.04). The level of cortisol in the first and second trimesters did not differ significantly depending on the gender of the fetus, whereas in the third trimester it was significantly higher in BM (696.0 compared to 611.0 in GM, p=0.04). The analysis of progesterone levels in women with normal pregnancy revealed no statistically significant differences in subgroups with different fetal gender (p>0.05). The level of unconjugated estriol was higher in the second trimester in GM (16.8 compared to 12.9 in BM, p=0.02). A similar distribution of data was noted in the assessment of placental lactogen levels: its values were higher in the second trimester in GM (2.6 compared to 1.8 in BM, p=0.02).
The analysis of the hormonal profile in pregnant women with gestational diabetes revealed that the absolute levels of 6-SOMT in the second and third trimesters were higher in GM (15.0 compared to 13.0 in BM, p=0.04, in the second trimester and 14.4 compared to 12.5 in BM, p=0.03, in the third trimester) (Table 2).
In contrast to women with normal pregnancy, patients with gestational diabetes, namely GM, demonstrated a significantly higher ACTH level in the first trimester (39.0 compared to 25.5 in BM, p=0.01), whereas in the third trimester it was higher in BM (32.0 compared to 23.0 in GM, p=0.01). Cortisol levels in the subgroups of pregnant women with different fetal gender did not significantly differ in the dynamics of the entire pregnancy (p>0.05).
The analysis of steroid hormones showed a significantly higher progesterone level in the third trimester of pregnancy in GM (124.9 compared to 115.8 in BM, p=0.04), as well as the level of estriol (62.0 compared to 31.3 in BM, p=0.01).
The level of placental lactogen in the first trimester of pregnancy was significantly higher in GM (8.3 compared to 6.7 in BM, p=0.02).
Thus, we revealed significant differences in the absolute levels of certain hormones of the hormonal profile in women carrying fetuses of different genders; these differences indicate the existence of different programs of gestational restructuring in the FSMPF when carrying fetuses of male and female genders.
The next stage of the study was devoted to the analysis of the character of integrative processes in the hormonal system of women carrying fetuses of different genders. A nonparametric correlation analysis was carried out.
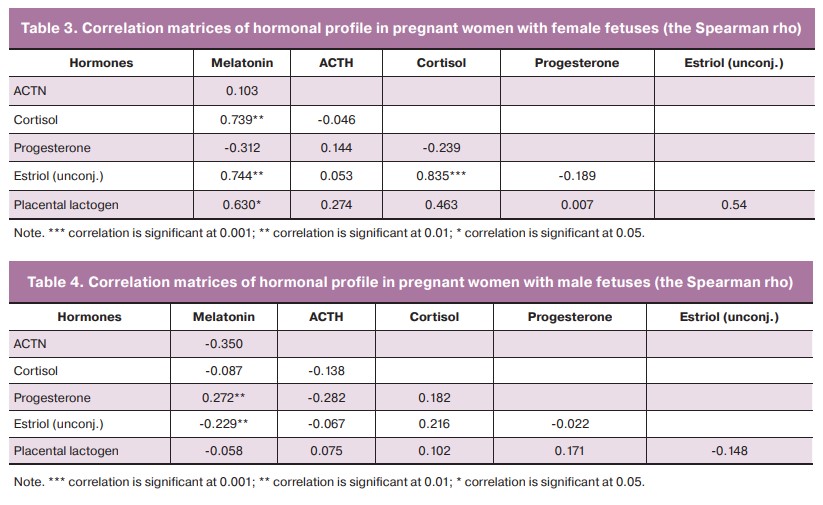
Since gestational diabetes manifests more frequently in the second trimester, special attention was paid to the results of the analysis of the correlation matrices of the hormonal profile in women at the previous stage, that is, in the first trimester.
The correlation matrices of GM had significant positive correlations of average strength between cortisol and melatonin (r=0.7), unconjugated estriol and melatonin (r=0.7), unconjugated estriol and cortisol (r=0.8), placental lactogen and melatonin (r=0.6) (Table 3).
The situation was different in BM: the number of significant correlations was minimal; these connections were weak and negative, namely between progesterone and melatonin (r=0.2) and between unconjugated estriol and melatonin (r=-0.2) (Table 4).
The results which were obtained during the correlation analysis were confirmed and detailed in the process of multivariate analysis «Decision trees» using the CRT method (»Classification and regression trees», where the dependent variable is GDM group; independent variables are melatonin, ACTH, cortisol, progesterone, unconjugated estriol, placental lactogen). On the basis of its results, we determined the hierarchy of hormonal subsystems involved in the maintenance of gestational processes in mothers carrying fetuses of different genders.
The multifactorial analysis «Decision trees» for pregnant women carrying female fetuses (I trimester) resulted in establishing the following rule:
If the placental lactogen level is >0.44 and the cortisol level is ≤374.5, then GDM is predicted [relative risk is 52.25 (confidence interval: 9.76–639.03); odds ratio is 616 (confidence interval: 35.77–10608.67); sensitivity is 91.7% (confidence interval: 67.1–99.3); specificity is 98.2% (confidence interval: 93.2–99.8)].
The multifactorial analysis «Decision trees» for pregnant women carrying male fetuses (I trimester) resulted in establishing the following rule:
- If the level of melatonin is >19.5, then GDM is predicted;
- If the level of melatonin is ≤19.5 and the level of placental lactogen is >0.405, and the level of progesterone is ≤98.1, then GDM is predicted [relative risk is 15.74 (confidence interval: 3.7–180.57); odds ratio is 900.00 (confidence interval: 53.17–15.235); sensitivity is 98.4% (confidence interval: 93.4–99.9): specificity is 93.8% (confidence interval: 74.8–99.4)].
Thus, the patients with female fetuses had a more remarkable activation of the stress-liberating and contrinsular links of the hormonal profile in the processes of gestational hormonal restructuring in the first trimester prior to the manifestation of GDM (II trimester), whereas the patients with male fetuses demonstrated a more remarkable activation of the steroid link and melatonin metabolism.
In this regard, the study of GDM incidence, clinical analysis of obstetric complications etiology and the condition of neonates born to women with GDM were of particular interest.
When determining GDM incidence depending on the gender of the fetus (group 0), it was found that this pathology was registered 2.1 times more often in BM compared to GM (219/1114 (19.7%) in BM compared to 88/934 (9.4%) in GM, p=0.03).
The analysis of obstetric complications etiology in patients with GDM depending on the gender of the fetus revealed that placental insufficiency was more often registered in pregnant women carrying boys (363/1114 (32.6%) in BM compared to 181/934 (19.4%) in GM, p=0.001). Gestation was more often accompanied by mild anemia in pregnant women carrying girls (270/934 (28.9%) compared to 163/1114 (14.6%) in BM, p=0.001). The incidence of fetal growth retardation depending on the gender of the fetus did not significantly differ (281/1114 (25.2%) in BM in comparison with 174/934 (18.6%) in GM, p=0.10).
The frequency of vaginal delivery depending on the gender of the fetus did not significantly differ in groups Ia and Ib (674/934 (72.2%) in GM versus 807/1114 (72.4%) in BM, p=0.93); the frequency of cesarean section delivery did not significantly differ in subgroups with different gender of the fetus either (260/934 (27.8%) in GM versus 307/1114 (27.6%) in BM, p=0.96).
The analysis of gestational age at delivery showed that the higher frequency of preterm labor was noted in patients with GDM carrying boys (325/1114 (29.2%) compared to 118/934 (12.6%) in GM, p=0.001). Unripe cervix was significantly more often observed in GM (164/934 (17.6%) compared to 80/1114 (97.2%) in BM, p=0.03) and labor abnormalities were also noted more frequently in GM (155/934 (16.6%) compared to 77/1114 (6.9%) in BM, p=0.04).
Among the postpartum and early postpartum complications, BM had more frequent cases of deep attachment of the placenta and retention of placental tissue in the uterine cavity, postpartum hypotonic bleeding accompanied by a higher (1.6 times) frequency of non-radical hemostasis methods. The analysis of injuries of the soft tissues of the birth canal revealed that woman carrying girls had cervical ruptures significantly more often compared to women carrying boys (148/934 (15.8%) in GM compared to 52/1114 (4.7%) in BM, p=0.04); I-II degree perineal ruptures were more often detected in BM (118/1114 (10.6%) compared to 26/934 (2.8%) in GM, p=0.03), which could be associated with more frequent detection of fetal macrosomia in patients with GDM (437/1114 (39.2%) in BM compared to 250/934 (26.8%) in GM, p=0.001).
The condition of newborns on the Apgar scale in case of GDM depending on the gender of the fetus also indicated lower (7 points and below) parameters at the 1st minute after birth mainly in boys (241/1114 (21.6%) compared with 90/934 (9.6%) in girls, p=0.001).
The revealed differences in obstetric complications in patients with GDM depending on the gender of the fetus require further study of their pathogenesis.
Discussion
When a woman becomes pregnant, almost all systems of the female body undergo gestational transformations which are accompanied by significant changes in the structural and functional parameters in these systems. Recent studies indicate that the gender of the fetus is a significant factor that plays an important role in the gestational restructuring of functional processes in the mother’s body. The changes in the mother’s body associated with the gender of the fetus are influenced by mutual biochemical and hormonal signals from the «mother» and «fetus» subsystems, which act as triggers in the functional processes of the fetal and maternal relationship. Maternal signals are often educational in relation to the fetus.
Gestational restructuring of hormonal status in normal pregnancy itself has the character of «physiological metabolic syndrome» and it is aimed at creating the necessary supply of nutrients for the fetus and mother; a number of genetic and constitutional factors associated with restructuring can transform the existing anabolic nature of metabolism of a pregnant woman into the «metabolic breakdown» in the form of GDM. According to the previous studies and their results, the gender of the fetus can refer to a number of such modulating factors. The frequency of GDM in pregnant women carrying boys which is 2.1 times higher indicates that such a type of sexual dimorphism contributes to the higher risk of developing GDM.
The analysis of the available data on the asymmetrically dominant principle of the organization of the female reproductive system, the most interesting findings are morpho-functional asymmetries, which are systemic and evolutionarily deterministic in nature [4, 20]. The previous studies on this subject have already shown that the morpho-functional asymmetries of the female reproductive system is a model demonstrating the biological and physiological processes in the fetoplacental system and the maternal body, and that right-sided placental lateralization prevails in GM, while the ambilateral position of the placenta prevails in BM. The dominance of the ambilateral position of the placenta in BM, in our opinion, is an adaptive manifestation of the improvement of the nutritional processes and oxygenation of the fetus from two uterine arteries at once, due to the more distinct trophic needs of male fetuses. Based on the principles of central and peripheral integration, this distribution of placental lateralization in BM is accompanied by partial dominance and activation of right-hemisphere exchange-associated structures of the brain with subsequent activation of sympatho-adrenal, vasopressor and anabolic right-hemisphere effects in FSMPF, changes in hormonal and glycemic status, increased generalized forms of uterine activity, the development of GDM, placental insufficiency and preterm birth [24].
It is due to the predominance of the ambilateral placental position in BM that the processes of central and peripheral functional symmetry begin to prevail; this predominance is the basis for the appearance of a bilateral generalized form of uterine activity, characteristic of both urgent and preterm labor.
The high frequency of fetal macrosomia in BM and the generalized form of uterine contractions of high intensity cause more frequent perineal ruptures; the predominance of asymmetry of uterine activity in GM is more often accompanied by unripe cervix and discoordination of labor because the dynamics of the cervix in childbirth is largely determined by an increase in intraamnial pressure, which is present only in conditions of generalized uterine activity. It is obvious that there are other mechanisms that require research activity in terms of interpreting the etiology and pathogenesis of various obstetric complications associated with GDM depending on the gender of the fetus.
The previous studies contribute to the analysis of the characteristics of fetal-maternal relations in the process of gestational restructuring depending on the gender of the fetus, namely, the hormonal system of women that changes most dynamically during pregnancy. GDM more frequently occurs in BM, and at the early stages of gestation, there are differences in the degree of involvement of various hormonal subsystems in ensuring hormonal restructuring in the mother’s body. The high incidence of GDM in pregnant women carrying boys was found to be accompanied by a decrease in the activity of the stress-liberating link of the hormonal system and an increase in the activity of melatonin metabolism in the first trimester. Stress-liberating and contrinsular hormones are more actively involved in gestational restructuring in women carrying girls; such hormones contribute to a greater resistance of their body and a lower frequency of GDM development and associated obstetric complications.
The role of melatonin in the hormonal composition of the gestational process in women with GDM carrying the fetuses of different genders is of particular interest for further study. The obtained results also determine the prospect of developing individual programs of patient’s support and prevention of gestational diabetes taking into account the gender of the fetus.
Conclusion
- In case of a male fetus, there is a decrease in the functional activity of the subsystem of stress-liberating hormones and an increase in the activity of melatonin metabolism in the mother’s body already in the first trimester which contribute to the subsequent manifestation of GDM. An algorithm for predicting GDM depending on the gender of the fetus was developed based on the results of the multifactorial analysis «Decision Trees».
- When a woman is pregnant with a boy, the incidence of gestational diabetes mellitus increases 2.1 times, placental insufficiency increases 1.6 times, and premature birth increases 1.8 times, while a pregnant woman carrying a girl is twice as likely to have mild anemia.
3. The gender of the fetus in women with GDM causes differences in the following complications in childbirth: unripe cervix, cervical ruptures and labor abnormalities are significantly more often observed in pregnant women carrying girls, whereas I-II degree perineal ruptures are significantly more often observed in pregnant women carrying boys, which is explained by the higher frequency of fetal macrosomia in this group.
References
- Dedov I.I., Shestakova M., ed. Diabetes mellitus: Variety of clinical forms. M.: Medical Information Agency; 2016. 224p. (in Russian).
- Dedov I.I. Sukhikh G.T., Filippov O.S., Arbatskaya N.Yu., Borovik N.V., Burumkulova F.F. et al. Gestational diabetes mellitus: diagnosis, treatment, postpartum care. Problemy reprodukcii/Problems of reproduction. 2018; 24(Suppl. 6): 115-27. (in Russian).
- Belotserkovtseva L.D., Konchenkova E.N., Kilicheva I.I., Ivannikov S.E.
Management of pregnancy in gestational diabetes mellitus. Voprosy ginekologii, akusherstva i perinatologii/Issues of gynecology, obstetrics and perinatology. 2020; 19(3): 40-4. (in Russian). https://dx.doi.org/10.20953/
1726-1678-2020-3-40-44.
- Radzinsky V.E., Botasheva T.L., Kotaysh G.A., ed. Diabetes. Pregnancy. Versions and contraverses. Clinical practices. Prospects. Moscow: GEOTAR-Media. 2020; 528р. (in Russian).
- Orazmuradov A.A., Akhmatova A.N., Arakelyan G.A., Savenkova I.V. Obesity and gestational weight gain in the development of gestational diabetes mellitus and its complications. Akusherstvo i ginekologiya: Novosti, Mneniya, Obuchenie/Obstetrics and gynecology: News, Opinions, Training. 2020; 8(3(29)): 86-9. (in Russian). https://dx.doi.org/10.24411/2303-9698-2020-13013.
- Abramova M.E., Khodzhaeva Z.S., Gorina K.A., Muminova K.T., Goryunov K.V., Ragozin A.K. et al. Gestational diabetes mellitus: screening and diagnostic criteria in early pregnancy. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 5: 25-32. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.5.25-32.
- Mateykovich E.A. Adverse pregnancy outcomes and gestational diabetes: from the hapo study to current data. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 2: 13-20. (in Russian). https://dx.doi.org/10.18565/aig.2021.2.13-20.
- Cosma V., Imbernon J., Zagdoun L., Boulot P., Ренард Э., Brunet et al. Prospective cohort study of postpartum glucose metabolic disorders in early versus standard diagnosed gestational diabetes mellitus. Sci. Rep. 2021; 11(1): 10430. https://dx.doi.org/10.1038/s41598-021-89679-2.
- Shi P., Liu A., Yin X. Association between gestational weight gain in women with gestational diabetes mellitus and adverse pregnancy outcomes: a retrospective cohort study. BMC Pregnancy Childbirth. 2021; 21(1): 508.
https://dx.doi.org/10.1186/s12884-021-03982-4.
- Aylamazyan E.K., ed. Diabetes mellitus and the female reproductive system: a guide for doctors. : GEOTAR-Media; 2017. 432p. (in Russian).
- Lipatov I.S., Tezikov Y.V., Azamatov A.R., Shmakov R.G. Identity of preeclampsia and metabolic syndrome clinical manifestations: searching for substantiation. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 3: 81-9.
(in Russian). https://dx.doi.org/10.18565/aig.2021.3.81-89.
- Chen F., Gan Y., Li Y., He W., Wu W., Wang K., Li Q. Association of gestational diabetes mellitus with changes in gut microbiota composition at the species level. BMC Microbiol. 2021; 21(1): 147. https://dx.doi.org/10.1186/
s12866-021-02207-0.
- Kaiser K., Nielsen M.F., Kallfa E., Dubietyte G., Lauszus F.F. Metabolic syndrome in women with previous gestational diabetes. Rep. 2021; 11(1): 11558.
- Serov V.N., Prilepskaya V.N., Ovsyannikova T.V. Gynecological endocrinology. Management. M.: MEDpress-inform; 2015. 512 p. (in Russian).
- Olmos-Ortiz A., Flores-Espinosa P., Díaz L., Velázquez P., Ramírez-Isarraraz С., Zaga-Clavellina V. Immunoendocrine dysregulation during gestational diabetes mellitus: The central role of the placenta. Int. J. Mol. Sci. 2021; 22(15): 8087. https://dx.doi.org/10.3390/ijms22158087.
- Zhang W.X., Chen S.Y., Liu C. Regulation of reproduction by the circadian rhythms. Sheng Li Xue Bao (Acta Physiol. Sinica). 2016; 68(6): 799-808.
- Epishkina-Minina A.A., Khamoshina M.B., Startseva N.M., Damirova S.F., Zyukina Z.V., Anikeev A.S. Gestational diabetes mellitus and anaemia: the contraversions of pathogenesis. Akusherstvo i ginekologiya: Novosti, Mneniya, Obuchenie/Obstetrics and gynecology: News, Opinions, Training. 2020; 8(S3(29)): 86-93. (in Russian). https://dx.doi.org/10.24411/2303-9698-2020-13914.
- Pokusaeva V.N., Amalitskiy V.Y., Ogareva A.S., Krivenko A.S. The effect of the first-trimester pregnancy hyperglycemia on the risks of developing fetal macrosomia. Medicinskij al'manah/Medical almanac. 2021: 1(66): 24-8. (in Russian).
- Khodzhaeva Z.S., Snetkova N.V., Muminova K.T., Gorina K.A., Abramova M.Ye., Esayan R.M. Clinical characteristics of pregnancy in women with gestational diabetes mellitus. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2020; 7: 47-52. (in Russian). https://dx.doi.org/10.18565/aig.2020.7.47-52.
- Palieva N.V., Botasheva T.L., Petrov Y.A., Pogorelova N., Drukker N.A., Levkovich M.A. et al. Carbohydrate metabolism and hemostatic system in women with gestational diabetes mellitus, preeclampsia, and fetal growth restriction Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 2: 69-76. (in Russian). https://dx.doi.org/10.18565/aig.2021.2.69-76.
- Savelyeva G.M., Serov V.N., Sukhikh G.T. Obstetrics and gynecology. Clinical guidelines. M: GEOTAR-Media; 2016. 1024p. (in Russian).
- Glavnova O.B., Shelygin M.S., Salukhova A.V. Gestational diabetes mellitus: prevention of reproductive losses. Farmateka/Pharmateka. 2021; 28(4): 34-7. (in Russian). https://dx.doi.org/10.18565/pharmateca.2021.4.34-37.
- Grigoryan O.R., Mikheev R.K., Kurinova A.N., Chernova M.O., Sazonova D.V., Akhmatova R.R. et al. Comparative impact analysis of risk factors on the course and outcomes of pregnancy with gestational diabetes mellitus. Problemy endokrinologii/Problems of Endocrinology. 2021; 67(3): 78-86. (in Russian). https://dx.doi.org/10.14341/probl12756.
- Botasheva T.L., Rymashevsky A.N., Fabrikant A.D., Petrov Yu.A., Palieva N.V., Andreeva V.O. et al. Features of the glycemic status, pro- and contrinsular factors in pregnant women with gestational diabetes mellitus, depending on the gender of the fetus. Glavnyj vrach Yuga Rossii/Chief Physician of the South of Russia. 2022; 1: 6-9. (in Russian).
- Gualdani E., Di Cianni G., Seghieri M., Francesconi P., Seghieri G. Pregnancy outcomes and maternal characteristics in women with pregestational and gestational diabetes: a retrospective study on 206,917 singleton live births. Acta Diabetol. 2021;58(9):1169-76. https://dx.doi.org/10.1007/s00592-021-01710-0.
- Shindo R., Aoki S., Nakanishi S., Misumi T., Miyagi E. Impact of gestational diabetes mellitus diagnosed during the third trimester on pregnancy outcomes: a case-control study. BMC Pregnancy and Childbirth. 2021; 21(1): 246.
https://dx.doi.org/10.1186/s12884-021-03730-8.
- Di Renzo G.C., Roura L.C., Facchinetti F., Helmer H., Hubinont C., Jacobsson B. et al. Preterm Labor and Birth Management: Recommendations from the European Association of Perinatal Medicine. Matern. Fetal Neonatal Med. 2017; 30(17): 2011-30. https://dx.doi.org/10.1080/14767058.2017.1323860.
- Palieva N.V., Botasheva T.L., Linde V.A., Avrutskaya V.V., Zheleznyakova E.V. Features of some vasoactive hormones and vascular factors in women with metabolic syndrome and their infuence on the development of obstetric complications. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2017; 6: 48-54. (in Russian). https://dx.doi.org/10.18565/aig.2017.6.48-54
- Botasheva T.L., Palieva N.V., Khloponina A.V., Vasiljeva V.V., Zheleznyakova E.V., Zavodnov O.P. et al. Fetal sex in the development of gestational diabetes mellitus and endothelial dysfunction. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2020; 9: 56-64. (in Russian). https://dx.doi.org/10.18565/aig.2020.9.56-64.
- Gonzalez T.L., Sun T., Koeppel A.F., Lee B., Wang E.T., Farber C.R. et al. Sex differences in the late first trimester human placenta transcriptome. Sex Differ. 2018; 9(1): 4. https://dx.doi.org/10.1186/ s13293-018-0165-y.
Received 06.06.2022
Accepted 06.09.2022
About the Authors
Tatyana L. Botasheva, Dr. Med. Sci., Professor, Chief Researcher at the Obstetrics and Gynecology Department of the Research Institute of Obstetrics and Pediatrics,Rostov State Medical University, Ministry of Health of Russia, +7(906)424-81-03, t_botasheva@mail.ru, https://orcid.org/0000-0001-5136-1752,
344012, Russia, Rostov-on-Don, Mechnikov str., 43.
Vera O. Andreeva, Dr. Med. Sci., Chief Researcher at the Obstetrics and Gynecology Department of the Research Institute of Obstetrics and Pediatrics;
Professor at the Department of Obstetrics and Gynecology No. 2, Rostov State Medical University, Ministry of Health of Russia, +7(903)401-01-51, vandreyeva@mail.ru,
https://orcid.org/0000-0002-7534-134X, 344012, Russia, Rostov-on-Don, st. Mechnikov str., 43.
Aleksandr N. Rymashevskiy, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology No. 1, Rostov State Medical University, Ministry of Health
of Russia, +7(918)553-25-00, rymashevskyan@mail.ru, https://orcid.org/0000-0002-3881-1613, 344012, Russia, Rostov-on-Don, Mechnikov str., 43.
Yuri V. Tezikov, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology, Institute of Clinical Medicine, Samara State Medical University,
Ministry of Health of Russia, +7(927)685-44-85, yra.75@inbox.ru, https://orcid.org/0000- 0002-8946-501X, Researcher ID: С-6187-2018, SPIN: 2896-6986,
Author ID: 161372, Scopus Author ID: 66037875954, 443099, Russia, Samara, Chapaevskaya str., 89.
Igor S. Lipatov, Dr. Med. Sci., Professor, Professor at the Department of Obstetrics and Gynecology, Institute of Clinical Medicine, Samara State Medical University,
Ministry of Health of Russia, +7(927)262-92-70, i.lipatoff2012@yandex.ru, https://orcid.org/0000-0001-7277-7431, Researcher ID: С-5060-2018, SPIN-код: 9625-2947,
Author ID: 161371, Scopus Author ID: 6603787595, 443099, Russia, Samara, Chapaevskaya str., 89.
Anna D. Fabrikant, Resident at the Department of Obstetrics and Gynecology No. 1, Rostov State Medical University, Ministry of Health of Russia,
+7(988)536-94-56, annutka944@mail.ru, https://orcid.org/0000-0002-4376-8111, 344012, Russia, Rostov-on-Don, Mechnikov str., 43.
Elizaveta Yu. Lebedenko, Dr. Med. Sci., Associate Professor, Professor at the Department of Obstetrics and Gynecology No. 3, Rostov State Medical University,
Ministry of Health of Russia, +7(863)252-24-65, lebedenko08@mail.ru, https://orcid.org/0000-0003-2602-1486, 344029, Rostov-on-Don, Russia, 1st Cavalry Army str., 33.
Elena V. Zheleznyakova, PhD, Researcher at the Obstetrics and Gynecology Department of the Research Institute of Obstetrics and Pediatrics, Rostov State Medical University, Ministry of Health of Russia, +7(908)175-40-58, elena.Gel.1961@yandex.ru, https://orcid.org/0000-0003-4496-6387,
344012, Russia, Rostov-on-Don, Mechnikova str., 43.
Corresponding author: Tatyana L. Botasheva, t_botasheva@mail.ru
Authors’ contributions: Botasheva T.L., Andreeva V.O., Tezikov Yu.V. – developing the concept and design of the study; Fabrikant A.D., Zheleznyakova E.V. – collecting and processing the material; Lipatov I.S., Lebedenko E.Yu. – statistical data processing; Andreeva V.O., Botasheva T.L., Rymashevskiy A.N. – writing the text; Tezikov Yu.V., Lipatov I.S. – editing the text.
Conflicts of interest: The authors declare that there are no conflicts of interest.
Funding: The study was carried out as a part of the research “Determination of clinical and diagnostic markers of complicated pregnancy”.
Ethical Approval: The study was approved by the Ethical Review Board of Rostov State Medical University, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Botasheva T.L., Andreeva V.O., Rymashevskiy A.N., Tezikov Yu.V., Lipatov I.S., Fabrikant A.D., Lebedenko E.Yu., Zheleznyakova E.V. The role of fetal gender in the pathogenesis of gestational diabetes mellitus and obstetric complications.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 9: 33-41 (in Russian)
https://dx.doi.org/10.18565/aig.2022.9.33-41



