Expression of ephrin receptor A3 in glandular epithelial cells of uterine endometrium in patients with endometriosis and endometrial cancer
Objective: Evaluation of ephrin receptor A3 (EphA3) expression in glandular epithelial cells in uterine endometrium in women without endometrial pathology, with endometriosis and endometrial cancer (EC).Chuprynin V.D., Muftaydinova Sh.K., Senina D.N., Fayzullina N.M., Asaturova A.V., Buralkina N.A., Fayzullin L.Z., Ovodenko D.L., Kozachenko A.V.
Materials and methods: The study included 46 patients of reproductive age who underwent treatment in National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Health of Russia in the period from 2020 to 2021. The patients were divided into four groups: group 1 comprised 20 women with deep infiltrating endometriosis (DIE), group 2 comprised 21 women with peritoneal endometriosis (PE), group 3 consisted of 6 women with EC, and group 4 (control group) consisted of 9 women without endometriosis, who underwent surgery for tubal and peritoneal factors infertility, and the results of histological tests of these women showed no pathological changes in the endometrium. Additionally, the groups were divided into subgroups depending on the stage of the menstrual cycle prior to surgery. Semi-quantitative evaluation of EphA3 expression in glandular epithelial cells of uterine endometrium was performed by immunohistochemistry, using rabbit polyclonal antibodies (ab126261) and open-source image analysis software platform ImageJ.
Results: Immunohistochemical examination showed that EphA3 expression in glandular epithlelial cells of normal endometrium and eutopic endometrium both in PE and DIE is significantly higher in the secretory phase versus the proliferative phase. Significantly high EphA3 expression in glandular epithelial cells of ectopic endometrium versus regular expression was detected in both phases of the cycle, in PE and DIE. There was no significant difference between EphA3 expression in glandular epithelial cells affected by endometrial cancer and in normal endometrium.
Conclusion: The results of this study showed that EphA3 is overexpressed in ectopic endometrium, in PE and DIE, but not in EC.
Keywords
Endometriosis is one of the most common gynecological disorders that decreases the quality of patients’ life [1]. Endometriosis is defined as the presence of viable endometrial-like tissues, including endometrial glands and stroma outside the uterine cavity, and is associated with pelvic pain, dysmenorrhea, and infertility. [2, 3]. Histologically, endometriosis has 3 different forms: ovarian endometriosis, peritoneal endometriosis (PE) and deep infiltrative endometriosis (DIE) [4].
Despite the fact that endometriosis is considered a benign disease, it has many similar characteristics with malignant cells, including invasiveness, adhesiveness, and metastatic potential [5]. Moreover, epidemiological data show that endometriosis may cause malignant tumors [6]. The similarity between the processes in cancer and endometriosis, especially DIE, suggests a possibility of using similar therapeutic approaches. In this respect, ephrin receptor A3 (EphA3) is of special interest, which experimentally proved to be a potential target in cancer treatment [7].
Eph receptors are the largest family of receptor tyrosine kinases that play an important role in embryogenesis, providing intercellular communications, formation of the actin cytoskeleton, and cell motility [8]. Eph receptors are located on the cell surface, and their function is activated when ephrin ligands bind to the extracellular domain. There are 2 classes of ephrin receptors – EphA and EphB, that differ by the spectrum of ephrins, with which they interact. In turn, EphA receptors are subdivided into 9 subclasses (EphA1–EphA8 and EphA10), which bind to five class A ephrins (ephrin-A1–ephrin-A5), EphB receptors are subdivided into 5 subclasses (EphB1– EphB4 and EphB6), which bind to three class B ephrins (ephrin-B1–ephrin-B3) [9]. Receptors and ephrins participate in many important human physiological processes, such as cell proliferation, apoptosis, adhesion, migration, angiogenesis, directed blood vessels and axons growth. The pathological changes in the function of receptor/ephrin system are associated with many pathophysiological processes – cancer, cardiovascular diseases, diabetes mellitus, Alzheimer's disease and other [10]. Recently, Eph has attracted much attention as potential therapeutic target in cancer treatment [11].
EphA3 is a member of the Eph receptor family, which is expressed at high levels in embryonic tissues [12]. EphA3 controls cell-shape changes, their migration during development, participates in the branching pattern of embryonic tissues of embryonic origin, in particular, controls the correct branching of neural circuits. The data on expression if EphA3 neither in endometrial cancer (EC) nor endometrisosis in searching systems was found in searching systems.
Due to this, the aim of the study was comparative analysis of EphA3 expression in glandular epithelial cells in normal uterine endometrium, in endometrium of different stages and endometrial cancer (EC).
Material and methods
The study was carried out in the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
The study included 46 women, who were divided into four groups: group 1 comprised 20 women with deep infiltrating endometriosis (DIE), group 2 comprised 21 women with peritoneal endometriosis (PE), group 3 (the comparison group) consisted of 6 women with endometrial cancer (EC) (histological type IaT1aNxMO), and group 4 (the control group) consisted of 9 women without endometriosis. Group 4 included the patients, who underwent surgery for tubal and peritoneal factors infertility due to pelvic adhesions, and the absence of pathological changes in the endometrium and the absence of endometriosis was confirmed by histological test results.
In each patient, the color of 3–5 glands was assessed. The exclusion criteria were: the presence of severe forms of extragenital diseases and other oncopathology. The study was approved by the local Ethics Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. All patients have signed informed consent to participate in the study.
For quantitative assessment of EphA3 expression, immunohistochemical staining of deparaffinized samples was performed using monoclonal antibodies (Abcam Inc.) to EphA3 receptor – rabbit polyclonal antibodies, clone ab126261, at dilution of 1:200. The secondary antibody was Goat anti-Rabbit IgG H&L (HRP), clone ab205718. The quantitative assessment of EphA2 expression was performed using ImageJ, open source software for processing and analyzing the images. Due to the fact that EphA3 predominantly stained the cell surface, the mouse cursor highlighted the area of the gland, and the maximum staining intensity was fixed. In each patient, the staining of 5–6 glands was evaluated. The sections with no specific antibodies staining were used as negative controls.
Statistical analysis
Statistical analysis was performed using MedCalc Statistical Software 11.5.0. The significance of differences between the compared groups was determined using nonparametric Mann–Whitney U test. The data are shown as median (Ме) and 1st and 3rd quartiles (Q1; Q3) – Ме (Q1; Q3). The level of statistical significance of null hypothesis testing was at p<0.5.
Results
The results presented in the Table show that cell-surface expression of EphA3 in glandular epithelial cells in normal endometrium is significantly higher in the secretory phase than in the proliferative phase. This indicates the absence of a direct connection between the activity of this receptor and proliferative processes in the endometrium (28.0 and 20.0 relative units (RU), respectively, р=0.03). The images illustrating these differences can be seen in Figure (А; B). It seems possible that the absence of direct association between EphA3 and cell proliferation explains low expression of EphA3 receptors on glandular epithelial cells in the endometrium in EC. Expression of this receptor in cancerous glandular cells was 20.0 RU (Table) and significantly differed from expression in epithelial cells in the normal endometrium in the proliferative phase of the menstrual cycle.
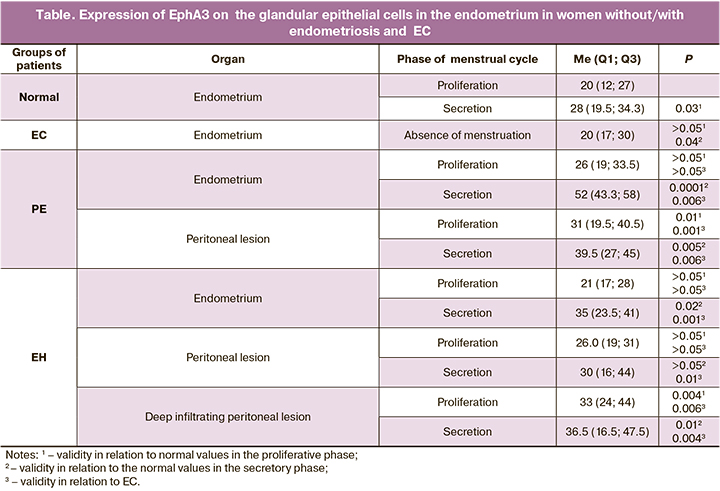
There was no significant difference between EphA3 expression in the eutopic endometrium and normal endometrium in the proliferative phase of the menstrual cycle both in PE and DIE (26.0 и 21.0, respectively, Table). At the same time, expression of EphA3 in glandular epithelial cells in the eutopic endometrium in the secretory phase both in PE and EH was significantly higher than in normal endometrium in the secretory phase (52.0 and 39.5 RU versus 28.0 RU, respectively. Table). Moreover, EphA3 expression in epithelial glandular cells in the eutopic endometrium in the secretory phase both in PE and EH was significantly higher than in the cells of the cancerous endometrium. Figure (C; D) shows the images illustrating these differences.
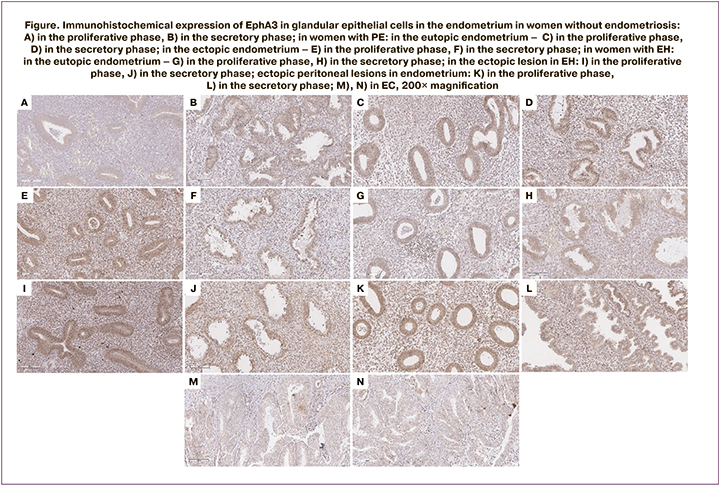
Expression of EphA3 in the ectopic endometrium was evaluated both in superficial peritoneal lesions and in deep infiltrating peritoneal lesions. In patients with PE, in both phases of the cycle EphA3 expression in glandular cells in peritoneal lesions was significantly higher than in the corresponding phases in normal endometrium. EphA3 expression in deep infiltrating lesions of ectopic endometrium in the intestinal tissues was significantly higher in both phases than in normal endometrium (33.0 RU in the prolipherative phase and 36.5 RU in the secretory phase (Table, Figure E; F).
No statistically significant differences in EphA3 expression were detected in the eutopic endometrium of patients with GE compared to normal endometrium in the proliferative phase and endometrial cancer (Figure G), but such differences were detected in the secretion phase compared to both normal proliferative endometrium and endometrial cancer (Figure H). In the ectopic endometrial focus, the assessment of the deep endometriosis focus in female patients with GE revealed statistically significant differences in both the proliferative and secretion phases compared to both normal endometrium in the proliferative phase and normal endometrium in the secretion phase and endometrial cancer (Figure I, J). In the ectopic peritoneal focus, statistically significant differences were detected only when comparing the focus in the secretion phase with Eph3 expression in endometrial cancer (Figure K–N).
Discussion
Our study showed that in normal endometrium, EphA3 expression in glandular cells does not correlate with the proliferative activity in the tissue: in the secretory phase of the menstrual cycle, receptor expression is significantly higher than in the proliferative phase. Similar results have been previously obtained by the group of researchers from Austria, who reported a higher expression of EphA3 in epithelial and mesenchymal cells, and in tissues of growing vessels in the endometrium in the secretory phase compared to the proliferative phase [13]. This proves that expression of EphA3 is not associated with cell division and endometrial growth, and is obviously determined by the functional status of the uterine mucous membrane in the secretory phase. Endometrial receptivity is formed in the secretory phase and is accompanied by activation of multiple receptors, which are necessary for migration of blastocyst to the site of attachment, adhesion to the endometrial surface and implantation (invasion) into the uterine cavity [14]. The model with cancer cells showed that EphA3 plays an active role in regulation of migration, adhesion, and invasion [15, 16].
EphA3 overexpression was found in various types of cancer, including lung cancer, leukemia, melanoma, lymphoma, and esophageal cancer [17]. At the same time, other publications report that high receptor activity suppresses tumor growth, and, on the contrary, low receptor activity increases proliferation and suppresses apoptosis [18, 19]. This suggests a controversial role of EphA3 in carcinogenesis. Andretta et al. demonstrated that neither experimentally induced overexpression nor inactivation affect the growth, motility, or metastatic potential in cancer cells in in vivo experiments [19]. Moreover, immunohistochemical analysis of EphA3 tumor levels found no association with survival or clinical and pathological features in patients with colorectal cancer. Wang et al. showed high expression of EphA3 protein in normal renal tubules, while in tissue samples of clear cell renal cell carcinoma, the level of expression was minimal [20]. Additionally, decreased level of EphA3 protein expression at the late stage of cancer directly correlated with increased tumor diameter. Also, EphA3 tumor-suppressing activity was detected in development of other oncological diseases [17].
These conflicting results underline the complexity of the biological role of EphA3 in formation of malignant tumors, especially when receptor functions may differ depending on the cellular context and coexisting hormones, cytokines, and growth factors. Apparently, EphA3 expression may vary depending on the stage of the disease. Particularly in breast cancer, high expression of EphA3 is in metastatic tissue, but not in the primary tumor [21]. Perhaps, EphA3 may become a promising marker for differentiation of the primary tumor from its derivatives resulting from metastasis.
The findings in our study did not show increased EphА3 expression level in the glandular cells in EC, and it was comparable to receptor expression level in similar cells in normal endometrium. It can be explained by the fact, that for our study, we have chosen the patients with early-stage cancer, and none of them had metastasis. The absence of high receptor expression level in endometrial epithelial cells in EC can be further used for differentiation between DIE and early stages of EC.
Both in normal endometrium and ectopic endometrium in the secretory phase, EphA3 expression was significantly higher versus the proliferative phase. Moreover, despite the severity of endometriosis, receptor expression in glandular cells in the secretory phase was significantly higher compared to increased expression in normal endometrium in the secretory phase. Overexpression of EphA3 level makes it possible to consider it as a promising target for endometriosis prevention and treatment.
Conclusion
Out findings demonstrated that EphA3 did not exhibit increased expression at the membrane site in glandular epithelial cells affected by EC. Apparently, this was associated with development of cancer from early stage to metastasis. At the same time, in PE as well as in DIE, significantly increased EphA3 expression at the membrane in glandular epithelial cells both in eutopic and ectopic endometrium was detected in the secretory phase of the cycle. Overexpression of EphA3 in ectopic endometrium suggests that it can be a promising target for drug development with purpose of endometriosis prevention and treatment.
References
- Лисовская Е.В., Хилькевич Е.Г., Чупрынин В.Д., Мельников М.В., Ипатова М.В. Качество жизни женщин с глубоким инфильтративным эндометриозом. Акушерство и гинекология. 2020; 3: 116-26.[Lisovskaya E.V., Khilkevich E.G., Chuprynin V.D., Melnikov M.V., Ipatova M.V. Quality of life in patients with deep infiltrating endometriosis. Obstetrics and Gynecology. 2020; 3: 116-26. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.3.116-126.
- Bulun S.E., Yilmaz B.D., Sison C., Miyazaki K., Bernardi L., Liu S. et al. Endometriosis. Endocr. Rev. 2019; 40(4): 1048-79. https://dx.doi.org/10.1210/er.2018-00242.
- Адамян Л.В., Мартиросян Я.О., Асатурова А.В. Этиопатогенез эндометриоз-ассоциированного бесплодия (обзор литературы). Проблемы репродукции. 2018; 24(2): 28-33. [Adamyan L.V., Martirosyan Y.O., Asaturova A.V. Etiopathogenesis of endometriosis-associated infertility (a review). Russian Journal of Human Reproduction. 2018; 24(2): 2833. (in Russian)]. https://doi.org/10.17116/repro201824228-33.
- Адамян Л.В., Андреева Е.Н., Беженарь В.Ф. Эндометриоз: диагностика, лечение и реабилитация. Клинические рекомендации по ведению больных. М.; 2013. 58с. [Adamyan L.V., Andreeva E.N., Bezhenar V.F. Endometriosis: diagnosis, treatment and rehabilitation. Clinical recommendations for the management of patients. M.; 2013. 58p. (in Russian)].
- Kajiyama H., Suzuki S., Yoshihara M., Tamauchi S., Yoshikawa N., Niimi K. et al. Endometriosis and cancer. Free Radic. Biol. Med. 2019; 133: 186-92. https://dx.doi.org/10.1016/j.freeradbiomed.2018.12.015.
- Щеголев А.И., Быков А.Г., Туманова У.Н., Павлович С.В. Эндометриоз и развитие опухолей. Акушерство и гинекология. 2016; 11: 49-56. [Shchegolev A.I., Bykov A.G., Tumanova U.N., Pavlovich S.V. Endometriosis and the development of tumors. Obstetrics and Gynecology. 2016; 11: 49-56. (in Russian)]. https://dx.doi.org/10.18565/aig.2016.11.49-56.
- Lodola A., Giorgio C., Incerti M., Zanotti I., Tognolini M. Targeting Eph/ephrin system in cancer therapy. Eur. J. Med. Chem. 2017; 142: 152-62. https://dx.doi.org/10.1016/j.ejmech.2017.07.029.
- Kaczmarek R., Zimmer K., Gajdzis P., Gajdzis M. The role of eph receptors and ephrins in corneal physiology and diseases. Int. J. Mol. Sci. 2021; 22(9): 4567. https://dx.doi.org/10.3390/ijms22094567.
- Pitulescu M.E., Adams R.H. Eph/ephrin molecules--a hub for signaling and endocytosis. Genes Dev. 2010; 24(22): 2480-92. https://dx.doi.org/10.1101/gad. 1973910.
- Kania A., Klein R. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat. Rev. Mol. Cell Biol. 2016; 17(4): 240-56. https://dx.doi.org/10.1038/nrm.2015.16.
- Tang F.H.F., Davis D., Arap W., Pasqualini R., Staquicini F.I. Eph receptors as cancer targets for antibody-based therapy. Adv. Cancer Res. 2020; 147: 303-17. https://dx.doi.org/10.1016/bs.acr.2020.04.007.
- Janes P.W., Slape C.I., Farnsworth R.H., Atapattu L., Scott A.M., Vail M.E. EphA3 biology and cancer. Growth Factors. 2014; 32(6): 176-89.https://dx.doi.org/10.3109/08977194.2014.982276.
- To C., Farnsworth R.H., Vail M.E., Chheang C., Gargett C.E., Murone C. et al. Hypoxia-controlled EphA3 marks a human endometrium-derived multipotent mesenchymal stromal cell that supports vascular growth. PLoS One. 2014; 9(11): e112106. https://dx.doi.org/10.1371/journal.pone.0112106.
- Lessey B.A., Young S.L. What exactly is endometrial receptivity? Fertil. Steril. 2019; 111(4): 611-7. https://dx.doi.org/10.1016/j.fertnstert.2019.02.009.
- Chen X., Lu B., Ma Q., Ji C.D., Li J.Z. EphA3 inhibits migration and invasion of esophageal cancer cells by activating the mesenchymalepithelial transition process. Int. J. Oncol. 2019; 54(2): 722-32. https://dx.doi.org/10.3892/ijo.2018.4639.
- La Rocca F., Airoldi I., Di Carlo E., Marotta P., Falco G., Simeon V. et al. EphA3 targeting reduces in vitro adhesion and invasion and in vivo growth and angiogenesis of multiple myeloma cells. Cell. Oncol. (Dordr.). 2017; 40(5): 483-96. https://dx.doi.org/10.1007/s13402-017-0338-4.
- London M., Gallo E. Critical role of EphA3 in cancer and current state of EphA3 drug therapeutics. Mol. Biol. Rep. 2020; 47(7): 5523-33. https://dx.doi.org/10.1007/s11033-020-05571-8.
- Lahtela J., Pradhan B., Närhi K., Hemmes A., Särkioja M., Kovanen P.E. et al. The putative tumor suppressor gene EphA3 fails to demonstrate a crucial role in murine lung tumorigenesis or morphogenesis. Dis. Models Mech. 2015; 8(4): 393-401. https://dx.doi.org/10.1242/dmm.019257.
- Andretta E., Cartón-García F., Martínez-Barriocanal Á., de Marcondes P.G., Jimenez-Flores L.M., Macaya I. et al. Investigation of the role of tyrosine kinase receptor EPHA3 in colorectal cancer. Sci. Rep. 2017; 7: 41576. https://dx.doi.org/10.1038/srep41576.
- Wang X., Xu H., Cao G., Wu Z., Wang J. Loss of EphA3 protein expression is associated with advanced TNM stage in clear-cell renal cell carcinoma. Clin. Genitourin. Cancer. 2017; 15(2): e169-73. https://dx.doi.org/10.1016/j.clgc.2016.07.028.
- Vecchi M., Confalonieri S., Nuciforo P., Viganò M.A., Capra M., Bianchi M. et al. Breast cancer metastases are molecularly distinct from their primary tumors. Oncogene. 2008; 27(15): 2148-58. https://dx.doi.org/10.1038/sj.onc.1210858.
Received 12.03.2022
Accepted 03.06.2022
About the Authors
Vladimir D. Chuprynin, PhD, Head of the Surgical Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-35-75, v_chuprynin@oparina4.ru, 117997, Russia, Moscow, Oparin str., 4.Shakhnoza K. Muftaydinova, graduate student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health
of Russia, +7(495)438-78-33, 117997, Russia, Moscow, Oparin str., 4.
Daria N. Senina, graduate student, I.M. Secenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), +7(495)438-78-33, 438-78-33, 119435, Russia, Moscow, B. Pirogovskaya, 2.
Nafisa M. Fayzullina, Ph.D., Senior Researcher at the 1st Pathology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(916)453-40-87, 117997, Russia, Moscow, Oparin str., 4.
Alexandra V. Asaturova, Dr. Med. Sci., Head of the 1st Pathology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(926)994-43-14, a_asaturova@oparina4.ru, 117997, Russia, Moscow, Oparin str., 4.
Natalya A. Buralkina, Dr. Med. Sci., Senior Researcher at Surgical Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-78-33, n_buralkina@oparina4.ru, 117997, Russia, Moscow, Oparin str., 4.
Leonid Z. Fayzullin, PhD, Leading Researcher at the Laboratory of Molecular Genetic Methods, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(916)710-67-89, l_faizullin@oparina4.ru, 117997, Russia, Moscow, Oparin str., 4.
Dmitry L. Ovodenko, Dr. Med. Sci., Head of the Department of Clinical Work, Oncologist at the Department of Innovative Oncology and Gynecology, Academician
V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-09-88, d_ovodenko@oparina4.ru, 117997, Russia, Moscow, Oparin str., 4.
Andrey V. Kozachenko, Dr. Med. Sci., Leading Researcher, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,
Ministry of Health of Russia, +7(916)614-41-32, a_kozachenko@oparina4.ru, 117997, Russia, Moscow, Oparin str., 4.
Authors’ contributions: Chyprynin V.D., Fayzullin L.Z., Buralkina N.A. – the concept and design of the study; Asaturova A.V., Fayzullina N.M. – immunohistochemical examination and imaging analysis; Muftaydinova Sh.K., Senina D.N., Ovodenko D.L., Kozachenko A.V. – material collection and processing; Fayzullin L.Z., Buralkina N.A., Asaturova A.V. – writing the article.
Conflicts of interest: The authors declare that they have no conflicts of interest.
Funding: The study was carried out without any sponsorship.
Ethical Approval: The study was approved by the local Ethics Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data and associated images.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Chuprynin V.D., Muftaydinova Sh.K., Senina D.N., Fayzullina N.M., Asaturova A.V., Buralkina N.A., Fayzullin L.Z., Ovodenko D.L., Kozachenko A.V.
Expression of ephrin receptor A3 in glandular epithelial cells of uterine endometrium
in patients with endometriosis and endometrial cancer.
Akusherstvo i Gynecologia/Obstetrics and Gynecology. 2022; 6: 98-104 (in Russian)
https://dx.doi.org/10.18565/aig.2022.6.98-104



