Risk factors for gestational diabetes mellitus
Objective. To identify significant risk factors for developing gestational diabetes mellitus (GDM) in women with hypothalamic dysfunction (HD) and metabolic disorders of puberty for predicting adverse pregnancy outcomes.Zhukоvets I.V., Levakov S.A., Leshchenko O.Ya.
Materials and methodsю This prospective study of 170 female adolescents with HD in puberty was conducted from 2000 to 2014. Seventy-two pregnant women with a history of HD were examined for socioeconomic and biomedical risk factors of GDM. Relative risk (RR) and 95% confidence interval (95%CI) were used to estimate the effects of specific risk factors.
Results The predictors with the best accuracy for predicting the probability of developing GDM were diabetes mellitus among family members (OR = 5.6) and induced pregnancy (OR = 1.4).
Conclusions. Women with a history of HD and metabolic disorders, who plan to use assisted reproductive technologies to treat infertility, should be considered a high‐risk group for GDM.
Keywords
Gestational diabetes mellitus (GDM) is the most common metabolic disorder in pregnancy, affecting up to 20% in the general population [1-3]. Globally, the prevalence of GDM varies between 1 and 22% depending on the genetic background of the studied population, the diagnostic criteria, social and environmental factors [1, 4].
The annual increase in the number of pregnant women with GDM is associated both with the improvement of diagnostics and increasing GDM incidence [1, 5, 6]. About 80% of women with GDM have a pregnancy and childbirth complications [1, 2, 6]. According to international population-based studies, women with GDM have an 8-fold higher risk of preeclampsia, abnormal labor, and newborn macrosomia, and 50% of them later develop type 2 diabetes.
Several risk factors for GDM have been identified [1]. Maternal pre-gravid obesity is most extensively studied modifiable predictor of GDM [5, 7]. Despite this, there is an ongoing search to develop additional markers for GDM risk prediction [3, 7, 8]. Recently, visceral fat mass emerged as the most important variable for predicting GDM [3]. However, the effect of metabolic disturbances of puberty on the development of pregnancy complications has not been sufficiently explored.
An estimated 25% of adolescent girls develop hypothalamic dysfunction (HD) during puberty [9–11]; 83% of them are overweight and obese [12, 13]. The active identification of risk factors for GDM in women with a history of HD during puberty will provide an opportunity not only to predict this complication during pregnancy but also to take timely preventive measures.
This study aimed to identify significant risk factors for developing GDM in women with a history of HD and metabolic disorders during puberty for predicting adverse pregnancy outcomes.
Material and methods
This prospective study comprised 170 female adolescents with HD during puberty. Of them, 66 (38.8%) and 104 (61.2%) girls were overweight and obese, respectively.
The study was conducted from 2000 to 2014, the mean follow-up was 4.7(1,7) years. Out of 170 adolescent girls with HD in the puberty, 86 women of reproductive age were enrolled in the study according to the inclusion and exclusion criteria. Seventy-two pregnant women with a history of HD were examined for the presence of GDM risk factors (Fig. 1).
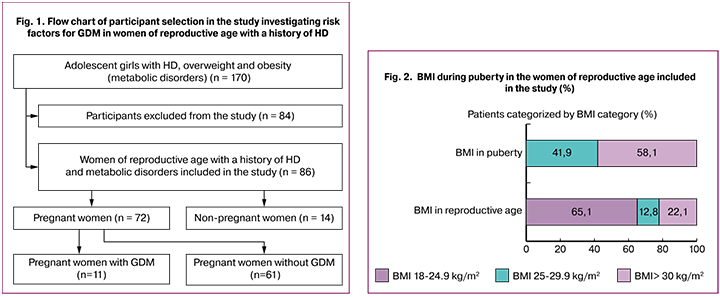
The inclusion criteria for the study participants of reproductive age were as follows: age over 18 years, HD (E 23.3) manifested by neuroendocrine (overweight or obesity) and neurotrophic (white and pink stretch marks) disorders during puberty. Exclusion criteria from the study were tubal infertility (N 97.1) and women of reproductive age who did not plan to get pregnant. Eighty-four women were excluded from the study, 22 of them due to the change of residence, 13 had tubal infertility (N 97.1), 26 were under 18 years of age at the time of enrollment in the study, and 23 did not plan a pregnancy.
The study was conducted using a puberty questionnaire for girls, based on the questionnaire developed in Norway by the University of Bergen (The Health Behavior in School-aged Children). The study was conducted in accordance with the World Medical Association’s Declaration of Helsinki, 2008 and the Rules of Clinical Practice in the Russian Federation, approved by order of the Ministry of Health of the Russian Federation dated June 19, 2003 No. 266.
BMI was calculated using the G. Brey (1978) equation; in adolescent girls, calculations were made based on age and the corresponding BMI standard deviations. According to the World Health Organization recommendations, obesity in adolescents was defined as a BMI equal to or more than 2.0 SDs BMI and overweight – from +1.0 to +2.0 SDs.
Fasting serum glucose was measured all in pregnant women at 7–8 weeks’ gestation by the enzymatic glucose oxidase method with orthotolidine oxidation. The oral glucose tolerance test included determination of serum glucose before and 120 minutes after a 75-g oral glucose load. Threshold values for the diagnosis of GDM were fasting serum glucose levels > 5.1 <7.0 mmol/l or > 8.5 mmol/l two hours after glucose load [1].
Statistical analysis was performed using Microsoft Excel and the Statistica 6.0 statistical software package in compliance with the general recommendations for medical and biological research. Quantitative variables were expressed as means (M) and standard deviation (SD). Risks were estimated by using 2×2 contingency tables. The impact of a specific factor was assessed by relative risk (RR) with 95% confidence interval (CI). Multivariate analysis was performed using logistic regression models. Logistic regression was used to estimate the odds ratios (OR) with 95% CI for factors that were considered potentially significant relative to the tested hypotheses and the contribution of independent variables to the model.
Results and discussion
The 6.1 (0.46) years follow-up of the adolescent girls with HD included in the study allowed an estimate of reproductive function in 86 women of reproductive age with a history of HD and metabolic disorders. The mean age of women was 21.95 (0.2) years, BMI 24.7 (3.9) kg/m2 (36 (41.9%) and 50 (58.1 %) women were overweight and obese, respectively).
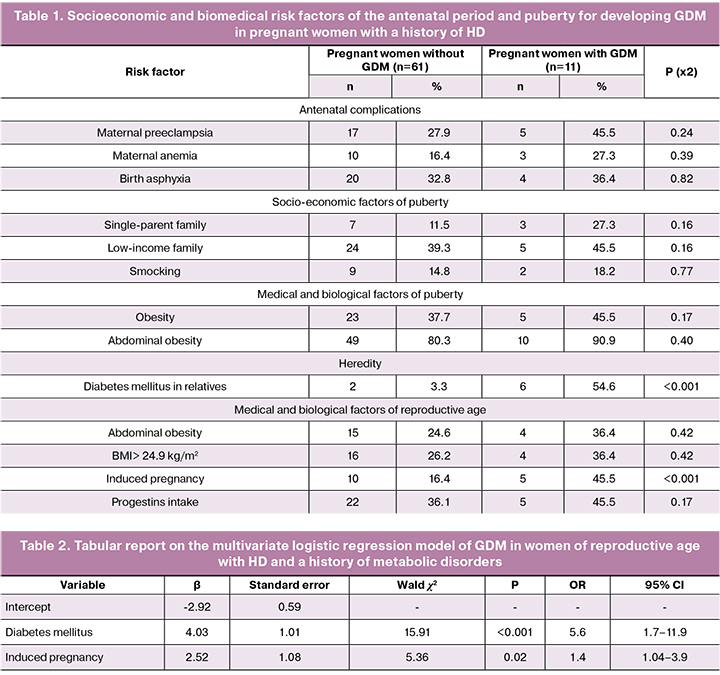
 Sixty-three (73.3%) of the 86 women of reproductive age with a history of HD and metabolic abnormalities had a lower BMI than that in puberty; thereof 56 (88.9%) had normal weight and 7 (11.1%) were overweight. In 15 (17.4%) women of reproductive age, BMI did not change as compared with BMI during puberty; 4 (26.7%) and 11 (73.3%) of them were overweight and obese, respectively. An increase in BMI> 30.0 kg/m2 in reproductive age in comparison with the puberty was observed in 8 (9.3%) women. Therefore, in women of reproductive age with a history of HD and metabolic disorders during puberty, normal body weight, overweight and obesity were found in 65.1% (95% CI: 54.6–74.35), 12.8% (95% CI : 7.29–21.5), and 22.1% (95% CI: 14.62–31.94) of women, respectively(Fig. 2).
Sixty-three (73.3%) of the 86 women of reproductive age with a history of HD and metabolic abnormalities had a lower BMI than that in puberty; thereof 56 (88.9%) had normal weight and 7 (11.1%) were overweight. In 15 (17.4%) women of reproductive age, BMI did not change as compared with BMI during puberty; 4 (26.7%) and 11 (73.3%) of them were overweight and obese, respectively. An increase in BMI> 30.0 kg/m2 in reproductive age in comparison with the puberty was observed in 8 (9.3%) women. Therefore, in women of reproductive age with a history of HD and metabolic disorders during puberty, normal body weight, overweight and obesity were found in 65.1% (95% CI: 54.6–74.35), 12.8% (95% CI : 7.29–21.5), and 22.1% (95% CI: 14.62–31.94) of women, respectively(Fig. 2).
Forty-six (53.5%, 95% CI: 43.03–63.66) of the 86 women of reproductive age with a history of HD and metabolic abnormalities used to be fertile, while 26 (30.2%, 95% CI: 21.54 –40.61) and 14 (16.3%, 95% CI: 9.95–25.49) had primary and secondary infertility, respectively. Pregnancy occurred in 64 (74.4%) of 86 women after a comprehensive examination and treatment of infertility, including ovulation stimulation and assisted reproductive technology (ART) in every fourth of them (n = 16; 25.0%).
During the first trimester, 12 (16.7%), 8 (11.1%), 8 (11.1%), and 6 (8.3%) pregnant women with a history of HD experienced a threatened miscarriage (O20.0), spontaneous miscarriage (O03), and anemia and vomiting of pregnancy, respectively. In the second and third trimesters (Fig. 3), 6 (9.4%), 11 (17.2%), and 11 (17.2%) women had threatened preterm labor (O60.0), intrauterine hypoxia (P20) and anemia of pregnancy, respectively. In the third trimester, a significant proportion of women (n = 13; 20.3%) had preeclampsia (O14) and edema due to proteinuria (O12.2) (n = 11; 17.2%).
Every fifth pregnant woman (n = 11, 17.2%) was diagnosed with GDM (О24.4). All pregnant women with a history of HD were examined for the presence of socioeconomic and biomedical risk factors of the antenatal period and puberty for developing GDM (Table 1).
An increased risk of developing GDM (RR>1) was associated with an adolescent living in a single-parent and low-income family (RR - 2.3; 95% CI: 0.74–7.31), (RR - 1.2; 95% CI: 0.41–3.67), smoking (RR - 1.2; 95% CI: 0.31–4.95), obesity (RR - 1.32; 95% CI: 0.42–4.12), including abdominal obesity (RR - 2.2; 95% CI: 0.31–15.7), pregnancy in the mother complicated by pre-eclampsia (OR - 1.9; 95% CI: 0.64 –5.5), anemia (RR - 1.7; 95% CI: 0.52–5.5), birth asphyxia (OR - 1.14; 95% CI: 0.37–3.52). A significant biomedical risk factor for developing GDM was family history of diabetes mellitus (RR, 9.6; 95% CI: 3.7–18.3).
Also, an increased risk (RR>1) of developing GDM among the women of reproductive age with a history of HD was associated with waist circumference> 80 cm (RR 1.6; 95% CI: 0.52–4.84) and BMI> 24.9 kg m2 (RR - 1.5; 95% CI: 0.49–4.5). Besides, we evaluated the role of fertility treatments in the development of GDM. A significant risk factor for GDM was induced pregnancy (ovulation stimulation/ART) (RR 3.16; 95% CI: 1.11–8.97). Vaginal administration of gestagens during pregnancy increased the risk of GDM (RR - 1.4; 95% CI: 0.47–4.1) but was not significant.
The association between gestagen therapy during pregnancy and the risk of developing GDM has long been a subject of debate in the medical literature. In our study, taking gestagens during pregnancy did not significantly increase the risk of GDM, which was consistent with previous retrospective studies conducted in Haifa (Israel) and Vienna, (Austria). These studies have shown that daily use of vaginal progesterone was not associated with an increased risk of GDM. [14, 15]. However, the route of progesterone administration was reported to be a risk factor for GDM. Administration of injectable progesterone during the first 10-12 weeks of pregnancy was associated with an approximately twofold increased risk of developing GDM (OR 2.28, 95% CI 1.27-4.09) compared to vaginal progesterone [8].
Based on risk factors of GDM identified in women with a history of HD, a multiple logistic regression model was built. The dependent variable was the presence of GDM in women with a history of HD; all independent variables in the model were binary. The multiple logistic regression model identified a set of predictors (Table 2) that best predicted the likelihood of GDM in women with a history of HD (χ2 = 22.11, p <0.001): a family history of diabetes mellitus (OS = 5.6; 95% CI: 1.7–11.9) and induced pregnancy (OR = 1.4; 95% CI: 1.04–3.9). Correctly classified correlation in the group was 91.6%.
Therefore, our findings emphasize the importance of genetic predisposition, which is a well-known risk factor for GDM [1, 6, 13]. In recent years, many studies confirmed the doubtless role of genetic abnormalities in the pathogenesis of diabetes mellitus [16]. Genetic mechanisms either contribute to the development or reduce the possibility of compensating for various disorders [16, 17], including GDM. Current literature is lacking studies addressing the risk of GDM associated with ovulation stimulation, including in ART programs. M. Ashrafi et al. (2014) reported a twofold increase in the risk of GDM in patients after ART [2]. The European Association of Perinatal Medicine in 2014 published data from a retrospective study of women who became pregnant after ART (n = 1292) or stimulated ovulation (n = 1988), which showed that pregnancies conceiving following ovulation induction and ART are at an increased risk for GDM (RR - 1.77) [18]. A growing body of literature suggests that ART not only increase the risk of developing GDM in a pregnant woman but shows that individuals conceived by in vitro fertilization may be at increased risk of cardio-metabolic disorders [19], which implies the need for more research in this area.
Conclusion
In this prospective study, we showed that in women with a history of HD and metabolic disorders during puberty, a family history of diabetes mellitus (OR = 5.6) and induced pregnancy (OR = 1.4) are the best predictors of developing GDM. Women with a history of HD, who plan to use ART and ovulation stimulation to treat infertility, should be considered a high‐risk group for GDM. The growing number of women, who became pregnant after ART, and the findings of this study suggest that patients should be aware of potential risks associated with pregnancy, including the risk of GDM.
References
- Сухих Г.Т., Краснопольский В.И., Рунихина Н.К. Переход на новый уровень ведения гипертензивных и метаболических осложнений при беременности: современные критерии диагностики гестационного сахарного диабета. Акушерство и гинекология. 2013; 3: 5-9. [Sukhikh G.T., Krasnopol’skij V.I., Runihina N.K. Transition to a new level of management of hypertensive and metabolic complications during pregnancy: current criteria for the diagnosis of gestational diabetes. Obstetrics and gynecology. 2013; 3: 5-9. (in Russian)]
- Ashrafi M., Gosili R., Hosseini R., Arabipoor A., Ahmadi J., Chehrazi M. Risk of gestational diabetes mellitus in patients undergoing assisted reproductive techniques. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014; 176: 149-52.
- Balani J., Hyer S.L., Shehata H., Mohareb F. Visceral fat mass as a novel risk factor for predicting gestational diabetes in obese pregnant women. Obstet. Med. 2018; 11(3): 121-5.
- Domanski G., Lange A.E., Ittermann T., Allenberg H., Spoo R.A., Zygmunt M. et al. Evaluation of neonatal and maternal morbidity in mothers with gestational diabetes: a population-based study. BMC Pregnancy Childbirth. 2018; 18(1): 367.
- Peaceman A.M., Clifton R.G., Phelan S. Lifestyle interventions limit gestational weight gain in women with overweight or obesity: LIFE-moms prospective meta-analysis. Obesity (Silver Spring). 2018; 26(9): 1396-404.
- Pastorino S., Bishop T., Crozier S.R., Granström C., Kordas K., Küpers L.K. et al. Associations between maternal physical activity in early and late pregnancy and offspring birth size: remote federated individual level meta-analysis from eight cohort studies. BJOG. 2019; 126(4): 459-70.
- Torres-Espínola F.J., Berglund S.K., García S., Pérez-García M., Catena A., Rueda R. et al. Visual evoked potentials in offspring born to mothers with overweight, obesity and gestational diabetes. PLoS One. 2018; 13(9): e0203754.
- Kouhkan A., Khamseh M.E., Moini A., Pirjani R., Valojerdi A.E., Arabipoor A. et al. Predictive factors of gestational diabetes in pregnancies following assisted reproductive technology: a nested case-control study. Arch. Gynecol. Obstet. 2018; 298(1): 199-206.
- Zhukovets I., Leshchenko O., Atalyan A., Podoshvelev D. Diagnostic markers of primary infertility in women of reproductive age with hypothalamic dysfunction in the pubertal period. Int. J. Biomed. 2017; 7(3): 213-7.
- Kolesnikova L.I., Kolesnikov S.I., Korytov L.I., Suslikova M.I., Darenskaya M.A., Grebenkina L.A. et al. Oxidative stress as a mechanisms of reduced glucose absorption under conditions of immobilization stress. Bull. Exp. Biol. Med. 2017; 164(2): 132-5.
- Zhukovets I., Leshchenko O., Atalyan A., Podoshvelev D. The main risk factors of menstrual disorders among adolescent girls with hypothalamic dysfunction. In: Gynecological Endocrinology. Abstracts from the ISGE World Congress. 2-5 March 2016, Firenze, Italy. 2016: 73, abstr. P36.
- Darenskaya M.A., Rychkova L.V., Kolesnikov S.I., Gavrilova O.A., Kravtsova O.V., Grebenkina L.A., Kolesnikova L.I. Оxidative stress parameters in adolescent boys with exogenous-constitutional obesity. Free Rad. Biol. Med. 2017; 112: 129-30.
- Leshchenko O., Zhukovets I., Atalyan A. Risk of оbesity in аdult women with hypothalamic dysfunction in puberty. Observational Prospective Study. In: Abstracts of the 99th Annual Meeting of Endocrine Society. April 1-4, 2017, Orlando, USA. Endocr. Rev. 2017; 38(Suppl. 3): 552.
- Zipori Y., Lauterbach R., Matanes E., Beloosesky R., Weiner Z., Weissman A. Vaginal progesterone for the prevention of preterm birth and the risk of gestational diabetes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018; 230: 6-9.
- Rosta K., Ott J., Kelemen F., Temsch W., Lahner T., Reischer T. et al. Is vaginal progesterone treatment associated with the development of gestational diabetes? A retrospective case-control study. Arch. Gynecol. Obstet. 2018; 298(6): 1079-84.
- Butler M.., McGuire A., Manzardo A.M. Clinically relevant known and candidate genes for obesity and their overlap with human infertilityand reproduction. J. Assist. Reprod. Genet. 2015; 32(4): 495-508.
- Franca-Neto A.H., Amorim M.M., Oliveira Barros V. Is newborn abdominal adiposity associated with maternal factors. Obstet. Gynecol. 2014; 123(1): 51- 9.
- Silberstein Т., Levy А., Harlev А. Perinatal outcome of pregnancies following in vitro fertilization and ovulation induction. J. Matern. Fetal Neonatal Med. 2014; 27(13): 1316-99.
- Chen M., Wu L., Wu F. Impaired glucose metabolism in response to high fat diet in female mice conceived by in vitro fertilization (IVF) or ovarian stimulation alone. PLoS One. 2014; 9(11): e113155.
Received 30.10.2018
Accepted 07.12.2018
About the Authors
Zhukovets, Irina V., PhD, head of the Department of Obstetrics and Gynecology, Amur State Medical Academy of Minzdrav of Russia.Tel.: +79143811706. E-mail: zhukovec040875@mail.ru.
Levakov, Sergey A., MD, head of the Department of Obstetrics and Gynecology, I.M. Sechenov First Moscow State Medical University of Minzdrav of Russia
(Sechenov University). Tel.: +79255063144. E-mail: levakoff@yandex.ru
Leshchenko, Olga Ya., MD, chief researcher at the Scientific Center of Family Health Problems and Human Reproduction, Irkutsk, Russia.
664003, Russia, Irkutsk. Tel.: +73952207636. E-mail: loyairk@mail.ru.
For citation: Zhukovets I.V., Levakov S.A., Leshchenko О.Ya. Risk factors for gestational diabetes mellitus. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (5): 57-62. (in Russian)
https://dx.doi.org/10.18565/aig.2019.5.57-62



