The role of fatty acid synthase in the development of fetal macrosomia in women with gestational diabetes mellitys and threatened late miscarriage in the second trimester of pregnancy
Currently, there has been an increased incidence of macrosomia, that negatively affects newborn’s future life and health. There is no data in literature on the effect of serum fatty acid synthase (FAS) on threatened miscarriage in women with gestational diabetes mellitus (GDM). Serum FAS concentrations have not been previously assessed to predict macrosomia. Objective: To compare serum FAS concentrations between women with GDM and threatened miscarriage that was diagnosed in the second trimester of pregnancy and women without GDM and with threatened miscarriage that was diagnosed in the second trimester. Materials and methods: The main group included 25 patients with GDM and miscarriage, diagnosed in the second trimester of pregnancy. The comparison group consisted of 30 women with miscarriage diagnosed in the second trimester of pregnancy. Results: The number of microsomic babies was higher in the main group versus the comparison group (15/25 (60.0%), 4/30 (13.33%), p=0.002). Serum concentrations in women in the main group was higher (1.64 ng/ml (1.13; 1.98); 0.8 ng/ml (0.3075; 1.55); p<0.001). The comparison between pregnant women with fetal macrosomia and women without fetal macrosomia showed high diagnostic ROC AUC value=0.867 (95% CI 0.67–0.96). Sensitivity was 87.5% (95% CI 70.3–93.4), specificity was 88.9% (95% CI 58.3–99.4), the prognostic value of positive results was 93.3% (95% CI 75.0–99.6), and the prognostic value of negative results was 80.0% (95% CI 52.5–89.5). Conclusion: It is probable that detection of serum FAS concentration can be considered as a promising method for prediction of macrosomia. However further studies are necessary in this area. Authors' contributions: Malyshkina A.I., Sotnikova N.Yu. – concept and design of the study, editing the text of the article; Afonina V.A., Kroshkina N.V. – data collection and processing; Afonina V.A., Batrak N.V. – statistical data processing, article writing. Conflicts of interest: The authors declare that they have no conflicts of interest. Funding: The study was carried out without any sponsorship. Ethical Approval: The study was approved by the local Ethics Committee of Ivanovo Research Institute of Maternity and Childhood named after V.N. Gorodkov, Ministry of Health of Russia (protocol No. 3 dated 20.11.2020). Patient Consent for Publication: All patients provided informed consent for the publication of their data. Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator. For citation: Malyshkina A.I., Sotnikova N.Yu., Kroshkina N.V., Batrak N.V., Afonina V.A. The role of fatty acid synthase in the development of fetal macrosomia in women with gestational diabetes mellitys and threatened late miscarriage in the second trimester of pregnancy. Akusherstvo i Gynecologia/Obstetrics and Gynecology. 2023; (5): 84-91 (in Russian) https://dx.doi.org/10.18565/aig.2023.32Malyshkina A.I., Sotnikova N.Yu., Kroshkina N.V., Batrak N.V., Afonina V.A.
Keywords
Macrosomia is a fetal condition characterized by birth weight or estimated body weight greater than or equal to 4000 g or greater than the 90th percentile according to the Intergrowth-21st. Increased morbidity associated with this pathology and high incidence of obstetric and perinatal pathology make fetal macrosomia one of the main contemporary obstetrical problems [1, 2].
This complication is a risk factor for obstetric conditions, such as premature rupture of membranes, weakness of primary and secondary uterine contractions, and clinically narrow pelvis. In women with large fetuses, pregnancy outcomes may be operative vaginal or abdominal delivery, postpartum hemorrhage, and birth trauma. During labor the fetus may suffer from meconium aspiration in the amniotic fluid, asphyxia, and birth injuries [3].
Macrosomia negatively affects the newborn’s future life and health. There is a proven evidence that long-term consequences for the offspring, such as obesity, diabetes, pathology of the cardiovascular system are associated with macrosomia. These newborns are more likely to have acute lymphoblastic leukemia at an older age [1, 4–6].
Macrosomia is often associated with impaired carbohydrate metabolism in pregnant women. According to literature, gestational diabetes mellitus (GDM), the incidence of which also tends to increase [7], is also a risk factor for fetal macrosomia [1, 8–10], and the incidence in women with GDM reaches 48% [11, 12]. In 53.9% of cases, complication of pregnancy in women with GMS is threatened miscarriage [13, 14]. Currently, there are no published data on the influence of threatened miscarriage on the condition of the mother with GDM and the fetus.
Fatty acid synthase (FAS) is one of the elements in the formation of insulin resistance. Its function in the cell is to "assemble" fatty acids from acyl-CoA and malonyl-CoA simple precursors [15]. FAS is involved in metabolism of complex saturated lipids, triglycerides, low and very low-density lipoproteins [15–17].
Elevated FAS concentration in patients with obesity, cancer, and diabetes is reported in literature [15–18]. FAS is widely used as a marker for pancreatic cancer [19] and prostate adenocarcinoma [20].
FAS concentration in placenta in pregnant women with GDM was studied. The study found that expression of FAS was more pronounced in patients with GDM [17, 21]. Another study showed high levels of placental and serum FAS in pregnant women with large fetuses [22].
However, there are no published data on the influence of threatened miscarriage on serum FAS concentration. Assessment of serum FAS concentration to predict fetal macrosomia in patients with GDM and threatened miscarriage that was diagnosed in the second trimester of pregnancy, has not been previously carried out.
Objective of the study was to compare serum FAS concentrations between women with GDM and threatened miscarriage that was diagnosed in the second trimester of pregnancy and women with threatened miscarriage that was diagnosed in the second trimester in the absence of GDM.
Materials and methods
A prospective observational study was performed in the clinic of Ivanovo Research Institute of Maternity and Childhood named V.N. Gorodkov of the Ministry of Health of Russia (the Institute). The study was approved by the local Ethics Committee of the Institute (Protocol No. 3 of November 20, 2020). The design of the study was in compliance with the requirements of the Declaration of Helsinki. Complex clinical, laboratory and instrumental examination was performed according to the Order No 1130n of the Ministry of Health of the RF “On approval of the procedure for providing health care in the profile “Obstetrics and Gynecology” of October 20, 2020. The diagnosis of GDM was made in accordance with the Letter No. 15-4/10/2-9478 “On Clinical Guidelines “Gestational diabetes mellitus: diagnosis, treatment, postpartum follow-up” (along with the Clinical Recommendations (treatment protocol) approved by the Russian Society of Obstetricians and Gynecologists).
The study included copying data from medical history, drawing blood samples from the cubital vein before treatment for analysis of serum FAS levels as the additional research method, assessment of anxiety and depression using Hospital Anxiety and Depression Scale (HADS) before starting therapy and evaluation of pregnancy outcomes.
Inclusion criteria were pregnant women aged 18–45 years, singleton pregnancy, threatened miscarriage diagnosed in the second trimester, informed consent of women to participate in the study.
Non-inclusion criteria were pregnancies that occurred as a result of using assisted reproductive technologies, multiple pregnancies, decompensated extragenital pathology, manifestation of the types of gestational diabetes mellitus. All women belonged to the Caucasian race, and had normal values of body mass index (BMI) before the onset of pregnancy; all women took dydrogesterone (10 mg/day) before admission to hospital.
Fifty seven patients matched the inclusion criteria and signed informed consent. Communication with two patients was lost, and due to this fact follow-up information on the course and outcome of pregnancy was not taken into account for the processing of the results obtained in the study. Thus, the study included 55 women. All patients were divided into 2 groups. The main group included 25 pregnant women with GDM and threatened miscarriage that was diagnosed in the second trimester. The diagnosis of GDM was primarily established when they were admitted to hospital. The comparison group included 30 pregnant women with threatened miscarriage, that was diagnosed in the second trimester. Blood sampling was done before starting treatment in hospital. Laboratory staff performing enzyme immunoassay was not informed about the clinical diagnosis in patients.
Peripheral venous blood samples (3 ml) were collected in the dry tubes from the cubital vein to determine serum FAS concentration. Blood coagulation occurred within 10–15 minutes, then the clot was centrifuged for 10 minutes. The obtained blood serum was placed in Eppendorf type tubes and stored at T0 -20°C. Serum FAS concentration was determined by enzyme immunoassay using test equipment (Multiscan FC Labsystems, China). The results were analyzed according to the manufacturer's protocol.
The criteria for comparison between the patients in the main and in the comparison group were: the age of women at the onset of pregnancy, height, BMI at the time of examination, family health history. In addition, pregnancy outcomes were compared. The absolute values of serum FAS concentration were assessed. Possible association between serum FAS level in women with GDM and threatened miscarriage that was diagnosed in the second trimester and the likelihood of fetal macrosomia was evaluated after childbirth.
Statistical analysis
The parameters of normal distribution were assessed using Shapiro–Wilk test, and equality of variances using Levene’s test. Normal distribution of quantitative values was shown as arithmetic mean (M) and standard deviation (SD). Non-normal distribution of the obtained results was shown as median, as well as lower and upper quartiles (Ме (Q1;Q3)). Non-parametric Mann–Whitney U test was used to compare the median between two independent samples. Qualitative characteristics were described as absolute numbers (n) and relative values (%) using Pearson's Chi-square test (χ2). Relative risk (RR) and confidence interval (CI) were calculated. The differences were considered to be statistically significant at р<0.05 (level of significance = 95%).
For evaluation of diagnostic methods, the following parameters were calculated and assessed: sensitivity, specificity, predictive value of positive and negative test results using ROC analysis to calculate the area under the curve (AUC); the diagnostic accuracy of the method was calculated according to the generally accepted formula and represented the percentage (%) of true results out of all obtained. Statistica for Windows 10.0, Microsoft Excel 2007, MedCalс and OpenEpi were used for statistical data processing.
Results and discussion
The anamnestic and clinical data characterizing the study groups are shown in Table 1. The age of women in the main group matched for age in the comparison group (30 (28; 35) and 29 (26,25; 31,75) years, p=0.062). The analysis of association between GDM and the occurrence of miscarriage showed the following risk factors for the development of GDM: higher BMI at the time of examination (28.36 kg/m2 (22.77; 31.48); 21.93 kg/m2 (20.82; 27.62), р=0.001), irregular working hours (RR( 4.00; 95% CI 1.23–12.97, р=0.022), arterial hypertension (AH) in first-degree relatives (RR 3.36; 95% CI 1.40–8.04, р=0.006), ischemic heart disease (IHD) in second-degree relatives (RR 4.00; 95% CI 1.23–12.97, р=0.022).
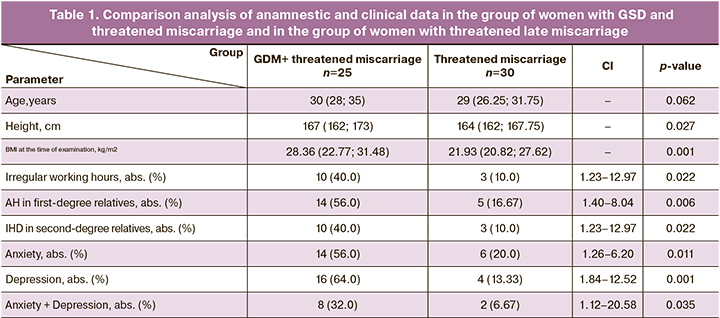
Assessment results of Hospital Anxiety and Depression Scale (HADS) were the following: the frequency of diagnostically significant level of anxiety (RR 2.80; 95% CI 1.26–6.20, р=0.011) and depression (RR 4.80; 95% CI 1.84–12.52, р=0.001), and their combination (RR 4.80; 95% CI 1.12–20.58, р=0.035) was higher in women with GDM.
The structure of GDM was analyzed. The diagnosis of GDM was primarily made at 19 weeks of pregnancy (15; 20). Blood sugar level in women with GDM was 5.92 (0,85) mmol/l. Compensation of endocrine disorders was achieved by diet therapy. There were no patients receiving insulin therapy.
We analyzed pregnancy outcomes in these women. Late miscarriages in the main group were at 21–22 weeks gestation, and at 18 weeks gestation in the comparison group. Preterm births in women with GDM and threatened late miscarriage were at 29.5 (5.92) weeks gestation, and at 31.5 (2.65) weeks gestation in women with threatened late miscarriage. There were cases of giving birth to babies with congenital malformation (CM) in both groups (1/25 (4.0%), 2/30 (6.67%), р=0.67). GDM was a risk factor in women in the main group [23], and a risk factor in the comparison group could be smoking and drinking alcohol during pregnancy [24]. The number of macrosomic newborns was higher in the main group versus the comparison group (15/25 (60.0%), 4/30 (13.33%), р=0.002) (Table 2).
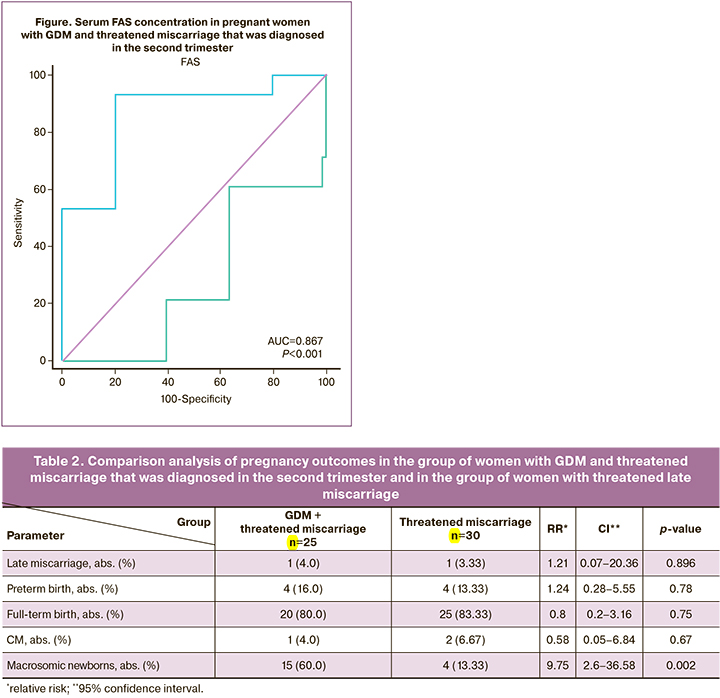
We assessed serum FAS concentration to clarify the mechanism of developing fetal macrosomia in women with GDM and threatened miscarriage, that was diagnosed in the second trimester. Serum FAS concentration was assessed in comparable terms of pregnancy (19 weeks (15; 20); 18.5 weeks (16; 20.75); р<0.744). The analysis of the obtained results showed high serum FAS concentration in women in the main group (1.64 ng/ml (1.13; 1.98) versus women without GDM (0.8 ng/ml (0.3075; 1.55); р<0.001).
Serum FAS concentration was assessed in 25 women in the main group. In 16 patients, it was lower or equal to 1.88 ng/ml. In 9 women in the main group, serum FAS concentration was higher, and only one of them had fetal macrosomia. Comparison between the group of women with fetal macrosomia, and the group of women without fetal macrosomia showed high diagnostic ROC AUC value = 0.867 (95% CI 0.67–0.96) (Figure). Sensitivity was 87.5% (95% CI 70.3–93.4), specificity was 88.9% (95% CI 58.3–99.4), the prognostic value of positive results was 93.3% (95% CI 75.0–99.6), and the prognostic value of negative results was 80.0% (95% CI 52.5–89.5).
This diagnostic method has the following advantages: possibility to predict development of fetal macrosomia starting from 13 weeks of pregnancy; easy implementation of the method. This method is characterized by individualized approach to management of patients with GDM and threatened miscarriage that was diagnosed in the second trimester. The use of this method provides the possibility of timely implementation of preventive measures.
The below examples demonstrate the practical use of this method.
Example 1
The patient A., aged 28 years, planned first pregnancy. The patient was admitted to Gynecology Department of the hospital. The woman complained of dragging pain in lower abdomen, spotting bloody vaginal discharge. Based on clinical and functional examination methods, the following diagnosis was made: 17 weeks of pregnancy; threatened late miscarriage; GDM.
According to the offered method, FAS concentration in peripheral venous blood was 0.99 ng/ml.
Medical conclusion was: prognosis for fetal macrosomia.
Conservation treatment and diet therapy with positive effect was prescribed. After 14 days, the woman was discharged from hospital with subsequent follow-up in women’s clinic. The diagnosis at the time of discharge was: 19 weeks of pregnancy. GDM.
During routine examination at 30 weeks gestation, based on clinical and functional methods the diagnosis was: 30 weeks of pregnancy. Fetal macrosomia. GDM.
Timely delivery in this primiparous woman was at 38 weeks and 4 days with cephalic fetal presentation before birth. Birth weight of the boy was 4620 g, length 56 cm (the Apgar score of 8/9 points). The diagnosis was made: term infant, large for gestational age.
It should be noted that FAS concentration was 0.99 ng/ml.
Example 2
The patient M., aged 32 years, planned third pregnancy. The woman had one spontaneous miscarriage at 6–7 weeks of pregnancy and one timely delivery in anamnesis. At 13 weeks gestation, the patient was admitted to Gynecology Department of the hospital. The woman had dragging pain in lower abdomen, spotting bloody vaginal discharge. Based on clinical and functional examination methods the diagnosis was made: 13 weeks of pregnancy. Threatened late miscarriage. GDM. Mild anemia.
According to the offered method, FAS concentration in the peripheral venous blood was 1.94 ng/ml. Judging by this value, it could be suggested that there was no fetal macrosomia in woman with GDM and threatened late miscarriage.
Conservation treatment and diet therapy with positive effect was prescribed. After 10 days, the woman was discharged from hospital with subsequent follow-up in women’s clinic. The diagnosis at the time of discharge was made: 14 weeks and 3 days of pregnancy. GDM.
Before delivery, the woman was regularly observed in the women’s clinic. According to the results of all ultrasound examinations and ultrasonographic fetometry, no signs of macrosomia were detected. The second timely delivery was at 39 weeks and 5 days with cephalic fetal presentation before birth. Birth weight of the boy was 3450 g, length 51 cm (the Apgar score of 8/9 points).
The diagnosis was: term infant, appropriate for gestational age.
Thus, with FAS value equal to 1,94 ng/ml, the baby was born with the normal body weight.
Example 3
The patient U., aged 37 years. planned fourth pregnancy. One medical abortion, one early miscarriage, one timely delivery was in anamnesis. The patient was admitted to Gynecology Department in the hospital at 20 weeks of pregnancy with complains of dragging pain in lower abdomen, spotting bloody vaginal discharge. Based on clinical and functional examination methods the diagnosis was made: 20 weeks of pregnancy. Threatened late miscarriage. GDM. Mild myopia.
According to the offered method, FAS concentration in the peripheral venous blood was 1.88 ng/ml. Judging by this value, the development of fetal macrosomia could be suggested.
Conservation treatment, diet therapy with a positive effect was started; After 12 days, the woman was discharged with subsequent follow-up in the women’s clinic. The diagnosis was made: 21 weeks and 5 days of pregnancy. GDM. Mild myopia.
During routine examination at 24 weeks gestation, based on clinical and functional methods the diagnosis was: 24 weeks of pregnancy. Fetal macrosomia. GDM. Mild myopia.
Timely second delivery was at 40 weeks and 1 day with sephalic felal presentation before birth. Birth weight of the girl was 4950 g, length 59 cm (the Apgar score of 8/9 points).
The diagnosis was made: term infant, large for gestational age.
It should be noted that FAS value was 1.88 ng/ml.
It is known about increased glucose uptake through utero-placental circulation in pregnant women with GDM. The reason is pregnancy-induced insulin resistance leading to the formation of macrosomia [14]. On the other hand, threatened miscarriage provokes stress that was assessed by HADS scores. Stress is a cause hyperactivity of the hypothalamic-pituitary-adrenal axis. Massive release of stress hormones leading to hyperglycemia exacerbates already existing insulin resistance and contributes to development of GDM [25].
It has been proven that FAS is released from the cell by adenosine monophosphate-activated protein kinase (AMPK) mechanism. With active fatty acids synthesis, FAS concentration increases in the cells, and AMPK actively releases FAS into the intracellular space [26–28]. However, increased insulin resistance during stress leads to reduction of energy substrate in the cell below the critical level. This is how the energy stress of the cell is formed, and AMPK phosphorylates metabolic enzymes, preventing their activity, and release from the cell [29, 30]. It is possible that FAS is also phosphorylated by AMPK, that, in turn, contributes to the growth of hyperglycemia and the development of macrosomia.
All patients received hormone therapy with 10 mg dydrogesterone before hospitalization. This fact rules out the role of this therapy in the pathogenesis of GDM. In addition, there is no evidence in published data showing the effect of dydrogesterone on serum FAS concentration.
Therefore, the stress that a woman experiences in case of threatened late miscarriage leads to increased activity of the hypothalamic-pituitary-adrenal axis system, which is accompanied by the release of stress hormones into blood, aggravating insulin resistance that was already formed by GDM. High glucose level is supplied to the fetus and provokes development of macrosomia. At the cellular level, decreased insulin sensitivity causes energy stress in the cell and phosphorylation of FAS by AMPK, preventing its release from the cell. Thus, reduced serum FAS concentration reflects sharply increased insulin resistance and high level of glucose supply to the fetus.
Conclusion
Therefore, comparison between serum FAS concentrations in women with GDM and threatened miscarriage that was diagnosed in the second trimester of pregnancy, and in women with threatened miscarriage diagnosed in the second trimester in the absence of GDM showed that serum FAS concentration in women in the main group was higher (1.64 ng/ml (1.13; 1.98) versus women in the group without GDM (0.8 ng/ml (0.3075; 1.55); р<0.001). Detection of FAS concentration had a high diagnostic accuracy – AUC = 0.867 (95% CI 0.67–0.96). Sensitivity was 87.5% (95% CI 70.3–93.4), specificity was 88.9% (95% CI 58.3–99.4), the prognostic value of positive results was 93.3% (95% CI 75.0–99.6), and the prognostic value of negative results was 80.0% (95% CI 52.5–89.5).
It is probable that detection of serum FAS concentration can be considered as a promising method for prediction of macrosomia. However further studies are necessary in this area.
References
- Виктор С.А., Курилович С.В. Фетальная макросомия: возможности антенатального прогнозирования (обзор литературы). Репродуктивное здоровье. Восточная Европа. 2019; 9(1): 97-106. [Viktor S.A., Kurilovich S.V. Fetal macrosomia: possibilities for antenatal prognosis (literature review). Reproductive Health. Eastern Europe. 2019; 9(1): 97-106. (in Russian)].
- Лысенко С.Н., Чечнева М.А., Бурумкулова Ф.Ф., Петрухин В.А., Панов А.Е., Плеханова М.А., Улятовская В.И., Зубкова Н.А., Тюльпаков А.Н. Ультразвуковые предикторы формирования макросомии при гестационном сахарном диабете. Сахарный диабет. 2019; 22(4): 358-66. [Lysenko S.N., Chechneva M.A., Burumkulova F.F., Petruhin V.A., Panov A.E., Plekhanova M.A., Ulyatovskaya V.I., Zubkova N.A., Tyul'pakov A.N. Ultrasound predictors of macrosomia formation in gestational diabetes mellitus. Diabetes Mellitus. 2019; 22(4): 358-66 (in Russian)].
- Геворкян Р.С., Рымашевский А.Н., Волков А.Е., Маркина В.В. Макросомия плода: современное состояние проблемы. Современные проблемы науки и образования. 2016; 6: 142. [Gevorkjan R.S., Rymashevskij A.N., VolkovA.E., Markina V.V. Fetal macrosomia: state of the art. Contemporary Issues of Science and Education. 2016; (6): 142. (in Russian)].
- Aguayo L., Li W., Joyce B.T., Leng J., Zheng Y., Shiau S., Liu H. et al. Risks of macrosomia associated with catechol-o-methyltransferase genotypes and genetic-epigenetic interactions among children with and without gestational diabetes exposure. Child. Obes. 2021; 17(5): 365-70. https://dx.doi.org/10.1089/chi.2020.0327.
- Biratu A.K., Wakgari N., Jikamo B. Magnitude of fetal macrosomia and its associated factors at public health institutions of Hawassa city, southern Ethiopia. BMC Res. Notes. 2018; 11(1): 888. https://dx.doi.org/10.1186/s13104-018-4005-2.
- Che H., Long D., Sun Q., Wang L., Li Y. Birth weight and subsequent risk of total leukemia and acute leukemia: a systematic review and meta-analysis. Front. Pediatr. 2021; 9: 722471. https://dx.doi.org/10.3389/fped.2021.722471.
- Ходжаева З.С., Снеткова Н.В., Клименченко Н.И., Абрамова М.Е., Дегтярева Е.И., Донников А.Е. Клинико-молекулярно-генетические детерминанты формирования гестационного сахарного диабета. Акушерство и гинекология. 2019; 4: 18-24. [Khodzhaeva Z.S., Snetkova N.V., Klimenchenko N.I., Abramova M.E., Degtyareva E.I., Donnikov A.E. Clinical and molecular genetic determinants of the development of gestational diabetes mellitus. Obstetrics and Gynecology. 2019; (4): 18-24. (in Russian)].https://dx.doi.org/10.18565/aig.2019.4.18-24.
- Czarnobay S.A., Kroll C., Schultz L.F., Malinovski J., Mastroeni S.S.B.S., Mastroeni M.F. Predictors of excess birth weight in Brazil: a systematic review. J. Pediatr. (Rio J). 2019; 95(2): 128-54. https://dx.doi.org/10.1016/j.jped.2018.04.006.
- Герасимов А.М., Батрак Н.В. Влияние гипотиреоза и избыточной массы тела беременных на течение гестационного периода, рождение крупного плода и функциональное состояние его щитовидной железы. Вестник Ивановской медицинской академии. 2013; 18(1): 39-42. [Gerasimov A.M., Batrak N.V. Effect of hypothyroidism and overweight in pregnant women on the course of the gestational period, the birth of a large foetus and the functional status of its thyroid gland. Bulletin of the Ivanovo Medical Academy. 2013; 18(1): 39-42 (in Russian)].
- Фролухина О.Б., Башмакова Н.В., Третьякова Т.Б., Дерябина Е.Г. Роль генов-кандидатов, как триггерных факторов развития гестационного сахарного диабета. Лечение и профилактика. 2019; 9(3): 39-46. [Frolukhina O.B., Bashmakova N.V., Tret'yakova T.B., Deryabina E.G. The role of candidate genes as trigger factors for the development of gestational diabetes mellitus. Treatment and Prevention. 2019; 9(3): 39-46. (in Russian)].
- Янкина С.В., Шатрова Н.В., Берстнева С.В., Павлов Д.Н. Особенности течения и исходы беременности у женщин с гестационным сахарным диабетом. Российский медико-биологический вестник имени академика И.П. Павлова. 2018; 1: 96-105. [Jankina S.V., Shatrova N.V., Berstneva S.V., Pavlov D.N. The course and outcome of pregnancy in women with gestational diabetes mellitus. Pavlov Russian Medical and Biological Bulletin. 2018; (1): 96-105. (in Russian)]. https://dx.doi.org/10.23888/PAVLOVJ201826196-105.
- Додхоева М.Ф., Пирматова Д.А. Гестационный сахарный диабет: современный взгляд на актуальную проблему. Вестник Авиценны. 2018; 20(4): 455-61. [Dodkhoeva M.F., Pirmatova D.A. Gestational diabetes mellitus: a modern view on an actual problem. Bulletin of Avicenna. 2018; 20(4): 455-61. (in Russian)]. https://dx.doi.org/10.25005/2074-0581-2018-20-4-455-461.
- Шапошникова Е.В., Ведмедь А.А., Бацунина О.В. Перинатальные исходы при гестационном сахарном диабете: особенности течения периода новорожденности, раннего детства. Смоленский медицинский альманах. 2017: 4; 57-60. [Shaposhnikova E.V., Vedmed' A.A., Bacunina O.V. Perinatal outcomes in gestational diabetes mellitus: features of the course of the neonatal period, early childhood. Smolensk Medical lmanac. 2017: (4); 57-60.(in Russian)].
- Карасева Е.В., Гузий Е.А. Гестационный сахарный диабет и макросомия. Журнал научных статей «Здоровье и образование в XXI веке». 2018; 20(3): 57-60. [Karaseva E.V., Guzij E.A. Gestational diabetes mellitus and macrosomia. Journal of Scientific Articles Health and Education in the 21st Century. 2018; 20(3): 57-60. (in Russian)]. https://dx.doi.org/10.26787/nydha-2226-7425-2018-20-3-57-60.
- De Silva G.S., Desai K., Darwech M., Naim U., Jin X., Adak S. et al. Circulating serum fatty acid synthase is elevated in patients with diabetes and carotid artery stenosis and is LDL-associated. Atherosclerosis. 2019; 287: 38-45.https://dx.doi.org/10.1016/j.atherosclerosis.2019.05.016.
- Carroll R.G., Zasłona Z., Galván-Peña S., Koppe E.L., Sévin D.C., Angiari S. et al. An unexpected link between fatty acid synthase and cholesterol synthesis in proinflammatory macrophage activation. J. Biol. Chem. 2018; 293(15): 5509-21. https://dx.doi.org/10.1074/jbc.RA118.001921.
- Афонина В.А., Батрак Н.В., Малышкина А.И., Сотникова Н.Ю. Взаимосвязь липидного обмена и инсулинорезистентности при гестационном сахарном диабете. Акушерство и гинекология. 2022; 7: 13-20. [Afonina V.A., Batrak N.V., Malyshkina A.I., Sotnikova N.Yu. Relationship between lipid metabolism and insulin resistance in gestational diabetes mellitus. Obstetrics and Gynecology. 2022; (7): 13-20. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.7.13-20.
- Wei X., Song H., Yin L., Rizzo M.G., Sidhu R., Covey D.F. et al. Fatty acid synthesis configures the plasma membrane for inflammation in diabetes. Nature. 2016; 539(7628): 294-8. https://dx.doi.org/10.1038/nature20117.
- Fazli H.R., Moradzadeh M., Mehrbakhsh Z., Sharafkhah M., Masoudi S., Pourshams A., Mohamadkhani A. Diagnostic significance of serum fatty acid synthase in patients with pancreatic cancer. Middle East J. Dig. Dis. 2021; 13(2): 115-20. https://dx.doi.org/10.34172/mejdd.2021.214.
- Wu X., Zayzafoon M., Zhang X., Hameed O. Is there a role for fatty acid synthase in the diagnosis of prostatic adenocarcinoma? A comparison with AMACR. Am. J. Clin. Pathol. 2011; 136(2): 239-46. https://dx.doi.org/10.1309/AJCP0Y5QWWYDKCJE.
- Balachandiran M., Bobby Z., Dorairajan G., Jacob S.E., Gladwin V., Vinayagam V., Packirisamy R.M. Placental accumulation of triacylglycerols in gestational diabetes mellitus and its ssociation with altered fetal growth are related to the differential expressions of proteins of lipid metabolism. Exp. Clin. Endocrinol. Diabetes. 2021; 129(11): 803-12. https://dx.doi.org/10.1055/a-1017-3182.
- Carreras-Badosa G., Prats-Puig A., Puig T., Vázquez-Ruíz M., Bruel M., Mendoza E. et al. Circulating fatty acid synthase in pregnant women: relationship to blood pressure, maternal metabolism and newborn parameters. Sci. Rep. 2016; 6: 24167. https://dx.doi.org/10.1038/srep24167.
- Ornoy A., Becker M., Weinstein-Fudim L., Ergaz Z. Diabetes during pregnancy: a maternal disease complicating the course of pregnancy with long-term deleterious effects on the offspring. A clinical review. Int. J. Mol. Sci. 2021; 22(6); 2965. https://dx.doi.org/10.3390/ijms22062965.
- Harris B.S., Bishop K.C., Kemeny H.R., Walker J.S., Rhee E., Kuller J.A. Risk factors for birth defects. Obstet. Gynecol. Surv. 2017; 72(2): 123-35.https://dx.doi.org/10.1097/OGX.0000000000000405.
- OuYang H., Chen B., Abdulrahman A.M., Li L., Wu N. Associations between gestational diabetes and anxiety or depression: a systematic review. J. Diabetes Res. 2021; 2021: 9959779. https://dx.doi.org/10.1155/2021/9959779.
- Bae S., Lee Y.H., Lee J., Park J., Jun W. Salvia plebeia R. Br. water extract ameliorates hepatic steatosis in a non-alcoholic fatty liver disease model by regulating the AMPK pathway. Nutrients. 2022; 14(24): 5379.https:/dx.doi.org/10.3390/nu14245379.
- Huang M., Koizumi A., Narita S., Inoue T., Tsuchiya N., Nakanishi H. et al. Diet-induced alteration of fatty acid synthase in prostate cancer progression. Oncogenesis. 2016; 5(2): e195. https://dx.doi.org/10.1038/oncsis.2015.42.
- Oliveras-Ferraros C., Vazquez-Martin A., Fernández-Real J.M., Menendez J.A. AMPK-sensed cellular energy state regulates the release of extracellular Fatty Acid Synthase. Biochem. Biophys. Res. Commun. 2009: 378(3): 488-93.https://dx.doi.org/10.1016/j.bbrc.2008.11.067.
- Соколова Л.К., Пушкарев В.М., Бельчина Ю.Б., Пушкарев В.В., Гончар И.В., Тронько Н.Д. Активность АМРК в лимфоцитах больных сахарным диабетом при действии сахароснижающих препаратов. Клінічна ендокринологія та ендокринна хірургія. 2017; 2: 82-90. [Sokolova L.K., Pushkarev V.M., Bel'china Ju.B., Pushkarev V.V., Gonchar I.V., Tronko N.D. AMРK activity in lymphocytes of diabetic patients under the action of antidiabetic drugs. Clinical Endocrinology and Endocrine Surgery. 2017; (2): 82-90. (in Ukrainian)]. https://dx.doi.org/10.24026/1818-1384.2(58).2017.105627.
- Wu L., Huang X.J., Yang C.H., Deng S.S., Qian M., Zang Y., Li J. 5'-AMP-activated protein kinase (AMPK) regulates progesterone receptor transcriptional activity in breast cancer cells. Biochem. Biophys. Res. Commun. 2011; 416(1-2): 172-7. https://dx.doi.org/10.1016/j.bbrc.2011.11.018.
Received 06.02.2023
Accepted 16.05.2023
About the Authors
Anna I. Malyshkina, Dr. Med. Sci., Professor, Director, V.N. Gorodkov Ivanovo Research Institute of Motherhood and Childhood, Ministry of Health of the Russian Federation, 153045, Russia, Ivanovo, Pobedy str., 20; Head of the Department of Obstetrics and Gynecology, Medical Genetics, Ivanovo State Medical Academy,Ministry of Health of the Russian Federation, 153012, Russia, Ivanovo, Sheremetevsky Ave., 8, ivniimid@inbox.ru, https://orcid.org/0000-0002-1145-0563
Natalya Yu. Sotnikova, Dr. Med. Sci., Professor, Honored Doctor of the Russian Federation, Head of the Laboratory of Clinical Immunology, V.N. Gorodkov Ivanovo Research Institute of Motherhood and Childhood, Ministry of Health of the Russian Federation, 153045, Russia, Ivanovo, Pobedy str., 20; Professor, Department of Pathophysiology and Immunology, Ivanovo State Medical Academy, Ministry of Health of the Russian Federation, 153012, Russia, Ivanovo, Sheremetevsky Ave., 8, ivniimid@inbox.ru,
https://orcid.org/0000-0002-0608-0692
Natalya V. Kroshkina, PhD, Researcher, V.N. Gorodkov Ivanovo Research Institute of Motherhood and Childhood, Ministry of Health of the Russian Federation,
153045, Russia, Ivanovo, Pobedy str., 20, +7(980)693-18-22, ivniimid@inbox.ru
Nataliya V. Batrak, PhD, Associate Professor at the Department of Obstetrics and Gynecology, Medical Genetics, Ivanovo State Medical Academy,
Ministry of Health of the Russian Federation, 153012, Russia, Ivanovo, Sheremetevsky Ave., 8, batrakn@inbox.ru, https://orcid.org/0000-0002-5230-9961
Viktorija A. Afonina, PhD Student, V.N. Gorodkov Ivanovo Research Institute of Motherhood and Childhood, Ministry of Health of the Russian Federation,
153045, Russia, Ivanovo, Pobedy str., 20; Teaching Assistant, Ivanovo State Medical Academy, Ministry of Health of the Russian Federation,
153012, Russia, Ivanovo, Sheremetevsky Ave., 8, +7(963)151-59-58, ezhevika23023@yandex.ru, https://orcid.org/ 0000-0002-3145-5679



