Fibrin monomer and D-dimer in infertile women undergoing assisted reproductive technology
Aim. To investigate the levels of fibrin monomer and D-dimer as markers of activation of the coagulation system in infertile women undergoing assisted reproductive technology (ART). Materials and methods. The study included 68 patients, who were divided into a group of infertile women (group 1, n=36) and a control group (group 2, n=32). Fibrin monomer and D-dimer were analyzed using a latex-enhanced immunoturbidimetric assay. Results. Patients undergoing ART had increased procoagulant activity of blood, as evidenced by an increase in fibrin monomer and D-dimer concentrations (p<0.001). There was a relationship between the studied parameters and an increase in the level of β-hCG in group 1 (p<0.001); no association was found with body mass index, age, and infertility factor. Conclusion. ART is associated with an increase in the levels of fibrin monomer and D-dimer. The rise in the fibrin monomer level was more significant compared with D-dimer. Patients with endometriosis-associated infertility had a relative increase in the fibrin monomer level, which requires further investigation. An increase in the fibrin monomer level in patients with a confirmed pregnancy demonstrates an increase in the procoagulant activity of blood at early gestational ages.Godzoeva A.O., Zazerskaya I.E., Vlasov V.S., Vavilova T.V., Gorelova I.V., Kustarov V.N., Zhambalova T.V.
Keywords
Pregnancy is associated with physiological changes in hemostasis, including an imbalance between the procoagulant, anticoagulant, and fibrinolytic activity. Hypercoagulability is a result of increased plasma levels of fibrinogen, factors VII, VIII, IX, and X, and a decreased plasma level of protein S along with increased resistance to activation of protein C [1, 2]. These changes are aimed at maintaining placental perfusion and preventing pathological blood loss during childbirth. However, they also confer significantly increased thrombotic risk and risk of obstetric complications due to impaired fetoplacental circulation. Therefore, pregnancy presents a hematologic paradox.
Venous thromboembolism can manifest as pulmonary embolism or deep vein thrombosis, and complicate 0.5–2.2 cases per 1000 births. Pregnant women are at a 5–10 times higher risk of developing thromboembolic complications than non-pregnant women, and in the postpartum period, the risk increases 15–35 times [3–5]. Venous thromboembolism is a leading cause of maternal morbidity and mortality. Identifying women who are at the greatest risk of venous thromboembolism is essential for timely prevention.
Risk factors for venous thromboembolism in obstetric risk groups were first stratified in the third edition of the Royal College of Obstetricians and Gynecologists guidelines for the management of thrombosis and embolism during pregnancy and the puerperium [6]. Assisted reproductive technology (ART) is classified as potentially reversible factors. Existing literature is contradictory regarding the association of ovarian stimulation cycles with a high incidence of venous thromboembolism. Among patients with pregnancy following ART, the risk of thrombosis and embolism is 0.2% for singleton and 0.3% for multiple pregnancies, which, according to various authors, is 3–10 times higher than in the population of women with healthy pregnancy [7]. The mechanism underlying venous thromboembolism among this patient category is poorly understood. It is only known that the basis for the formation of a hypercoagulable state can be the effect of super-physiological doses of sex steroids, which induces a synthetic liver function with an increase in the levels of the coagulation factors [8, 9]. However, there is no answer to the question of whether ART is an independent risk factor or it masks the risks associated with ovarian hyperstimulation, multiple pregnancies, preeclampsia, or worsening of the course of somatic diseases. The issue of prevention of venous thromboembolism also remains controversial. According to current research, low molecular weight heparins in prophylactic doses in patients undergoing ART might improve implantation rates and the number of live births. Still, there is no evidence to support the standard addition of low molecular weight heparins to treatment regimens for women with infertility [10–12].
The paradox of the problem lies in the fact that despite the great importance of studying the hemostasis system, the boundaries between the norm and pathology for patients undergoing ART at various stages of gestation remain the subject of debate. The preceding warrants the search for reliable markers of hypercoagulability and the need for assessing their dynamics to categorize patients into risk groups for the timely pharmacological prevention of thromboembolic complications.
The present study aimed to investigate the levels of fibrin monomer and D-dimer as markers of activation of the coagulation system in infertile women undergoing ART.
Materials and methods
The study was conducted at the Department of ART of the Almazov Federal Medical Research Centre from September 2018 to February 2019. A total of 68 patients were included in the study after signing informed consent approved by the Ethics Committee. The study participants were divided into a group of infertile women (group 1, n = 36) (N97.0, N97.1, N97.4, N97.8 and N97.9 according to ICD10) and a control group (group 2, n = 32).
Criteria for inclusion of patients in group 1:
1. Diagnosed infertility, not amenable to therapy, or the likelihood of successful in vitro fertilization (IVF) is higher than with other methods;
2. Absence of contraindications for IVF (following the Order of the Ministry of Health of Russia dated 30.08.2012 No. 107n «On the Procedure for Using Assisted Reproductive Technologies, Contraindications and Restrictions on Their Use» dated August 23, 2012).
Criteria for excluding patients from group 1:
1. Malignant neoplasms of any location;
2. Chronic systemic and other somatic diseases that can affect the level of coagulation and pro-inflammatory markers.
Before IVF, all patients underwent clinical and laboratory examinations following the Order of the Ministry of Health of Russia dated 30.08.2012 No. 107n «On the Procedure for Using Assisted Reproductive Technologies, Contraindications and Restrictions on Their Use» dated August 23, 2012).
The control group included healthy, non-pregnant women of reproductive age.
Exclusion criteria for patients from group 2:
1. A history of bleeding or thromboembolic complications;
2. Use of combined hormonal contraceptives within the last three months;
3. Smoking, drug addiction.
In both groups, past medical history was taken (age at menarche, nature of the menstrual cycle, clinical course and methods of treatment of past pelvic organ diseases, family history of hereditary diseases, occupational hazards, lifestyle, somatic diseases, and past surgical interventions).
Patients underwent controlled ovarian hyperstimulation with GnRH-antagonist based ovarian stimulation protocols. Gonadotropins (urinary or recombinant) were administered from 2–3 days of the menstrual cycle under the control of pelvic ultrasound. The doses were tailored individually, ranging from 150 to 300 IU/day, depending on the patient's age, body mass index (BMI), ovarian reserve, and ovarian response to stimulation. GnRH-ant was administered according to a flexible protocol after the largest follicles reached the diameter of 14 mm daily until the ovulation triggering was performed. For ovulation triggering, chorionic gonadotropin (hCG) at a dose of 10,000 IU or 0.2 mg triptorelin (gonadotropin-releasing hormone (GnRH) agonist) were used when the three largest follicles reached the diameter of ≥17 mm. When the thickness of the endometrium was ≤8 mm on the day of trigger administration, 2 mg daily transdermal estradiol hemihydrate was administered. The indication for changing the trigger and using a GnRH-a was a high risk of developing ovarian hyperstimulation syndrome. Transvaginal ovarian puncture and oocyte sampling was performed under intravenous anesthesia and ultrasound guidance 36 hours after the ovulation trigger was administered.
Luteal phase support with 600 mg daily micronized progesterone was started from the first day after transvaginal follicle puncture.
The transfer of one or two embryos was done on the 5th day of cultivation with a sterile flexible catheter under sterile conditions and pelvic ultrasound guidance. Two weeks after the embryo transfer, pregnancy was diagnosed by measuring serum beta subunit of human chorionic gonadotropin (beta-HCG) concentration.
At this time, blood samples of patients in the study group were taken to investigate the hemostatic status.
In the control group, the sampling was performed on days 20–22 of the menstrual cycle.
Laboratory investigations
Fasting blood samples were drawn from the antecubital vein into 4.5 ml vacuum tubes with 3.8% sodium citrate. Sample preparation for the determination of fibrin monomer and D-dimer included the following steps:
1. Centrifugation of the tubes at 2000g for 15 minutes;
2. Collection of supernatants in plastic Eppendorf tubes;
3. Repeated centrifugation at 2000g for 15 minutes;
4. Collection of supernatants and storing it as aliquots in 2 plastic Eppendorf tubes;
5. If the analysis was postponed, aliquots were frozen at -40°С and kept no longer than one month.
Plasma samples were defrosted after being removed from the freezer in a thermostat at 37° C until complete thawing occurs. Fibrin monomer and D-dimer were analyzed using a latex-enhanced immunoturbidimetric assay on an automatic STA-Compact analyzer (Diagnostica Stago SAS, France) using the STA-Liatest FM and STA-Liatest D-Di kits (Diagnostica Stago SAS, France) with appropriate calibration and control materials.
Statistical analysis
Statistical analysis was performed using the STATISTICA 10.0 software (StatSoft, Inc.). Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD) and presented as M (SD); otherwise, the median (Me) and the quartiles Q1 and Q3 in the Me (Q1; Q3) format were reported. Qualitative variables were summarized as counts and percentages in the n (%) format. Some quantitative variables, including fibrin monomer and D-dimer concentrations, age, and BMI, were logarithmized to obtain approximately normally distributed data; the normality of their distributions was confirmed by the Kolmogorov – Smirnov test. The Mann – Whitney U-test was used to compare two groups by variables not meeting normality assumptions. The relationship between the studied hemostatic markers was assessed using correlation analysis with the calculation of the Pearson correlation coefficient. Statistical significance was assumed for p < 0.05.
Results
The mean age of patients was 34.7 (4.5) and 30.8 (5.4) years in group 1 and 2, respectively. The majority of patients undergoing IVF (n=23) were at the most favorable childbearing age from 26 to 35; 13 women were at late reproductive age (over 35 years) (63.9% and 36.1%, respectively). Clinical and anamnestic data of the patients included in the study are presented in Table 1.
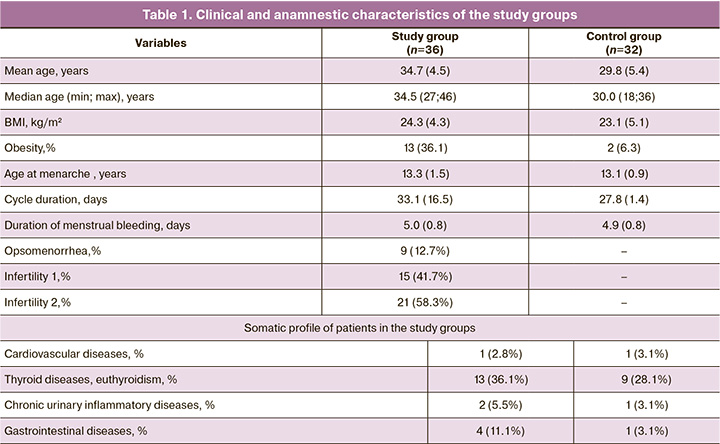
Among the patients of group 1, no significant differences were found between proportions of primary (n=30) and secondary (n=32) infertility (48.3% and 51.7%), respectively.
The duration of infertility in the examined women varied from 1 to 25 years and averaged 4.3 (2.7) years. Combined, tubal-peritoneal, male, anovulatory, extragenital endometriosis, and adenomyosis associated infertility were found in 29% (n=18), 24.2% (n=15), 19.4% (n=12), 17.7% (n=11), and 9.7% (n=6) of patients, respectively. The predominant etiological factor in anovulatory infertility was polycystic ovary syndrome.
Among women with secondary infertility, 26.8%, 16.1%, 17.9%, and 16.1% reported a history of an ectopic pregnancy, spontaneous miscarriage in the first trimester, missed miscarriage, and induced abortions, respectively. The full-term delivery rate in women with secondary infertility was 23.2%.
The gonadotropin starting dose among the patients included in the study was 150 IU (150; 200), the duration of ovarian stimulation was 9 days (7; 12), and the total dose of gonadotropins was 1400 IU (1050; 2000). Final oocyte maturation was triggered by hCG and GnRH in 26 (72.2%) and 10 (27.8%) women, respectively. In the post-transfer period, 30 patients (83%) were administered progesterone in combination with estradiol hemitartrate, and 6 patients received progesterone monotherapy. The total dose of estrogen and progesterone was 19 mg (9; 27) and 5400 mg (4200; 6000), respectively. Biochemical pregnancy was confirmed by a positive serum hCG test in 33.3% of patients.
Analysis of the hemostatic profile demonstrated statistically significant differences of fibrin monomer and D-dimer levels between the study groups (p <0.001). This observation indicates an increase in the blood procoagulant properties during the IVF (Fig. 1, 2). The median fibrin monomer level in both study groups did not exceed the reference range established by the manufacturer (2.14 μg/ml and 1.32 μg/ml in group 1 and 2, respectively). The median D-dimer was also higher in group 1 (0.6 μg/ml), exceeding the reference value in 61% of patients. In the control group, the median D-dimer was 0.32 μg/ml (Table 2). These variables showed normal distribution (tested by the Kolmogorov– Smirnov and Lilliefors tests).
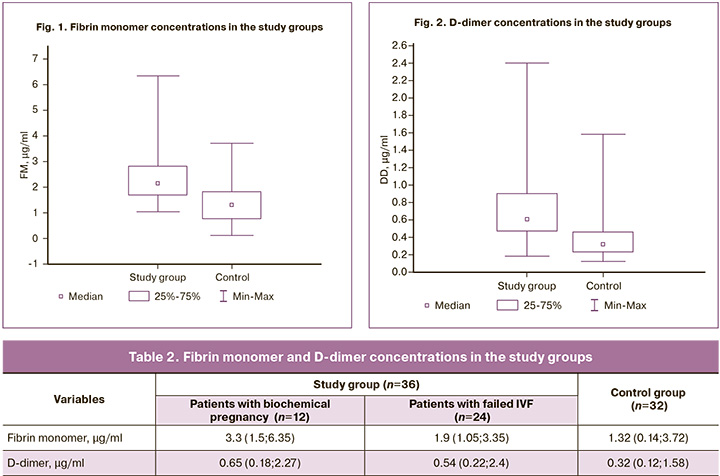
The formation of fibrin monomer and D-dimer is a physiologically related process in the chain of blood coagulation processes. There was a moderate, but statistically significant correlation between logs of fibrin monomer and D-dimer with the Pearson correlation coefficient r=0.37; p=0.03.
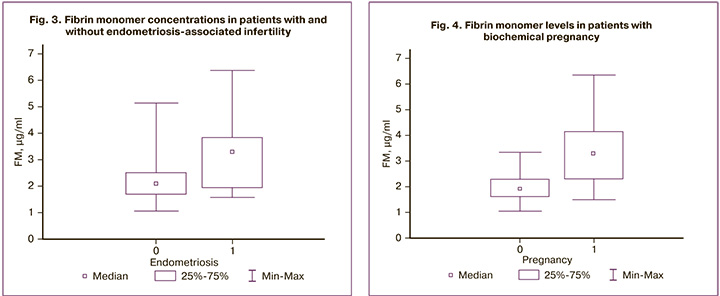
Considering the increase in the level of the studied parameters in the patients of group 1, we searched for possible clinical and anamnestic factors associated with the development of the hypercoagulable syndrome. Analysis of the relationship between blood hemostatic markers and BMI showed no differences among patients with and without obesity of different classes (p=0.7 and p=0.8). The factor of infertility, in particular anovulatory and tubo-peritoneal, also did not affect the level of both fibrin monomer and D-dimer (p=0.8 and p = 0.9, respectively). It should be noted that patients with endometriosis-associated infertility had higher levels of fibrin monomer than control subjects, but there were no statistically significant differences (p=0.09) (Fig. 3).
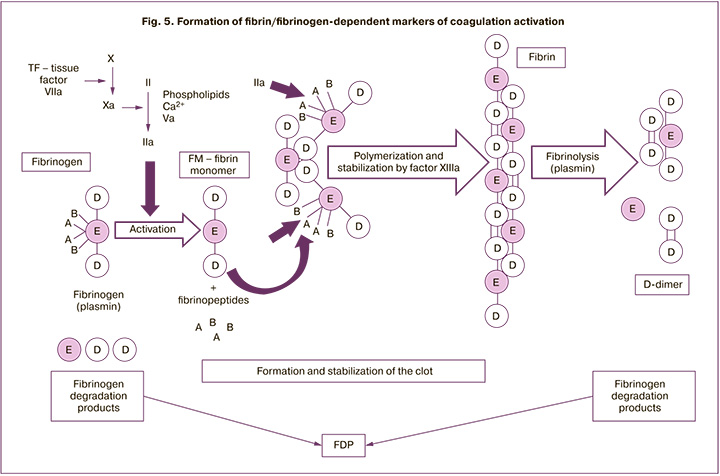
There was a statistically significant relationship between the level of fibrin monomer and an increase in the serum level of β-subunit of hCG in group 1 (Mann–Whitney U-test, p <0.001; Fig. 4). There were no differences in the D-dimer levels (p=0.96).
Discussion
Despite the plethora of published research, the time of onset and activity of hypercoagulable syndrome as a risk factor for venous thromboembolism in patients in ART is still under debate.
The risk of thromboembolic complications women in the first trimester among women undergoing ART is 0.17%, compared to 0.02% among women with a healthy pregnancy. These data are confirmed by information obtained by a group of researchers from the French Public Health Agency. In contrast, the results reported by Grandone E. et al. indicate a high risk of pulmonary embolism among patients with failed IVF, compared with patients who achieve a pregnancy [13–15].
Factors associated with to long-term activation of hemostasis and increased prothrombotic potential include high levels of estrogen and FSH due to an increase in the level of procoagulants such as von Willebrand factor, factor V, VIII, and fibrinogen, cooccurring with an increase the levels of proteins C and S, and antithrombin. According to Westerlund E. et al., high doses of FSH lead to 38 times higher endogenous thrombin potential compared with its minimal doses [8]. At the same time, there was an increase in markers of hemostasis activation, including fragments of prothrombin F1+2 and D-dimer. Besides, estradiol can directly reduce the peripheral arterial and venous tone, slowing down blood flow. These changes promote the release of tissue factor from circulating monocytes, activating the coagulation cascade without apparent damage to the vessel walls.
It should be noted that the clinical picture of venous thromboembolism during ovarian stimulation rarely develops before the administration of hCG. Somigliana E. et al. and Nelson S. reported that administration of hCG was associated with an increase in the levels of fibrinogen, factors II, V, VII, VIII, and IX. Following the activation of procoagulant mechanisms, fibrinolytic processes are triggered with a 2-day delay peaking after eight days [16–18].
The results of our study do not contradict the data indicating the activation of the hemostatic system during the IVF. A statistically significant increase in the concentrations of the studied indicators was established. Among patients in group 1, there was a more substantial increase in the fibrin monomer level than that of D-dimer. The appearance of D-dimer in the blood indicates the occurrence of intravascular fibrin formation. Besides, extravascular fibrin deposits can also be a source of D-dimer in plasma. The method is characterized by high sensitivity but low specificity. Therefore, it has a high negative diagnostic value; that is, it allows a reliable exclusion of venous thromboembolism in patients with normal D-dimer levels. However, an increased level of D-dimer is nonspecific and may be due to sepsis, malignant neoplasms, inflammation, surgery, and some systemic diseases. Increased levels of D-dimer are also known to be associated with pregnancy and gestational age [19–21].
Fibrin monomer is a biomarker of fibrin formation. The fibrin monomer level does not depend on the extravasate fibrin deposits. Under the proteolytic action of the critical clotting enzyme, thrombin, fibrinopeptide A is first cleaved from the fibrinogen molecule, resulting in the formation of a soluble fibrin monomer. Further, spontaneous polymerization is initiated by the end-to-side junction of the end part of one fibrin monomer with the central part of the other fibrin monomer at the cleavage site of fibrinopeptide A to form a two molecules wide linear polymer. At the next stage, fibrinopeptide B is cleaved, which provides the possibility of lateral growth of the polymer and the formation of a soluble fibrin polymer. The final stage of clot stabilization occurs under the influence of factor XIIIa, resulting in the formation of an insoluble fibrin clot (Fig. 5) [22].
What is the difference between the studied indicators? D-dimer is a fibrin degradation product that is formed immediately after thrombin-generated fibrin clots are degraded by plasmin. By measuring D-dimer concentration, we determine the products of fibrin clots degradation. That is, D-dimer may be considered as a post-thrombotic marker. Fibrin monomer can be used as a prethrombotic marker [23]. Besides, contrary to the primary markers of the hemostasis activation, which increase in pregnant women (fibrinogen, factor VIII activity, von Willebrand factor antigen, and D-dimer), the median fibrin monomer level during the whole pregnancy does not undergo significant changes and does not exceed general population level. According to other sources, it rises by the end of the first trimester in 22–28% of cases and does not undergo further significant changes. However, fibrin monomer is significantly increased in venous thromboembolism, suggesting the possibility of considering it as an early and specific marker of thrombotic episodes, including during pregnancy [22, 24].
Despite all of the above, thrombosis does not occur in all patients, which may indicate the effect of additional factors, such as hyperinsulinemia, obesity, metabolic disorders, and others. The literature describes a direct relationship between D-dimer levels and age, gestational age, BMI, and smoking. High levels of fibrin monomer have also been associated with overweight, as well as with chronic hypertension and cocaine use [13, 22, 25]. In this study, fibrin monomer and D-dimer were not statistically significantly associated with BMI, age, somatic diseases, and tubo-peritoneal and anovulatory factors of infertility. However, the participants of the study group did not have a premorbid background. BMI did not exceed 35 kg/m2, and cardiovascular diseases were presented in one case as corrected congenital heart disease without hemodynamic abnormalities. Patients with thyroid diseases had no thyroid dysfunction or were medically compensated. Therefore, this study did not include women with known high-risk factors.
Of note is the increase in fibrin monomer among patients with endometriosis-associated infertility, though not statistically significant, which can be explained by the small number of patients in this diagnostic category and requires additional study. The level of fibrin monomer in patients with the onset of pregnancy was 1.7 times higher than in patients who failed to achieve pregnancy and 2.4 times higher than in the control group. The increase in the fibrin monomer concentration in patients with biochemically confirmed pregnancy is consistent with reports of some other authors suggesting that the rise in the blood procoagulant properties begins very early in gestational age [25–27].
The question of the need for pharmacologic prevention of venous thromboembolism should be made on a case-by-case basis according to a combination of risk factors, since ART is not included in significant risk factors. To obtain evidence of the significance of pathophysiological disorders, sufficiently powered longitudinal studies are necessary with the definition of fibrin monomer in dynamics, with the use of various protocols and IVF regimens, including in comparison with a natural pregnancy.
Conclusion
ART is associated with a significant increase in the levels of fibrin monomer and D-dimer, which may suggest the development of a hypercoagulable state. The rise in the fibrin monomer level was more significant compared with D-dimer and may be considered as an earlier and specific marker of fibrin formation. No association was found between the studied parameters and BMI, age, and infertility factors. However, patients with endometriosis-associated infertility had a relative increase in the fibrin monomer level, which requires further investigation of the feasibility of pharmacological prevention of venous thromboembolism. An increase in the fibrin monomer level in patients with a confirmed pregnancy demonstrates an increase in the procoagulant activity of blood at early gestational ages.
References
- Hellgren M. Hemostasis during normal pregnancy and puerperium. Semin. Thromb. Hemost. 2003; 29(2): 125-30. https://dx.doi.org/10.1055/s-2003-38897.
- Refaai M., Riley P., Mardovina T., Bell P. The clinical significance of fibrin monomers. Thromb. Haemost. 2018; 118(11): 1856-66. https://dx.doi.org/10.1055/s-0038-1673684.
- Bates S., Middeldorp S., Rodger M., James A.H., Greer I. Guidance for the treatment and prevention of obstetric-associated venous thromboembolism. J. Thromb. Thrombolysis. 2016; 41(1): 92-128. https://dx.doi.org/10.1007/s11239-015-1309-0.
- Bain E., Wilson A., Tooher R., Gates S., Davis L.J., Middleton P. Prophylaxis for venous thromboembolic disease in pregnancy and the early postnatal period. Cochrane Database Syst. Rev. 2014; (2): CD001689. https://dx.doi.org/10.1002/14651858.cd001689.pub3.
- Шмаков Р.Г., Каримова Г.Н. Профилактика и лечение венозных тромбоэмболических осложнений во время беременности и после родов. Медицинский оппонент. 2019; 3: 58-62. [Shmakov R.G., Karimova G.N. Prevention and treatment of venous thromboembolic complications during pregnancy and after childbirth. Meditsinskiy opponent/Medical opponent. 2019; 3 (7): 58-62. (in Russian)].
- Thrombosis and embolism during pregnancy and the puerperium, reducing the risk. Green-top Guideline No. 37a. Royal College of Obstetricians and Gynaecologists; 2015.
- Sennström M., Rova K., Hellgren M., Hjertberg R., Nord E., Thurn L., Lindqvist P.G. Thromboembolism and in vitro fertilization - a systematic review. Acta Obstet. Gynecol. Scand. 2017; 96(9): 1045-52. https://dx.doi.org/10.1111/aogs.13147.
- Westerlund E., Henriksson P., Wallén H., Hovatta O., Wallberg K.R., Antovic A. Detection of a procoagulable state during controlled ovarian hyperstimulation for in vitro fertilization with global assays of haemostasis. Thromb. Res. 2012; 130(4): 649-53. https://dx.doi.org/10.1016/j.thromres.2011.11.024.
- Mahajan N., Naidu P., Gupta S., Rani K. Deep venous thrombosis in a patient undergoing In-vitrofertilization with oocyte donation. J. Hum. Reprod. Sci. 2015; 8(3): 182. https://dx.doi.org/10.4103/0974-1208.
- Grandone E., Villani M., Dentali F., Tiscia G.L., Colaizzo D., Cappucci F. et al. Low-molecular–weight heparin in pregnancies after ART - A retrospective study. Thromb. Res. 2014; 134(2): 336-9. https://dx.doi.org/10.1016/j.thromres.2014.06.004.
- Bates S. Anticoagulation and in vitro fertilization and ovarian stimulation. Hematology. 2014; 2014(1): 379-86. https://dx.doi.org/10.1182/asheducation-2014.1.379.
- Yefet E., Weiss A. Is anti-thrombotic prophylaxis warranted in fertility and in-vitro fertilization treatments? Harefuah. 2016; 155(7): 398-402.
- Grandone E., Di Micco P., Villani M., Colaizzo D.., Fernández-Capitán C., Del Toro J. et al. Venous thromboembolism in women undergoing assisted reproductive technologies: Data from the RIETE Registry. Thromb. Haemost. 2018; 118(11): 1962-8. https://dx.doi.org/10.1055/s-0038-1673402.
- Filipovic-Pierucci A., Gabet A., Deneux-Tharaux C., Plu-Bureau G., Olié V.Arterial and venous complications after fertility treatment: A French nationwide cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019; 237: 57-63. https://dx.doi.org/10.1016/j.ejogrb.2019.02.034.
- Соболева В.В., Трифонова Н.С., Александров Л.С., Ищенко А.И., Борисова Н.И., Жукова Э.В., Никонов А.П., Беришвили М.В., Жолобова М.Н.Состояние системы гемостаза во время беременности, наступившей с использованием вспомогательных репродуктивных технологий. Вопросы гинекологии, акушерства и перинатологии. 2017; 16(4): 64-70. [V.V. Soboleva, N.S. Trifonova, L.S. Aleksandrov, A.I. Ishchenko, N.I. Borisova, E.V. Zhukova, A.P. Nikonov, M.V. Berishvili, M.N. Zholobova. The state of the haemostasis system during pregnancy occurring with the use of assisted reproductive technologies. Gynecology, Obstetrics and Perinatology. 2017; 16(4): 64-70. (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2017-4-64-70.16.
- Nelson S. Venous thrombosis during assisted reproduction: Novel risk reduction strategies. Thromb. Res. 2013; 13(Suppl.1): S1-3. https://dx.doi.org/10.1016/s0049-3848(13)00023-6.
- Somigliana E., Peccatori F., Filippi F., Martinelli F., Raspagliesi F., Martinelli I. Risk of thrombosis in women with malignancies undergoing ovarian stimulation for fertility preservation. Hum. Reprod. Update. 2014; 20(6): 944-51. https://dx.doi.org/10.1093/humupd/dmu035.
- Бицадзе В.О., Акиньшина С.В., Макацария А.Д., Андреева М.Д. Вспомогательные репродуктивные технологии и ятрогенные тромботические осложнения. Вопросы гинекологии, акушерства и перинатологии. 2014; 13(1): 49-59. [Bicadze V.O., Akin'shina S.V., Makacariya A.D., Andreeva M.D. Assisted reproductive technologies and iatrogenic thrombotic complications. Questions of gynecology, obstetrics and perinatology. 2014; 13(1): 49-59. (in Russian)].
- Момот А.П., Молчанова И.В., Цывкина Л.П. Изменения системы гемостаза в цикле ЭКО и их влияние на эффективность процедуры. Бюллетень медицинской науки. 2017; 4: 77-81. [Momot A.P., Molchanova I.V., Tsypkina L.P. Alterations of the hemostatic system in the IVF cycle and their influence on the procedure efficiency. Bulletin of Medical Science. 2017;(4):77-81.(in Russian)].
- Момот А.П., Николаева М.Г., Сердюк Г.В., Елыкомов В.А., Мамаев А.Н., Романов В.В., Фадеева Н.И., Кудинова И.Ю., Белозеров Д.Е., Трухина Д.А., Максимова Н.В., Вахлова Ж.И. Оценка системы состояния гемостаза при физиологически протекающей беременности (методические рекомендации). Российский вестник акушера-гинеколога. 2018; 18(3-2): 2-37. [Momot A.P., Nikolaeva M.G., Serdyuk G.V., Ely'komov V.A. et al. Assessment of the hemostasis system in physiologically occurring pregnancy (guidelines). Russian Bulletin of obstetrician-gynecologist. 2018; 18(3-2): 2-37. (in Russian)].
- Мирашвили М.И., Зайнулина М.С., Коган И.Ю., Гзгзян А.М., Рзаева Р.Н. Ведение женщин с тромбофилией на этапе подготовки к экстракорпоральному оплодотворению. Журнал акушерства и женских болезней. 2012; 61(5): 60-7. [Mirashvili M.I., Zajnulina M.S., Kogan I.YU., Gzgzyan A.M., Rzaeva R.N. The management of women with thrombophilia at the stage of preparation for in vitro fertilization. Journal of obstetrics and women's diseases. 2012; 61(5): 60-7. (in Russian)].
- Grossman K., Arya R., Peixoto A., Akolekar R., Staboulidou I., Nicolaides K. Maternal and pregnancy characteristics affect plasma fibrin monomer complexes and D-dimer reference ranges for venous thromboembolism in pregnancy. Am. J. Obstet. Gynecol. 2016; 215(4): 466. e1-466. e8. https://dx.doi.org/10.1016/j.ajog.2016.05.013.
- Memon A., Sundquist K., PirouziFard M., Elf J.L., Strandberg K., Svensson P.J. et al. Identification of novel diagnostic biomarkers for deep venous thrombosis. Br. J. Haematol. 2018; 181(3): 378-85. https://dx.doi.org/10.1111/bjh.15206.
- Воробьева Н.А., Звездина Ю.М., Власов В.С. Определение фибрин-мономера в профилактике и диагностике тромботических осложнений при беременности. Тромбоз, гемостаз и реология. 2016; 3: 41-7. [Vorobyeva N.A., Zvezdina N.A., Vlasov V.S. Determination of fibrin monomer in prophylaxis and diagnostics of thrombotic complications at pregnancy. Tromboz, gemostaz i reologiya/ Thrombosis, hemostasis and rheology. 2016; 3: 41-7.(in Russian)].
- Hansen A., Kesmodel U., Juul S., Hvas A. Increased venous thrombosis incidence in pregnancies after in vitro fertilization. Hum. Reprod. 2014; 29(3): 611-7. https://dx.doi.org/10.1093/humrep/det458.
- Rova K., Passmark H., Lindqvist P. Venous thromboembolism in relation to in vitro fertilization: an approach to determining the incidence and increase in risk in successful cycles. Fertil. Steril. 2012; 97(1): 95-100. https://dx.doi.org/10.1016/j.fertnstert.2011.10.038.
- Henriksson P., Westerlund E., Wallén H., Brandt L., Hovatta O., Ekbom A. Incidence of pulmonary and venous thromboembolism in pregnancies after in vitro fertilization. Obstetric Anesthesia Digest. 2014; 34(1): 22-3. https://dx.doi.org/10.1097/01.aoa.0000443362.78919.bc.
Received 15.05.2020
Accepted 01.06.2020
About the Authors
Alina O. Godzoeva, Ph.D. Student at the Department of Obstetrics and Gynecology, V.A. Almazov NMRC of Minzdrav of Russia. Tel.: +7(988)870-86-76.Е-mail: godzoevaalina@mail.ru. 197341, Russia, Saint Petersburg, Akkuratova str., 2b.
Irina E. Zazerskaya, M.D., Dr.Med.Sci., Head of the Department of Obstetrics and Gynecology, V.A. Almazov NMRC of Minzdrav of Russia. Tel.: +7(921)948-83-40.
Е-mail: zazera@mail.ru. 197341, Russia, Saint Petersburg, Akkuratova str., 2b.
Vladimir S. Vlasov, Specialist at the Department of Laboratory Medicine and Genetics, V.A. Almazov NMRC of Minzdrav of Russia. Е-mail: vlasov1989vladimir@gmail.com. 197341, Russia, Saint Petersburg, Akkuratova str., 2b.
Tatyana V. Vavilova, M.D., Dr.Med.Sci., Head of Department of Laboratory Medicine and Genetics, V. A. Almazov NMRC of Minzdrav of Russia; Chief Specialist in Clinical Laboratory Diagnostics of the Ministry of Health of the Russian Federation.
Tel.: +7(921)948-83-40. Е-mail: vtv.lab.spb@gmail.com. 197341, Russia, Saint Petersburg, Akkuratova str., 2b.
Inga V. Gorelova, M.D., Ph.D., Head of the Department of Reproductive technologies, V.A. Almazov NMRC of Minzdrav of Russia. Е-mail: ivmosyagina@gmail.com.
197341, Russia, Saint Petersburg, Akkuratova str., 2b.
Vitaly N. Kustarov, M.D., Ph.D., Dr.Med.Sci., Professor at the Department of Obstetrics and Gynecology, V. A. Almazov NMRC of Minzdrav of Russia.
Е-mail: prof.kustarov@gmail.com. 197341, Russia, Saint Petersburg, Akkuratova str., 2b.
Tuyana V. Zhambalova, Ph.D. Student at the Department of Obstetrics and Gynecology, V.A. Almazov NMRC of Minzdrav of Russia. Е-mail: 2yana1@rambler.ru.
197341, Russia, Saint Petersburg, Akkuratova str., 2b.
For citation: Godzoeva A.O., Zazerskaya I.E., Vlasov V.S., Vavilova T.V., Gorelova I.V., Kustarov V.N., Zhambalova T.V. Fibrin monomer and D-dimer in infertile women undergoing assisted reproductive technology.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2020; 9: 73-81 (in Russian)
https://dx.doi.org/10.18565/aig.2020.9.73-81



