Expression of ephrin receptor A1 on the membrane of epitheliocyte glands of the uterine mucosa in patients with endometrial cancer and endometriosis
Objective: To assess the expression of the Epha1 receptor on the epithelial cell membrane of the glands of the eutopic and ectopic endometrium in women without endometrial pathology, with endometriosis of varying severity and endometrial cancer.Muftaydinova Sh.K., Senina D.N., Litvinova V.V., Fayzullina N.M., Asaturova A.V., Buralkina N.A., Ovodenko D.L., Fayzullin L.Z., Chuprinin V.D.
Materials and methods: The study included 46 patients of reproductive age who were treated in the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Health of the Russian Federation from 2020 to 2021. The patients were divided into four groups: Group 1 included 20 women with deep endometriosis (DE), Group 2 included 21 patients with peritoneal endometriosis (PE), Group 3 (comparison group) included 6 patients with endometrial cancer (EC), and Group 4 (control) included 9 patients without endometriosis (no pathological changes in the endometrium) and operated on for tubal-peritoneal infertility factor. The groups were additionally divided into subgroups depending on the phase of the menstrual cycle on the day before the operation. Semi-quantitative evaluation of EphA1 expression on the epithelial cells of the glands of the uterine mucosa was performed by immunohistochemistry, using rabbit polyclonal antibodies ab217363 and the ImageJ open source program for image analysis and processing.
Results: We have shown for the first time that in healthy endometrium, a significantly higher expression of the ephrin receptor EphA1 on the surface of glandular cells is observed in the secretory phase of the menstrual cycle (MC), compared to the proliferative one. The same pattern of receptor expression was found in the eutopic endometrium of patients with PE - higher in the secretory than in the proliferative phases. In the eutopic endometrium of patients with DE, a significantly increased level of EphA1 expression was found in the proliferative and secretory phases of MC compared to patients without endometriosis or with PE. In foci on the peritoneum in both PE and DE, a higher level compared to the norm was found only in the proliferative phase of the cycle. The highest hyperexpressed level was found on the membrane of the glandular cells of the endometrium affected by cancer and in deeply infiltrative foci on the intestine with DE in both phases of the cycle.
Conclusion: Overexpression of the ephrin receptor EphA1 in glandular cells of endometrial tissue in EC and DE makes it a promising target for the development of new technologies for the diagnosis and treatment of these diseases.
Authors' contributions: Muftaydinova Sh.K., Senina D.N., Litvinova V.V., Ovodenko D.L. – clinical characterization and group formation; Fayzullina N.M. – immunohistochemical reaction; Asaturova A.V. – immunohistochemical reaction and analysis; Buralkina N.A., Fayzullin L.Z. – problem setting and evaluation of the results obtained; Chuprynin V.D. – general organization and management of the study.
Conflicts of interest: Authors declare lack of the possible conflicts of interest.
Funding: Work done without sponsorship.
Ethical Approval: The study was approved by the Ethics Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: Patients have signed informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Muftaydinova Sh.K., Senina D.N., Litvinova V.V., Fayzullina N.M., Asaturova A.V.,
Buralkina N.A., Ovodenko D.L., Fayzullin L.Z., Chuprinin V.D. Expression of ephrin receptor A1
on the membrane of epitheliocyte glands of the uterine mucosa in patients with endometrial cancer and endometriosis.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (7): 74-80 (in Russian)
https://dx.doi.org/10.18565/aig.2023.82
Keywords
Endometriosis is a chronic inflammatory disease with a high prevalence and serious reproductive and health consequences [1, 2]. Endometriosis is one of the most common benign gynecologic neoplasms in premenopausal women and affects, according to various estimates, 10–15% of women of reproductive age [3]. Despite many years of research, the pathogenetic mechanisms of the disease remain unclear, and the methods of diagnosis and treatment are still ineffective. This disease is a significant public health problem, strongly affecting the quality of life of women, as well as being a significant economic burden.
Three phenotypes of the disease are distinguished according to clinical manifestation: peritoneal endometriosis (PE), ovarian endometrioma, and deep infiltrative endometriosis (DE) [4]. DE is manifested by an infiltration of endometriosis deeper than 5 mm beneath the peritoneum and is the most aggressive form of the disease. DE has all common signs of malignant diseases: increased proliferative and reduced apoptotic activity, invasiveness, adhesiveness propensity to metastasize; it can progress to malignant tumor [5, 6]. This suggests the possibility of using cancer therapies for DE treatment as well. In this regard, ephrin receptors (Eph) are of interest, which have been proven in experimental animal studies as a promising target for cancer treatment [7].
Ephrin receptors (Eph) are part of the family of receptor tyrosine kinases involved in embryogenesis, controlling organ growth by intercellular communications [8]. Eph are expressed on the surface of the cell membrane and their functional activation is realized by binding to the corresponding ligand, ephrin. Eph receptor tyrosine kinases and their ephrin ligands are critical regulators of cell contact-dependent signal signaling and pattern formation. Eph/ephrin binding can lead to activation of various biological functions such as adhesion or repulsion, or increased or decreased motility [9]. Pathologic increase or decrease in Eph expression is associated with various diseases: cancer, cardiovascular diseases, brain diseases, etc. [10]. Therefore, Ephs are of interest as therapeutic target in the treatment of various diseases [11].
The EphA1 receptor was first detected in an erythropoietin-producing human liver cancer cell in 1987, and its increased expression (overexpression) was noticed in various types of human cancer, including gastric carcinoma, colorectal cancer, ovarian, and breast cancer [12]. The receptor regulates many aspects of tumor biology, patient survival, and is considered as a therapeutic target for cancer treatment [13]. At the same time, we did not find works on EphA1 expression in EC. We also found no works on receptor expression in eutopic and ectopic endometrium in endometriosis.
In this regard, the aim of this work was to conduct a comparative study of EphA1 expression on the membrane of epitheliocytes of the glands of the uterine mucosa in patients with endometriosis and EC by immunohistochemistry.
Material and methods
The study was performed at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Four groups of 46 patients were formed for the study: Group 1 consisted of 20 women with DE (10 – in proliferative, 10 – in secretory phase of menstrual cycle (MC)), Group 2 consisted of 21 women with PE (12 – in proliferative, 8 – in secretory phase of MC), Group 3 included 6 women with EC (high-grade endometrial carcinomas stage FIGO Ia pT1aNxM0), and Group 4 (control) included 9 women without endometriosis (no pathological changes in the endometrium) and operated on for tubal-peritoneal infertility factor. The groups were additionally divided into subgroups depending on the phase of the menstrual cycle on the day before the operation. Exclusion criteria were the presence of severe extragenital diseases and other oncopathology. All patients signed informed consent for participation in this study. The study was approved by the Ethics Committee of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Health of the Russian Federation.
Semi-quantitative evaluation of EphA1 expression, the standard technique of immunohistochemical staining of deparaffinized preparations with antibodies was used, as described previously [14]. To detect EphA1, we used antibodies from Abcam Inc: rabbit polyclonal antibodies ab217363 at a dilution of 1:200, and goat anti-rabbit IgG H&L immunoglobulin (HRP) clone ab205718 as secondary antibodies. Slices stained without specific antibodies were used as a negative control.
Statistical analysis
Statistical analysis was performed using the MedCalc Statistical Software 11.5.0 software package. Statistical significance between the compared groups was performed using the Mann–Whitney U test. Data are presented as median (Me) and quartiles of Q1 and Q3 in Me format (Q1;Q3). The level of statistical significance in testing the null hypothesis was considered to be p<0.5.
Results
The data presented in the Table 1 show that the expression of EphA1 receptor on the membrane of epithelial cells of the glands of the uterine mucosa in patients without endometrial pathology is significantly higher in the secretory phase than in the proliferative phase (35.0 and 22.0 c.u., respectively, p=0.003, Figure A, B).
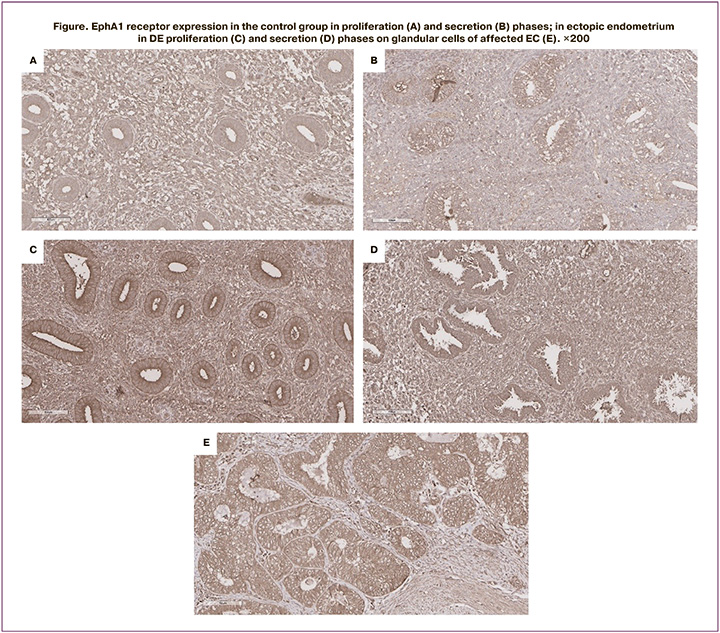
The expression level of the receptor on the membrane of epithelial cells of the glands in endometrial cancer cells significantly higher than that in normal endometrial cells both in the proliferative and secretory phases (46.0 c.u., p=0.003, Table 1, Figure B)), which indicates abnormally elevated (overexpressed) level of EphA1 in cancer cells (Figure E).
In PE, the expression of EphA1 on the membrane of gland epitheliocytes in eutopic endometrium did not significantly differ from that in the control group in the corresponding phases of the cycle. At the same time, increased expression of the receptor on superficial peritoneal lesions was detected in the proliferative phases of the cycle (Table).
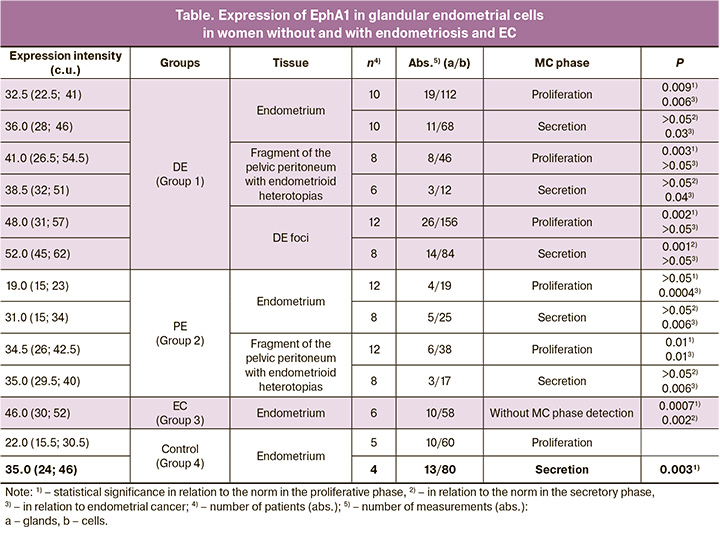
In patients with DE on the membrane of epithelial glands in the eutopic endometrium, increased EphA1 expression was detected only in the proliferative phase: 32.5 c.u. compared to 22.0 c.u. norm (p=0.009). In the secretory phase of the cycle, EphA1 expression on the membrane of the epithelial cells of the glands of the eutopic endometrium was at the same level as in the healthy endometrium – 36.0 c.u. In the same patients, on the membrane of epithelial cells of glands in ectopic foci on the peritoneum, representing a superficial form of endometriosis, increased EphA1 expression was also detected only in the proliferative phase (41.0 c.u., p=0.003), and not in the secretory phase (Table).
Significantly increased expression of EphA1 both in the proliferative and secretory phases was detected on glandular cells of the endometrium in deeply infiltrative foci in DE (48.0, p=0.002 and 52.0, p=0.001, respectively). At the same time, the expression in both phases of the cycle was at the level of expression on the surface of gland epitheliocytes affected by EC, which is shown in Figure E.
Discussion
Ephrin receptors and their ephrin ligands are located on the cell surface, and their interaction through intercellular contact activates a wide range of signaling pathways aimed at proliferation, apoptosis, differentiation, cell adhesion, and migration [15]. EphA1, together with its main ligand EphrinA1, plays an important role in reproductive function by regulating folliculogenesis, ovulation, embryo transport, implantation, and placenta formation [16]. EphA1 is expressed on the outer membrane of endometrial epithelial cells, while its ligand EphrinA1 is seen on the outer membrane of blastocyst cells, which mediates the uterine implantation and ensures the proliferation and invasion of trophoblast into the thickness of decidual tissue [17]. The process of embryo attachment happens in the secretory phase of the menstrual cycle, which explains the found increased expression of EphA1 in this particular phase. In the secretory phase, the endometrial receptivity is formed, an important factor of which is the overgrowth of the blood vascular system in the endometrium, provided by the increased expression of angiogenic factors during this period [18]. Chinese researchers from Wenzhou showed that EphA1 activates angiogenesis by direct stimulation of the SDF-1/CXCR4 signaling system that controls the growth of the vascular system in the tissue [19].
Increased expression of EphA1 has been detected in many types of cancer, including ovarian and cervical cancer [16, 20]. Targeted inhibition of this expression in experimental models is often accompanied by inhibition of various stages of the cancer process. In this regard, ephrin receptors have recently been considered as promising targets for the therapy of various cancers [21]. We did not find any publications on EphA1 expression in malignant endometrial lesions. Our study has shown that in EC, in particular in early stage of cancer (high-grade endometrial carcinomas stage FIGO Ia pT1aNxM0), the expression of EphA1 is significantly increased compared to norm. This finding can be used for the development of therapy for the disease.
Endometriosis, especially DE, has a number of biological processes that are common with malignant diseases where ephrin receptors are involved [5]. We have previously shown that EphA2 in DE overexpressed on the surface of the epithelial cells of the glands of the ectopic endometrium similar to the level in endometrial cancer cells [22]. Similarly, EphB4 has been shown to be overexpressed in ectopic endometrium compared to eutopic [23]. Moreover, experiments with transplanted mice have shown that the suppression of EphB4 expression leads to inhibition of the growth of endometrioid cells by inhibiting angiogenesis [24].
Our study revealed an increased expression of EphA1 in the eutopic endometrium in the proliferative phase of the cycle in DE, while it was not found in the control (healthy) group or in patients with PE, which may be due to the increased level of systemic inflammatory response in DE. However, we found no increase in receptor expression in the eutopic endometrium of patients with DE during the secretory phase. To date, there are no publications on EphA1 expression in endometrial tissues, and further studies are needed to understand its function. However, the revealed overexpression of EphA1 in the endometrium of women with DE in the proliferative phase can be used to differentiate severity of endometriosis.
In ectopic lesions on the peritoneum in PE and DE, increased expression of the receptor was revealed only in the proliferative phase, but not in the secretory one. At the same time, on the membrane of epithelial cells of the glands in the infiltrative foci of the endometrium, a significantly increased expression of EphA1 was revealed in both phases of the cycle, which was similar to the expression in the same tissues affected by EC. This overexpression confirms the presence of common mechanisms in cancer and endometriosis.
Conclusion
The findings provide new insights into the molecular processes involved in the pathogenesis of endometriosis and EC. The overexpression of EphA1 makes it a promising target for the treatment of DE and EC. Further research is needed to understand the mechanism of pathologic processes underlying infertility and pain symptoms in women with endometriosis.
References
- Адамян Л.В., Мартиросян Я.О., Асатурова А.В. Этиопатогенез эндометриоз-ассоциированного бесплодия (обзор литературы). Проблемы репродукции. 2018; 24(2): 28-33. [Adamyan L.V., Martirosyan Y.O., Asaturova A.V.Etiopathogenesis of endometriosis-associated infertility (a review). Russian Journal of Human Reproduction. 2018; 24(2): 28 33. (in Russian)].https://dx.doi.org/10.17116/repro201824228-33.
- Bulun S.E., Yilmaz B.D., Sison C., Miyazaki K., Bernardi L., Liu S. et al. Endometriosis. Endocr. Rev. 2019; 40(4): 1048-79. https://dx.doi.org/10.1210/er.2018-00242.
- Shafrir A.L., Farland L.V., Shah D.K., Harris H.R., Kvaskoff M., Zondervan K., Missmer S.A. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract. Res. Clin. Obstet. Gynaecol. 2018; 51: 1-15. https://dx.doi.org/10.1016/j.bpobgyn.2018.06.001.
- Koninckx P.R., Ussia A., Adamyan L., Tahlak M., Keckstein J., Wattiez A.,Martin D.C. The epidemiology of endometriosis is poorly known as the pathophysiology and diagnosis are unclear. Best Pract. Res. Clin. Obstet. Gynaecol. 2021; 71: 14-26. https://dx.doi.org/10.1016/j.bpobgyn.2020.08.005.
- Kajiyama H., Suzuki S., Yoshihara M., Tamauchi S., Yoshikawa N., Niimi K. et al. Endometriosis and cancer. Free Radic. Biol. Med. 2019; 133: 186-92.https://dx.doi.org/10.1016/j.freeradbiomed.2018.12.015.
- Щеголев А.И., Быков А.Г., Туманова У.Н., Павлович С.В. Эндометриоз и развитие опухолей. Акушерство и гинекология. 2016; 11: 49-56. [Shchegolev A.I., Bykov A.G., Tumanova U.N., Pavlovich S.V. Endometriosis and the development of tumors. Obstetrics and Gynecology. 2016; (11): 49-56. (in Russian)]. http://dx.doi.org/10.18565/aig.2016.11.49-56.
- Buckens O.J., El Hassouni B., Giovannetti E., Peters G.J. The role of Eph receptors in cancer and how to target them: novel approaches in cancer treatment. Expert Opin. Investig. Drugs. 2020; 29(6) 567-82. https://dx.doi.org/10.1080/13543784.2020.1762566.
- Kaczmarek R., Zimmer K., Gajdzis P., Gajdzis M. The role of Eph receptors and ephrins in corneal physiology and diseases. Int. J. Mol. Sci. 2021; 22(9): 4567. https://dx.doi.org/10.3390/ijms22094567.
- Pitulescu M.E., Adams R.H. Eph/ephrin molecules--a hub for signaling and endocytosis. Genes Dev. 2010; 24(22): 2480-92. https://dx.doi.org/10.1101/gad. 1973910.
- Kania A., Klein R. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat. Rev. Mo.l Cell Biol. 2016; 17(4): 240-56.https://dx.doi.org/10.1038/nrm.2015.16.
- Tang F.H.F., Davis D., Arap W., Pasqualini R., Staquicini F.I. Eph receptors as cancer targets for antibody-based therapy. Adv. Cancer Res. 2020; 147: 303-17. https://dx.doi.org/10.1016/bs.acr.2020.04.007.
- Ieguchi K., Maru Y. Roles of EphA1/A2 and ephrin-A1 in cancer. Cancer Sci. 2019; 110(3): 841-8. https://dx.doi.org/10.1111/cas.13942.
- Wang Y., Yu H., Shan Y., Tao C., Wu F., Yu Z. et al. EphA1 activation promotes the homing of endothelial progenitor cells to hepatocellular carcinoma for tumor neovascularization through the SDF-1/CXCR4 signaling pathway. J. Exp. Clin. Cancer Res. 2016; 35(1): 65. https://dx.doi.org/10.1186/s13046-016-0339-6.
- Чупрынин В.Д., Муфтайдинова Ш.К., Сенина Д.Н., Файзуллина Н.М., Асатурова А.В., Буралкина Н.А., Файзуллин Л.З., Оводенко Д.Л., Козаченко А.В. Экспрессия эфринового рецептора A3 на мембране эпителиоцитов желез слизистой тела матки у пациенток с эндометриозом и раком эндометрия. Акушерство и гинекология. 2022; 6: 98-104. [Chuprynin V.D., Muftaydinova Sh.K., Senina D.N., Fayzullina N.M., Asaturova A.V., Buralkina N.A., Fayzullin L.Z., Ovodenko D.L., Kozachenko A.V. Expression of ephrin receptor A3 in glandular epithelial cells of uterine endometrium in patients with endometriosis and endometrial cancer. Obstetrics and Gynecology. 2022; (6): 98-104. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.6.98-104.
- Gucciardo E., Sugiyama N., Lehti K. Eph - and ephrin - dependent mechanisms in tumor and stem cell dynamics. Cell. Mol. Life Sci. 2014; 71(19): 3685-710. https://dx.doi.org/10.1007/s00018-014-1633-0.
- Wu Y., Du Z., Mou J., Qiu X., Chen J., Cai S. et al. The Functions of EphA1 receptor tyrosine kinase in several tumors. Curr. Med. Chem. 2023; 30(20): 2340-53. https://dx.doi.org/10.2174/0929867329666220820125638.
- Fujii H., Tatsumi K., Kosaka K., Yoshioka S., Fujiwara H., Fujii S. Eph–ephrin a system regulates murine blastocyst attachment and spreading. Dev Dyn. 2006; 235(12): 3250-8. https://dx.doi.org/10.1002/dvdy.20977.
- Bourlev V., Volkov N., Pavlovitch S., Lets N., Larsson A., Olovsson M. The relationship between microvessel density, proliferative activity and expression of vascular endothelial growth factor-A and its receptors in eutopic endometrium and endometriotic lesions. Reproduction. 2006; 132(3): 501-9.https://dx.doi.org/10.1530/rep.1.01110.
- Wang S., Gao S., Li Y., Qian X., Luan J., Lv X. Emerging importance of chemokine receptor CXCR4 and its ligand in liver disease. Front. Cell. Dev. Biol. 2021;9:716842. https://dx.doi.org/10.3389/fcell.2021.716842.
- Wu Y., Du Z., Mou J., Qiu X., Chen J., Cai S. et al. The functions of EphA1 receptor tyrosine kinase in several tumors. Curr. Med. Chem. 2023;30(20):2340-53. https://dx.doi.org/10.2174/0929867329666220820125638.
- Lodola A., Giorgio C., Incerti M., Zanotti I., Tognolini M. Targeting Eph/ephrin system in cancer therapy. Eur. J. Med. Chem. 2017; 142: 152-62.https://dx.doi.org/10.1016/j.ejmech.2017.07.029.
- Shchegolev A., Muftaydinova S., Fayzullina N.M., Buralkina N., Fayzullin L.Z., Chuprynin V.D. Overexpression of ephrin receptor a2 in the ectopic endometrium of patients with deep infiltrative endometriosis. Virchow’s Archiv. 2019; 475(Suppl. 1): S318.
- Yerlikaya G., Balendran S., Pröstling K., Reischer T., Birner P., Wenzl R. et al. Comprehensive study of angiogenic factors in women with endometriosis compared to women without endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016; 204: 88-98. https://dx.doi.org/10.1016/j.ejogrb.2016.07.500.
- Rudzitis-Auth J., Fuß S.A., Becker V., Menger M.D., Laschke M.W. Inhibition of erythropoietin-producing hepatoma receptor B4 (EphB4) signalling suppresses the vascularisation and growth of endometriotic lesions. Br. J. Pharmacol. 2020; 177(14): 3225-39. https://dx.doi.org/10.1111/bph.15044.
Received 04.04.2023
Accepted 06.07.2023
About the Authors
Shaxnoza K. Muftaydinova, doctoral student (DSc), Tashkent Medical Academy, Tashkent, Uzbekistan, +998(90)540-85-87,100109, Uzbekistan, Tashkent, Farabi str., 2.
Daria N. Senina, graduate student of the Surgical Department, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia, 119992, Russia, Moscow, Trubetskaya str., 8-2; Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(904)189-30-63, seninadasha1995@gmail.com
Valentina V. Litvinova, Resident, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, Valentina_li@list.ru, https://orcid.org/0000-0001-9437-2005, Spin-code: 9540-7046, AuthorID: 1089646, 117997, Russia, Moscow, Ac. Oparina str., 4.
Nafisa M. Fayzullina, Ph.D., Senior Researcher at the 1st Pathology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(916)453-40-87, 117997, Russia, Moscow, Ac. Oparina str., 4.
Alexandra V. Asaturova, Dr. Med. Sci., Head of the 1st Pathology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(926)994-43-14, a_asaturova@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Natalya A. Buralkina, Dr. Med. Sci., Senior Researcher at Surgical Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-78-33, n_buralkina@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Leonid Z. Fayzullin, PhD, Leading Researcher at the Laboratory of Molecular Genetic Methods, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(916)710-67-89, l_faizullin@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Dmitry L. Ovodenko, Dr. Med. Sci., Head of the Department of Clinical Work, Oncologist at the Department of Innovative Oncology and Gynecology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-09-88, d_ovodenko@oparina4.ru,
117997, Russia, Moscow, Ac. Oparina str., 4.
Vladimir D. Chuprynin, PhD, Head of the Surgical Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-35-75, v_chuprynin@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.



