Antenatal and intrapartum risk factors associated with fetal hypoxia in labor
Objective: To identify the risk factors associated with fetal hypoxia in labor. Materials and methods: A prospective cohort study included 657 women. Of them, 119 women delivered babies with fetal hypoxia at birth and were included in the study group. Cases without fetal hypoxia (n=538) constituted the control group. The diagnosis of fetal hypoxia at birth was verified by determining the acid-base composition of the arterial cord blood. Criteria for acidosis were pH<7.12 and/or BE≥12.4 mmol/L. To identify risk factors for fetal hypoxia in labor, we compared age, patient anthropometric parameters, medical history, and pregnancy complications by collecting anamnesis, studying ambulatory records and physical examination, as well as the characteristics of labor and delivery modes during labor management, evaluating fetal monitoring, and determining the composition of the blood acid-base. Results: Patients in the fetal hypoxia group were more likely to have anemia in the first half of pregnancy, gestational diabetes mellitus, extragenital infections, edema of pregnant women, placental insufficiency, and fetal growth restriction. Hypertensive complications of pregnancy as an indication of induction of labor were observed more frequently in the fetal hypoxia group [3 (0.6%) vs 7 (5.9%), p=0.0006]. Gestational age 37–38 and 41 weeks or more at delivery was more common in the fetal hypoxia group, while gestational age 39–40 weeks at delivery was more common in the control group. There were no differences in the rates of preterm prelabor rupture of membranes (9.9 and 13.4%), amniotomy (15.1 and 9.2%), epidural analgesia (51.3 and 55.5%), and mean length of ruptured membranes. Tachysystole was more common in the fetal hypoxia group (RR=7.12 (95% CI 4.8; 10.5), p<0.0001). The relative risk of fetal hypoxia in the presence of labor dystocia was RR=3.17 (95% CI 2; 5), p<0.0001, oxytocin labor stimulation was RR=1.6 (95% CI 1.3; 2), p<0.0001. Conclusion: Antenatal risk factors have low specificity. Intrapartum risk factors including gestational age 37–38 weeks, 41 weeks or more, labor dystocia, oxytocin, labor stimulation, and tachysystole have higher predictive value, especially when combined with CTG. However, in the presence of clinical intrapartum risk factors, the absence of a pathological type of CTG does not guarantee an asphyxia-free birth. Intrapartum risk factors are modifiable in most cases. A rational choice of gestational age and method of labor induction and careful labor management are the reserves for reducing the incidence of fetal hypoxia in labor.Baev O.R., Prikhodko A.M., Ziganshina M.M., Evgrafova A.V., Khomyakova E.V.
Keywords
Birth asphyxia occurs in 6.8–20% of births [1–3]. The WHO estimates suggest that perinatal asphyxia ranks third among the causes of neonatal mortality [4]. Fetal distress occurs in 15–20% of all births; 2–2.1% of newborns require resuscitation, and 1.1–6% develop hypoxic ischemic encephalopathy [5, 6]. In the Russian Federation, antepartum and intrapartum hypoxia are the most frequent causes of stillbirth (up to 80.5% and 5.0% of all stillbirths in 2020) [7].
Abnormalities of newborn condition can have different causes, and umbilical cord acid-base analysis is fundamental for assessing intrapartum hypoxia, which allows the diagnosis of metabolic acidosis. In 1999, the International Cerebral Palsy Task Force defined the criteria for metabolic acidosis metabolic acidosis in intrapartum fetal, umbilical arterial cord, or very early neonatal blood samples (pH < 7.00 and base deficit >12 mmol/l), which are combined with early onset of severe or moderate neonatal encephalopathy [8]. Some researchers use pH=7.05 as a critical limit below which the development of metabolic acidosis that is associated with severe neurological injury to the child, is diagnosed [9, 10].
Criteria for severe asphyxia at birth also include the 5-minute Apgar score <7 [11] or the 10-minute Apgar score <5, the need for resuscitation > 10 min [5, 12].
Despite the introduction of modern methods of fetal evaluation during pregnancy and delivery, the incidence of hypoxic ischemic encephalopathy and cerebral palsy has not decreased in the past 30 years and even shows an increasing trend [13]. In this sense, the search for predictors of fetal hypoxia, which will allow its prediction, the development of preventive measures, and appropriate obstetric strategies, remains relevant.
This study aimed to identify the risk factors associated with fetal hypoxia in labor
Materials and methods
We conducted a prospective cohort study investigating clinical and anamnestic data, course of pregnancy and labor in 657 women who delivered at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia in 2017–2020. The inclusion criteria were full-term singleton pregnancy, 18 years or older. Non-inclusion criteria were congenital fetal malformations, prematurity, multiple pregnancies, fetal malposition, uterine scar, acute phase or exacerbation of chronic infectious diseases, severe non-obseteric pathology in the mother (decompensated somatic diseases, transplanted organs, cancer). The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
The diagnosis of fetal hypoxia at birth was verified by determining the acid-base composition of fetal umbilical artery blood. The acidosis criteria were pH<7.12 and/or BE≥12.4 mmol/L. Fetal hypoxia at birth occurred in 119 observations, which constituted the study group, and 538 cases without fetal hypoxia were included in the control group. To identify risk factors, the age, anthropometric parameters of the patients, the somatic history and pregnancy complications were compared by collecting their medical history, reviewing their ambulatory records, physical examination, as well as the specifics of labor and delivery management, evaluating the results of fetal monitoring and determining the acid-base balance in the blood.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 statistical software (GraphPad Software, USA). The distribution of continuous variables was tested for normality using the generalized D'Agostino–Pearson test. Parametric data were presented as mean (standard deviation). Nonparametric data were presented as median (interquartile range). Categorical variables were presented as counts and percentages. We used the t test (for parametric data), the Mann–Whitney test (for nonparametric data) and Fisher exact test with relative risk (RR) calculation (for categorical variables) to compare numerical data between groups. Differences were considered statistically significant at p<0.05.
Results
The age of the patients in the compared groups did not differ significantly, except for the group under 20 years, in which fetal hypoxia was more frequent [2 (0.37%) vs 4 (3.36%), p=0.01]. Although there were no differences in age, baseline weight was on average higher and obesity was more common in the group with fetal hypoxia [63.9 (0.4) kg vs 70.8 (2.3) kg, p<0.015; 67 (12.5%) vs 31 (26%), p<0.0001]. We found no differences between the groups in the incidence of nonobstetric diseases. However, a history of polyps and endometrial hyperplastic processes was more common in patients with fetal hypoxia (15 (2.8%) vs 8 (6.7%), p=0.04).
Primiparas women [470 (87.3%) and 94 (78.9%)] were found to be predominant in both the control and fetal hypoxia groups. There were no differences in ectopic pregnancy rates, induced and early spontaneous miscarriages, except for the failure to conceive, which occurred more frequently in the fetal hypoxia group [51 (9.5%) vs 22 (18.5%), p<0.05].
Women whose labor was complicated by fetal hypoxia were more likely to have anemia in the first half of pregnancy, gestational diabetes mellitus, extragenital infections (angina, RRVI, pneumonia, pyelonephritis), prenatal edema, as well as placental insufficiency and fetal growth restriction (Table). At the same time, threatened preterm labor, hypertensive complications, and lower genital tract infections during pregnancy (aerobic or candidiasis vaginitis, bacterial vaginosis) were not associated with subsequent fetal hypoxia during labor.
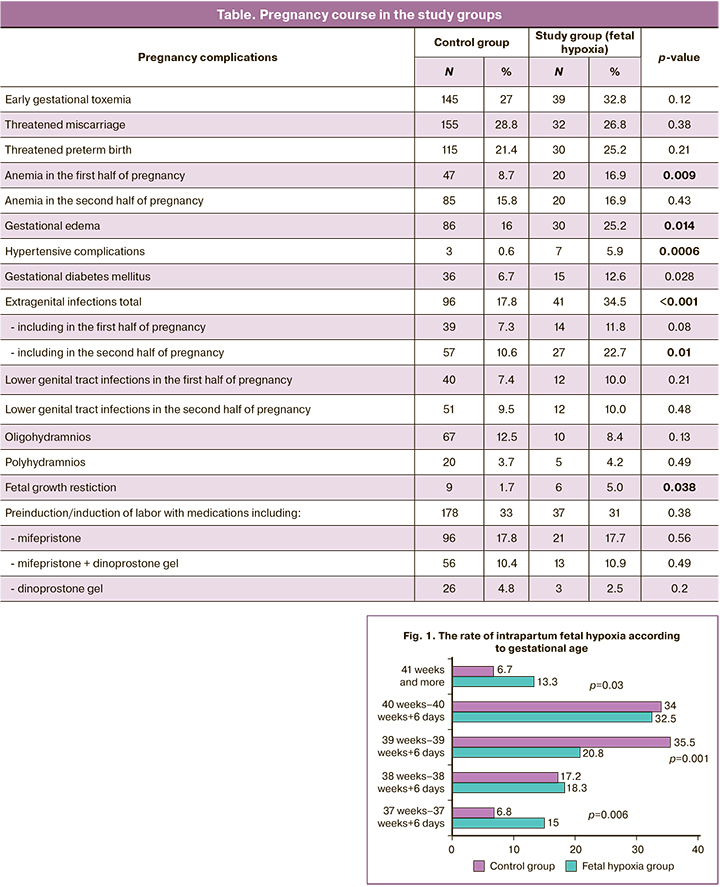
Induction of labor occurred with equal frequency in both groups [178 (33%) and 37 (31%)]. Hypertensive pregnancy complications as an indication for labor induction were more frequent in the fetal hypoxia group [3 (0.6%) vs 7 (5.9 %) p=0.0006]; there were no differences for other indications. There was also no association between the use of pharmacologic agents for cervical ripening and fetal hypoxia (Table).
Gestational age 37–38 and 41 weeks or more at delivery was more common in the fetal hypoxia group, while gestational age 39–40 weeks at delivery was more common in the control group (Fig. 1). The relative risk of intrapartum fetal hypoxia at 37–37 weeks and 6 days was RR=1.98 (95% CI 1.18; 3.38), p=0.009; at 41 weeks or more, RR=1.97 (95% CI 1.15; 3.36), p=0.013; at 39–39 weeks 6 days, RR=0.52 (95% CI 0.36; 0.76), p=0.0006.
There were no differences in the rates of preterm prelabor rupture of membranes (9.9 and 13.4%), amniotomy (15.1 and 9.2%), epidural analgesia (51.3 and 55.5%), and mean length of ruptured membranes (306 and 276 minutes). At the same time, labor dystocia and oxytocin labor stimulation were significantly more frequent in the fetal hypoxia group (Fig. 2). Despite the higher frequency of labor dystocia, the mean duration of labor was shorter in women in the study group [408 minutes (336–504) vs 438 minutes (327–576) in the control group, p=0.015]. The high frequency of contractions (tachysystole of 6 to 9 contractions per 10 minutes) was significantly more common in the fetal hypoxia group [RR=7.12 (95% CI 4.8; 10.5), p=0.0001] (Fig. 2). The risk of fetal hypoxia in the presence of labor dystocia was RR=3.17 (95% CI 2; 5), p=0.0001, and the stimulation of labor with oxytocin was RR=1.6 (95% CI 1.3; 2), p=0.0001.
Of note, a pathological type of CTG (category 3) was noted in the majority of fetal hypoxia observations (79.0% vs 7.4% in the control group, p=0.001) (Fig. 2). The suspicious type (category 2) occurred with equal frequency in 20.2%, while the normal type occurred in 81.6 and 1.7% of observations.
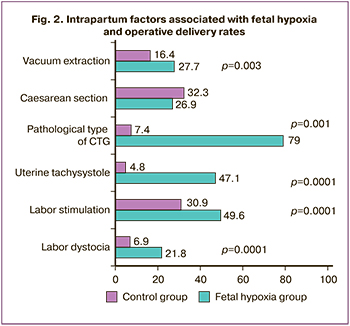
The overall surgical delivery rate did not differ between the groups, but the fetal vacuum extraction rate was significantly higher in the fetal hypoxia group [88 (16.4%) vs 33 (27.7%), p=0.003] (Fig. 2).
All babies were born alive. There were no differences in the anthropometric parameters of the newborns. Birth weight averaged 3347.4 (414.8) and 3334.30 (381.65) g, p=0.90; body length was 51.7 (2.2) and 51.3 (2.04) cm, p=0.06. There were no differences in the frequency of large [30 (5.5%) vs 3 (2.5%), p=0.118] and low birth weight fetuses [12 (2.2%) vs 4 (3.3%), p=0.47]. The median Apgar score was consistently lower in the fetal hypoxia group and was 8 points (7–8) versus 4 (3–6) at minute 1, p<0.001; 9 points (8–9) vs 7 (5–8) at minute 5, p<0.001.
Construction of models that predict intrapartum fetal hypoxia showed that factors such as age under 20 years, obesity, endometrial hyperplastic processes, and a history of failed pregnancies have high sensitivity but low specificity. Specificity is also low in the model based on factors that contribute to a complicated pregnancy including anemia, gestational diabetes mellitus, infections experienced during pregnancy, edema, and fetal growth restriction. Such clinical intrapartum factors as delivery gestational age of 41 weeks or more, labor dystocia, oxytocin labor stimulation, and tachycardia are associated with high sensitivity and moderate specificity. Complementing this model with an abnormal CTG curve showed both high sensitivity and high specificity.
Discussion
Fetal hypoxia develops as a result of impaired blood flow and gas exchange in the fetal-placental unit and is a trigger for a cascade of changes in the functioning of its organs and systems. Nerve cells are most susceptible to hypoxic damage. Depending on the severity of hypoxemia/hypoxia, the initial stages involve compensatory reactions of the cardiovascular system with redistribution of blood flow. However, severe and prolonged hypoxic insult leads to irreversible brain cell damage, intraventricular hemorrhage, which is manifested by hypoxic-ischemic encephalopathy after birth, and the development of cerebral palsy and can be fatal [14].
Age, medical history, and anthropometric data are available means of predicting the outcome in many pathological conditions, as they reflect the functioning of body systems and indicate preexisting disorders, which may reappear against a background of increased stress or strain. In our observational series, fetal hypoxia was associated with a too young age of the pregnant woman, history of previous endometrial hyperplastic processes, failed pregnancy, and obesity. Young age was of limited importance, as the vast majority of the women were between 25 and 35 years of age, and only 3% were less than 20 years of age. Obesity is polyetiological in nature, predisposes to, and accompanies a large number of pathologic conditions, making this a factor of low specificity. Failure to conceive is a factor that is theoretically associated with endometrial hyperplastic processes, as it is often caused by initial endometrial deficiency.
Despite the differences in the above factors and the difference in their origins, it is nevertheless the immaturity and/or endometrial deficiency, which often occurs in these conditions, that unites them into one group.
According to our data, there were no differences in the groups depending on the parity. At the same time, according to Locatelli A. et al. (2020) [5], first birth is the most common risk factor for birth asphyxia, which the authors attribute to a higher frequency of placentation disorders, as well as a longer course of labor and the associated increased number of fetal infections and surgical delivery. At the same time, they point to the results of the study of Liljestrom et al. (2018) [15], which showed a higher incidence of hypoxic-ischemic encephalopathy in multiparous women, which, in their opinion, indicates that intrapartum complications can also cause severe complications in low-risk women.
It is logical to assume that the complicated course of pregnancy may be the cause of the complicated course of labor. According to our study, anemia, gestational diabetes mellitus, pregnancy edema, and extragenital infectious diseases were found to be significant risk factors. It is noteworthy that anemia in the first half of pregnancy was associated with fetal hypoxia in labor, whereas there was no such association in the second half. A strong positive association with bacterial or viral infection at any gestational age, but the relationship was clearest in the second half of pregnancy.
Despite the significant association between the above factors and intrapartum hypoxia, they are of pathogenetic rather than prognostic value, explaining the possible mechanisms of placental abnormalities or placental damage later in pregnancy. The specificity of these factors in the prognosis is low, because they can be combined with a wide range of other complications. Thus, the specificity of antenatal risk factors according to the analysis was only 0.008–0.133. Thus, it is inappropriate to use these factors to predict fetal hypoxia. At the same time, if factors such as age or obesity are not modifiable, at least at the stage of pregnancy, then timely detection and correction of anemia and gestational diabetes and prevention of infections are of great preventive value in reducing the incidence of fetal hypoxia.
Unlike antenatal risk factors, intrapartum risk factors can most often be regarded as modifiable. These include delivery gestational age of 41 weeks or more, labor dystocia, oxytocin labor stimulation, and tachysystole. Our results suggest that the odds of fetal hypoxia in labor at 39 weeks–39 weeks 6 days gestation are 2 to 2.5 times lower than those at 37 weeks–37 weeks 6 days and 41 weeks or more.
We found no association between pharmacological agents for cervical ripening and labor induction (mifepristone, dinoprostone) and fetal hypoxia. A Cochrane review in 2020 showed that, compared to the expectant approach, induction of full-term delivery was associated with a reduction in perinatal mortality, cesarean section rates, and hospitalization in the intensive care unit [16]. In view of this, and given that delivery gestational age of 39 weeks–39 weeks 6 days has a protective effect against fetal hypoxia, expanding the indications for induction of labor at this gestational age will help to reduce the incidence of this complication.
Other modifiable risk factors for fetal hypoxia are interrelated and include labor dystocia, oxytocin stimulation, and uterine tachysystole. The regulation of these factors lies in the management of the labor strategy. During labor, the blood perfusion in the placental intervillous space decreases and the outflow of venous blood outflow slows down. Due to the increased pressure in the placental intervillous space, blood flow in the capillaries of the villi slows down. These mechanisms alter fetal gas exchange, which compensates for lack of oxygen by high levels of fetal hemoglobin, redistribution of blood flow, and slowing of heart rate [17].
Unjustified early diagnosis of labor dystocia and aggressive labor stimulation with high doses of oxytocin lead to tachysystole. At the same time, excessively strong, prolonged and frequent contractions do not allow the restoration of fetal oxygenation, and hypoxemia and hypoxia develop. Interestingly, up to 40% of tachysystole cases can lead to fetal hypoxia in the absence of other typical CTG abnormalities [5]. The above clinical intrapartum risk factors for fetal hypoxia should be considered in labor management, as they are no less important than the pathological nature of CTG, which is not as much a risk factor for hypoxia as a sign of its development. Therefore, adding the pathological type of CTG to the fetal hypoxia prediction model increases its specificity, while the sensitivity does not change. In addition, it should be taken into account that in every fifth observation of birth asphyxia at intrapartum, the pathological nature of the CTG curve was not recorded.
Conclusion
Despite the presence of antenatal risk factors, their low specificity does not allow their use to predict intrapartum fetal hypoxia. Intrapartum risk factors including delivery gestational age of 37–38 weeks, 41 weeks or more, labor dystocia, use of oxytocin, and tachysystole have higher predictive value, especially when combined with CTG. However, in the presence of clinical intrapartum risk factors, the absence of a pathological type of CTG does not guarantee an asphyxia-free birth. Intrapartum risk factors are modifiable in most cases. A rational choice of gestational age and method of labor induction and careful labor management are the reserves for reducing the incidence of fetal hypoxia in labor.
References
- Шилова Н.А., Харламова Н.В., Андреев А.В., Межинский С.С., Панова И.А., Дудов П.Р. Частота асфиксий и объем оказания реанимационной помощи новорожденным в родильном зале. Неонатология: новости, мнения, обучение. 2020; 8(2): 47-53. [Shilova N.A., Kharlamova N.V., Andreev A.V., Mezhinsky S.S., Panova I.A., Dudov P.R. Frequency of perinatal asphyxia and volume of provision of care to newborns in the delivery room. Neonatology: News, Opinions, Training. 2020; 8(2): 47-53. (in Russian)].https://dx.doi.org/10.33029/2308-2402-2020-8-2-47-53.
- Prior T., Kumar S. Expert review – identification of intra-partum fetal compromise. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015; 190: 1-6.http://dx.doi.org/10.1016/j.ejogrb.2015.04.002.
- Natsumi F., Junichi H., Haruka I., Chika H., Akiko K., Haruhiro K., Nao S. Accuracy of predicting neonatal distress using a five-level classification of fetal heart rate monitoring. J. Obstet. Gynaecol. Res. 2021; 47(1): 254-61.https://dx.doi.org/10.1111/jog.14490.
- World Health Organization. Global Health Observatory (GHO); WHO 2016. Available at: http://www.childmortality.org/ Accessed April 10, 2020.
- Locatelli F., Lambicchi L., Incerti M., Bonati F., Ferdico M., Malguzzi S. et al. Is perinatal asphyxia predictable? BMC Pregnancy Childbirth. 2020; 20(1): 186. https://dx.doi.org/10.1186/s12884-020-02876-1.
- Ravichandran L., Allen V.M., Allen A.C., Baskett T.F., Woolcott C.G. Incidence, intrapartum risk factors, and prognosis of neonatal hypoxic–ischemic encephalopathy among infants born 35 weeks’ gestation or more. J. Obstet. Gynaecol. Can. 2020; 42(12): 1489-97. https://dx.doi.org/10.1016/j.jogc.2020.04.020.
- Щеголев А.И., Туманова У.Н., Чаусов А.А., Шувалова М.П. Сравнительный анализ причин мертворождения в Российской Федерации в 2019 и 2020 годах. Акушерство и гинекология. 2022; 2: 80-90. [Shchegolev A.I., Tumanova U.N., Chausov A.A., Shuvalova M.P. Comparative analysis of stillbirth causes and rates in the Russian Federation in 2019 and 2020. Obstetrics and Gynecology. 2022; 2: 80-90. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.2.80-90.
- MacLennan A. A template for defining a causal relation between acute intrapartum events and cerebral palsy: international consensus statement. BMJ. 1999; 319(7216): 1054. https://dx.doi.org/10.1136/bmj.319.7216.1054.
- Bullens L.M., Smith J.S., Truijens S.E.M., van der Hout-van der Jagt M.B., van Runnard Heimel P.J., Oei S.G. Maternal hemoglobin level and its relation to fetal distress, mode of delivery, and short-term neonatal outcome: a retrospective cohort study. J. Matern. Fetal Neonatal Med. 2019; 33(20): 3418-24.https://dx.doi.org/10.1080/14767058.2019.1573221.
- Hulsenboom A.D.J., Verdurmen K.M.J., Vullings R., van der Hout–van der Jagt M.B., Kwee A., van Laar J.O.E.H. et al. Relative versus absolute rises in T/QRS ratio by ST analysis of fetal electrocardiograms in labour: A case-control pilot study. PLoS One. 2019; 14(3): e0214357. https://dx.doi.org/10.1371/journal.pone.0214357.
- Patel K.P., Makadia M.G., Patel V.I., Nilayangode H.N., Nimbalkar S.M. Urinary uric acid/creatinine ratio - A marker for perinatal asphyxia. J. Clin. Diagn. Res. 2017; 11(1): SC08-SC10. https://dx.doi.org/10.7860/JCDR/2017/22697.9267.
- Herrera Ch., Silver R.M. Perinatal asphyxia from the obstetric standpoint diagnosis and interventions. Clin. Perinatol. 2016; 43(3): 423-38.https://dx.doi.org/10.1016/j.clp.2016.04.003.
- Amankwah N., Oskoui M., Garner R., Bancej Ch., Manuel D.G., Wall R. et al. Cerebral palsy in Canada, 2011–2031: results of a microsimulation modelling study of epidemiological and cost impacts. Health Promot. Chronic Dis. Prev. Can. 2020; 40(2): 25-37. https://dx.doi.org/10.24095/hpcdp.40.2.01.
- Hill M.G., Reed K.L., Brown R.N. Perinatal asphyxia from the obstetric standpoint. Semin. Fetal Neonatal Med. 2021; 26(4): 101259.https://dx.doi.org/10.1016/j.siny.2021.101259.
- Liljestrom L., Wikstrom A.-K., Agren J., Jonsson M. Antepartum risk factors for moderate to severe neonatal hypoxic ischemic encephalopathy: a Swedish national cohort study. Acta Obstet. Gynecol. Scand. 2018; 97(5): 615-23. https://dx.doi.org/10.1111/aogs.13316.
- Middleton P., Shepherd E., Morris J., Crowther C.A., Gomersall J.C. Induction of labour at or beyond 37 weeks' gestation. Cochrane Database Syst. Rev. 2020; 7(7): CD004945. https://dx.doi.org/10.1002/14651858.CD004945.pub5.
- Turner J.M., Mitchell M.D., Kumar S.S. The physiology of intrapartum fetal compromise at term. Am. J. Obstet. Gynecol. 2020; 222(1): 17-26.https://dx.doi.org/10.1016/j.ajog.2019.07.032.
Received 23.06.2022
Accepted 08.07.2022
About the Authors
Oleg R. Baev, Dr. Med. Sci., Professor, Maternity Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia; Professor at the Department of Obstetrics, Gynecology, Perinatology, and Reproductology, I.M. Sechenov First MSMU, Ministry of Health of Russia, +7(495)438-11-88, o_baev@oparina4.ru,117997, Russia, Moscow, Ac. Oparina str., 4.
Andrey M. Prikhodko, PhD, Physician at the Maternity Department, Teaching Assistant at the Department of Obstetrics and Gynecology, Researcher at the Innovative Technologies Department of Obstetrics Institute, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-30-47, a_prikhodko@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Marina M. Ziganshina, PhD, Leading Researcher at the Laboratory of Clinical Immunology, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-11-83, mmz@mail.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Aleksandra V. Evgrafova, Postgraduate Student, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-30-47, a_evgrafova@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Ekaterina V. Khomyakova, Postgraduate Student, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-30-47, e_khomyakova@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Authors' contributions: Baev O.R., Prikhodko A.M. – conception and design of the study; Prikhodko A.M.,
Khomyakova E.V., Evgrafova A.V. – data collection and analysis; Prikhodko A.M. – statistical analysis; Baev O.R. – manuscript drafting; Ziganshina M.M. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Baev O.R., Prikhodko A.M., Ziganshina M.M., Evgrafova A.V., Khomyakova E.V. Antenatal and intrapartum risk factors associated with fetal hypoxia in labor.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 8: 47-53 (in Russian)
https://dx.doi.org/10.18565/aig.2022.8.47-53



