A tissue-engineered construct based on polydioxanone and multipotent stromal cells for plastic surgery repair of abdominal cavity and pelvic floor defects
Objective. To study the safety and efficiency of transplantation of the developed tissue-engineered prosthesis based on polydioxanone and cultured multipotent stromal cells (MSC) from the umbilical cord.Grinberg M.V., Arutyunyan I.V., Tsedik L.V., Makarov A.V., Elchaninov A.V., Lokhonina A.V., Fathudinov T.Kh.
Material and methods. In vivo biocompatibility of prostheses was evaluated in outbred rats, by modeling a full-layer defect of the anterior abdominal wall, the edges of which was sutured with a polydioxanone prosthesis that was unpopulated and populated by the cultured cells. A prosthesis based on the decellularized dermis (Permacol) was used in the comparison group. The animals were withdrawn from the experiment at 3, 10, 30, 60, and 180 days after surgery. Macroscopic, tensiometric, histomorphometric, and immunohistochemical studies were conducted.
Results. The polydioxanone-based prostheses were found to be more effectively integrated and grow their own tissues. The biomechanical properties of tissues in the field of transplantation in the long-term periods did not differ between the groups and the native tissue of the anterior abdominal wall. When a tissue-engineered construct was transplanted, there was a lower inflammatory response due to macrophage M2 polarization, as well as a more pronounced angiogenesis. The transplanted cells did not differentiate into blood vessel cells and were totally eliminated by a recipient’s macrophages. The registered effects appeared to be shown via paracrine mechanisms.
Conclusion. The addition of cultured MSCs to the prosthesis could substantially reduce the severity of an inflammatory response to rejection of a foreign body and stimulate angiogenesis and the rate of replacement by the recipient’s own tissues.
Keywords
The application of totally resorbable biological prostheses is becoming an ever more frequent choice among surgeons in hernioplasty operations and the fortification of the soft tissues of the small pelvis [1]. Despite the high cost, biological prostheses based on decellularized animal (Permacol) and human (Alloderm) tissue are being applied ever more often, especially in pediatric practice. For instance, it is used in the diaphragmatic hernia of newborns since the prosthesis is gradually replaced by one’s own tissues and it is able to “grow” along with the child’s tissues [2]. Full resorption of the prosthesis and its replacement with one’s own tissues must eliminate the potential causes of most mesh-associated complications based on the “no prosthesis, no complications” principle [3].
Unfortunately, the frequency of relapses does not differ in the use of biological and synthetic prostheses [4]; and according to some studies, it even exceeds in operations with biological prostheses [5]. This may be due to the fact that the speed of prosthesis resorption exceeds the speed at which one’s own tissues are replaced, which leads to extreme integration of prosthesis which is not durable [4]. There are two main approaches in solving this issue. The first is to use longer-lasting resorbable synthetic materials for prosthesis production. Such long-lasting resorbable polymers as polycaprolactone [6], polydioxanone [3], trimethylene carbonate [7, 8], and poly-4-hydroxybutyrate [9] are of great interest for the development of surgical prostheses and scaffolds in tissue engineering.
The second approach involves adding biologically active substances to the prosthesis. They ensure the acceleration of the replacement of prosthesis material with one’s own tissues. Technologies for impregnation of recombinant growth factors (such as FGF, BMP, VEGF, etc.) were experimentally and clinically approved. The releasing of the factors stimulates angiogenesis and regeneration in the transplantation field [10, 11]. A prospective method stimulating regeneration is the addition of multipotent stromal cells (MSCs) which are a cellular substrate for the replacement of the transplantation area with the connective tissue. The cells stimulate angiogenesis and regeneration due to paracrine factors and, moreover, must increase the biological compatibility of the prostheses suppressing the reaction to the foreign body [12]. In this study, we researched the effect MSCs have on biological compatibility and the functional properties of reticular prosthesis based on polydioxanone using an experimental model of a full-layered defect of the anterior abdominal wall in rats.
Materials and Methods
Experimental Reticular Prostheses Samples
Samples of reticular prostheses were manufactured based on monofilament polydioxanone (PDO) threads 3-0 (USP). The porosity of the networks was determined by way of low-temperature nitrogen absorption using a surface area SA 3100 (Beckman Coulter) analyzer according to the standard methods. The micro-hardness of the threads was measured on an atomic-powered ICON (Bruker) microscope before and after hydrolytic deconstruction in Na2HPO4·2H2O (рH = 7.32) solution within 30 days.
MSC Culture
The MSCs of a rat’s umbilical cord were used as the cellular component of the tissue engineering construction (TEC). The primary cultures were extracted out of an intervascular stroma by way of explants. The material of the cells from the MSCs was confirmed on the basis of the evaluation of their ability for clonogenic growth on the surface of an unprocessed cultural substratum, expression of specific surface antigens and the ability for a response to the action of differentiation inducers in the adipogenic, osteogenic, and chondrogenic directions [13].
The Creation of TEC Based on a Reticular PDO Carrier and Umbilical Cord MSC
The rat’s umbilical cord MSCs were labelled with a vital RKH26 Red Fluorescent Cell Linker Kit (Sigma-Aldrich) tracer, washed twice with the solution containing 0.9% sodium chloride and resuspended in the culture until the final concentration was 0.5×106 cells/ml. The reticular carrier was transferred into a bioreactor beaker (SPL Lifesciences) and 5 ml of the cellular suspension were added. The settlement of the carrier was conducted via a rotational method using an OS-20 orbital shaker (Biosan) (method involving speed control range of the platform 75 rotations per minute) under standard culture conditions within 24 hours. The effectiveness of the carrier’s settlement with the cells was evaluated using fluorescent microscopy.
Scanning Electron Microscopy
The samples were concentrated in a 2.5% glutaric aldehyde (Sigma-Aldrich) solution, incubated in a 1% osmium solution and dehydrated. After drying in the air within 24 hours, the samples were covered with a layer of gold using a vacuum evaporation installation (Eiko), stuck to the table with Dotite electricity conducting silver glue (Fujikura Kasei) and researched using SEM S-500 (Hitachi).
Experimental Model of the Anterior Abdominal Wall Defect
In carrying out the experiment the researchers were guided by the “Rules for Conducting Research Using Experimental Animals” in accordance with orders of Ministry of Health of the USSR No. 755 and 701 and the “Rules for Laboratory Practice in the Russian Federation”. In the study, 120 reproductive outbred male rats with a mass of 250-320 g were used. The animals were randomly divided into the following groups: rats that had transplanted prosthesis out of PDO (n = 24); those with transplanted prosthesis out of PDO settled with an MSC culture (n = 24); and animals with transplanted prosthesis out of Permacol (5001-100, Covidien) as the control group (n = 24).
The rats were operated under general anesthesia with zoletil (20 mg/kg) and rometar (5 mg/kg). The incision was performed along the medial line of the abdomen of 4 cm length. Full-layered defect of rectangular shape of 2 cm length and 1.5 cm width was dissected with scissors (penetrating the abdominal cavity). The incision was done in the middle of the anterior abdominal wall having the white line of the abdomen as the orienting point. Then a prosthesis was sutured to the edges of the defect leaving the tissues of the anterior abdominal wall over its edges. The wound was sutured and sterilized with an antiseptic. Postoperative anesthesia was given once with an intermuscular Baralgin M injection (10 mg/kg).
The animals were taken out of the experiment on days 3, 10, 30, 60, and 180 after the operation by way of ether anesthesia overdosage. Extracted tissues from each experimental animal were split into three parts and placed into: 1) 10% buffered formalin solution for preparing paraffin sections; 2) liquid nitrogen for preparing cryosections and conducting Western blots; 3) 70° ethyl spirits for conducting biomechanical tests.
Transplant Area: Macroscopic Research
A regular clinical survey was conducted of operation wounds of the animals. In revealing wound disruption, deformations in the abdominal wall, or eventration of prosthesis or organs, the animal was excluded out of the experiment. After euthanasia and opening the abdominal cavity, the transplantation area was thoroughly studied in order to reveal the formation of hematomas, seromas, abscesses, and adesions. The adhesion process was evaluated on the basis of scoring system [14].
Tensiometric Research
The universal electromechanical testing machine Scg-1kNa (Shimadzu) was used for tensiometry. The samples were placed into forceps, establishing a 10 mm distance between the jaws. The test was conducted with the speed of 5 mm/min. The limit of the sample’s stength was tested.
Histologic Study
After the standard histologic diagnosis, the material was poured into paraffin, sections of 5-7 µm width were prepared and stained with hematoxylin and eosin. The morphometric study was conducted using Leica DM 2500 microscope and Image Scope M (Leica Biosystems) software on microphotographs of preparations at magnification of ×400 and not less than 100 fields of view.
Immunohistochemical Study
The cryosections were stained with anti-CD68, macrophage specific (ab125212, Abcam), anti-CD206, M2 activated macrophage-specific (sc-34577, Santa Cruz Biotechnology), collagen I and III types (Imtec), anti-αSMA, smooth muscle cell actin-specific (ab5694, Abcam), von Willebrand factor, VWF, endothelial cell-specific (ab6994, Abcam), anti-Ki67, proliferation marker (ab15580, Abcam), and Troponin I antibody, marker for skeletal muscles (sc-15368, Santa Cruz Biotechnology) according to the producer’s protocols. The morphometric study was conducted using Leica DM 4000 B microscope and LAS AF v.3.1.0 build 8587 software (Leica Microsystems) on microphotographs of preparations at magnification of ×400. Relations of specifically stained cells in the transplantation area were calculated for a minimum of 100 fields of view per individual.
Statistical Analysis
The data were presented as the mean±standard deviation. For multiple comparisons, one-way dispersion analysis was used (ANOVA) for groups of normally distributed values and on ranks dispersion analysis was applied (ANOVA) for distributions differing from normal; p values <0.05 were considered significantly different. The data were analyzed using the Sigma Stat 3.5 (Systat Software) program.
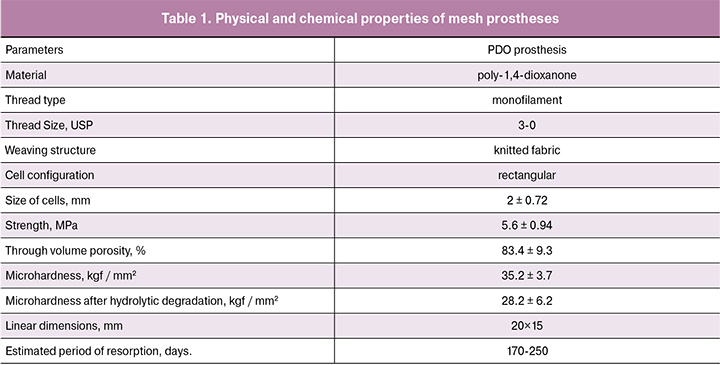
Results and Discussion
Experimental Samples of Reticular Prostheses
The appearance of the experimental samples is presented in Figure 1A. The characteristics of the samples are shown in Table 1. We demonstrated the lack of cytotoxicity of polydioxanone and reticular prostheses based on it in the previous study [15]. In the statistical settlement RKN26-labelled cells were equally stuck to the surface of the prosthesis material providing confluence of 86 ±12.3% of the material’s surface (Fig. 1B).
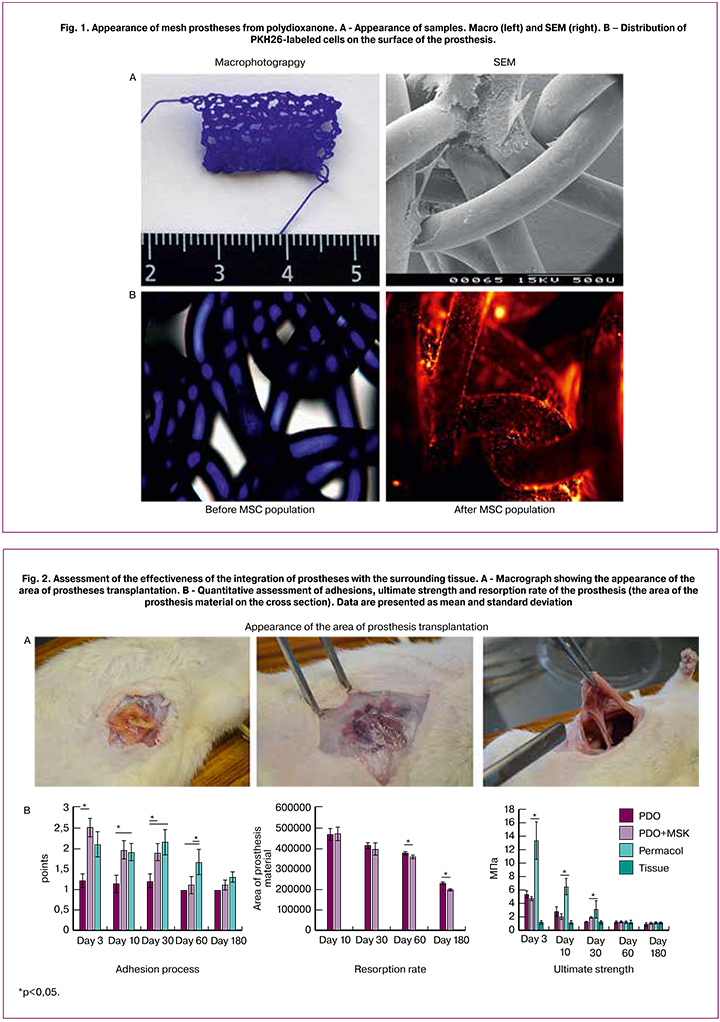
Macroscopic Study
All the animals that underwent the surgery had a satisfactory condition and 100% of the animals survived in the early and later time periods following the operation. No animals showed the signs of the formation of seromas or abscesses. At earlier stages, in the transplant area, one could observe, even in the macroscopic study, the growth of newly formed blood vessels, sometimes hematomas. In the PDO and PDO-MSCs groups, the growth of network cells of one’s own connective tissue was observed (Fig. 2A). The same type of integration was observed in the Permacol group, but only on day 30, during the earlier period the prosthesis integrated with surrounding tissues only in the sutures area (Fig. 2A).
The formation of adhesions of different intensity was observed in all groups and during all periods of the study (Fig. 2A and 2B). In some animals greater omentum as well as the loops of the small and large intestines was involved in the adhesion process. A more evident adhesion process was observed during the PDO+MSC transplantation but only before day 30. Thereafter, on day 60 there were less adhesions in the groups with reticular prostheses than in the Permacol group; on day 180 no differences were revealed between the groups (Fig. 2B). In the Permacol group, there were more adhesions, but it is worth noting that the contact area of the material with the abdominal cavity tissues was significantly larger since Permacol presents a plate with perforations. In the PDO+MSC group, high intensity of adhesion process was due to settled cells which facilitated the adhesion of the tissues to the graft. For this reason, the developed prosthesis should be placed between the muscular and fascial sublayers rather than intraperitoneally.
Determining the Biomechanical Properties of Prostheses after the Transplantation
To study the biomechanical properties of the native tissue we conducted a tensiometric study of the full-layered incised section of the anterior abdominal wall of the rat. When stretching the anterior abdominal wall of the rat, the strength limit was equal to 1.24 ± 0.26 MPa. While stretching the tissues in the transplantation area, the strength limit significantly changed with the resorption of the prosthesis material (Fig. 2B). The maximum limit of strength was noted in Permacol transplantation on days 3 and 10. On day 30, this parameter became equal in all groups and did not differ from one of the native tissues. On days 60 and 180 after the transplantation, this parameter did not change in any of the groups. Therefore, it can be asserted that the balance between the resorption speed of the prosthesis material and the speed of its replacement with one’s own connective tissue provided the biomechanical properties of the tissues in the transplantation area, compatible with the native tissues. However, in a number of clinical cases, it is more effective and safer to apply non-resorbable stronger prostheses; for instance, in severe forms of dysplasia of the connective tissue when the biomechanical properties of one’s own connective tissue facilitate the formation of the hernia.
Speed of Prosthesis Resorption
The speed of prosthesis resorption was evaluated according to the dynamics of change in the area of prosthesis structural elements. In the groups with transplanted PDO prostheses, the area of the transversely cut thick monofilament thread was measured. Both types of prosthesis were equally subjected to resorption and by day 180 day they decreased by 52.3 ± 8.1% and 58.1 ± 9.7%; differences could not be verified (Fig. 2B). Thus, it may be assumed that adding MSC culture into prosthesis components does not accelerate and does not decelerate resorption of prosthesis material.
Study of the Speed of Prosthesis Replacement with One’s Own Tissues
During all periods, the transplantation area was presented as loose fibrous connective tissue of varying degree of maturity with subsequent replacement with dense connective tissue (Fig. 3A). There were no significant qualitative differences in cellular components and structure of the extracellular matrix in various study groups. A connective tissue capsule formed around the prosthesis material. No evident inflammatory infiltration was observed; however, the infiltrate persisted mainly in the contact area with the prosthesis material and was presented primarily with macrophages and single giant cells of foreign bodies and Pirogov-Langhans cells (Fig. 4D). The attention was attracted by a more evident infiltration with inflamed cells, primarily with neutrophils, in the Permacol group; it persisted within all periods of observation. On day 180 after the transplantation, there were the remaining fragments of prosthesis materials, surrounded by dense connective tissue, which unimpededly, without any limit, transferred into the tissue surrounding the transplant. During these periods of time no signs of granulomatous inflammation were identified. At the later observation time intervals, among the newly formed connective tissue one could identify cells positively stained by Troponin I antibodies, (marker for skeletal muscles) (Fig. 3B). Meanwhile, such accumulations formed not in the edge zone of the transplant, but in its thickness, i.e. de novo, possibly on account of migrating myosatellite cells.

The indirect indicator of the speed of replacement with one’s own tissues can be the speed of the formation and remodeling of collagen fibers, as the primary structural elements of the extracellular matrix that define the biomechanical strength characteristics of the newly formed tissue. The remodeling and maturation of the fiber matrix can be evaluated as the ratio of I and III type collagens: the more type I collagen forms thicker and firmer bundles of collagen fibers, the more mature the extracellular matrix is. Collagen type 1 synthesis was noted already on day 3; meanwhile, on day 10 it was distinctly revealed in the components of the fibrous capsule and the surrounding connective tissue (Fig. 3B). The thickness of the collagen fibers and bundles of them grew during the maturation of connective tissue.
The Fate of the Transplanted MSCs in the Prosthesis Components
To study the survival rate, localization, and direction of the differentiation of the transplanted cells before injection, the MSC culture was labelled with the membrane fluorescent stain RKN26. The transplanted cells were identified at all stages of observation, but their number significantly decreased in dynamics, and on day 180 only single cells could be identified (Fig. 4A). Meanwhile, the cells were always located near the surface of the prosthesis material, featured fibroblast-like morphology and did not look damaged. Furthermore, the evidence of their viability was their migration to minor distance from the prosthesis surface. The decrease in the number of labelled cells was due to several reasons: their death and elimination by the host cells and discoloring of the label.
To evaluate the differentiation direction of the transplanted cells, histologic preparations (cryosections) were stained with antibodies for the von Willebrand factor (VWF, marker of endothelial cells) and anti-αSMA, smooth muscle cell actin-specific (Fig. 4B). The transplanted labelled cells showed differentiation neither in the blood vessel cells, in the endothelia of the intima, nor the smooth myocytes of the middle wall. The transplanted cells might be featured with differentiation in the fibroblasts and they synthesized components of the intercellular substance. However, proliferating labelled cells stained positively with Ki67 were not identified during the study (Fig. 4B).

To evaluate the elimination of the transplanted allogenic cells with its own macrophages, the cryosections with labelled cells were stained with anti-CD68. RKN26+ cells localized mainly in the capsule around the prosthesis material were identified during all periods of observation. Meanwhile, part of the labelled cells featuring the same localization was positively stained with AT for the marker of macrophages CD68 (Fig. 4B): this part increased during the later periods of the study. This testifies to active elimination of living transplanted cells or the phagocytosis of membrane structures of labelled cells that died. The number of CD68+ labelled cells increased in the dynamics and reached almost 100% by days 60 and 180. Total elimination of the transplanted allogenic cells agrees with the data we obtained in our other experimental models [16]. In the contemporary view, therapeutic (anti-inflammatory, angiogenic, reparative, etc.) effects of MSCs are implemented on account of the paracrine mechanism. In our experiment, the transplanted cells featured no differentiation in the cells of blood vessels, in the best case they transplanted in fibroblasts, and actively were eliminated by the macrophage system of the recipient; it appears that all identified therapeutic effects were due to paracrine factors.
Reaction to Foreign Bodies
For the characteristic of the evidence of reaction to foreign bodies, we counted the number of macrophages in the general population (CD68+) and M2-macrophages (CD206+). The latters are proregenerative, anti-inflammatory macrophages which are activated in the productive inflammation phase and regulate the synthesis of the extracellular matrix and angiogenesis [17]. The number of CD68+ cells reduced beginning from day 60 of observation, and did not differ among groups. During earlier periods (days 10 and 30) there were significantly more macrophages (in the general population) after the Permacol transplantation.
The M2 macrophages (CD206+cells) were also mainly localized in the capsule around thee prosthesis material, the amount of them was notably less than that of CD68+ cells, but on day 180 their number in the connective tissue surrounding the prosthesis increased in all groups (Fig. 4B). In the PDO and Permacol groups, their amount became verifiably less on day 10, which testifies to the polarization of macrophages in the M2 direction during MSC transplantation ind the prosthesis components and turning the alternative stage of inflammation into the productive one (Fig. 4E).
Thus, it can be asserted that regardless of the nature of prosthesis material, its transplantation causes evident macrophage infiltration, which persists for a long period of time, up to the period of 180 days. This testifies to the continuous chronic inflammation in response to the injection of a foreign body. However, the degree of the intensity and nature of such inflammation were different in the groups, which enables to select prosthesis with minimal proinflammatory properties. The use of MSC as a cellular component of combined prosthesis is worthwhile since it leads to the polarization of macrophages into anti-inflammatory, proangiogenic, and proregenerative cells; it leads to a reduction in the inflammation and acceleration of regeneration.
Another characteristic morphological sign of a reaction to a foreign body is granulomatous inflammation or the formation of a foreign body granuloma. In the case of implantation of foreign bodies large in size (in terms of the size of cells) and their undetermined antigenic properties (the lack of specific receptors on the surface of immunocompetent cells), there is an activation of in-born immunity cells, primarily macrophages. The latters actively migrate to the foreign body, proliferate, and enter the giant cells of the foreign bodies (polynuclear symplast with a central location of nuclei) or Pirogov-Langhast cells (with a peripheric location of nuclei). These cells, along with mononuclear leukocytes, can accumulate around a foreign body with the formation of non-immune (unspecialized) granules. Furthermore, such granules are encapsulated and replaced by a fibroid tissue, but in case of preserving the foreign body material (non-resorbed prosthesis), such granulomas may be preserved during the recipient’s entire live as the locus of chronic inflammation.
In our experiment, the cells of granulomatous inflammation (as the giant cells of foreign bodies, such as Pirogov-Langhans cells) were revealed in all groups (Fig. 4D). Meanwhile, the cells did not form granulomas, but were rather diffused, primarily located in the components of the forming fibrous capsule, but also they could be revealed some distance away from the prosthesis material as well. The number of such cells was among groups (Fig. 4E). The granulomatous inflammation in the area of the transplantation was more pronounced in the Permacol group on days 30 and 60. On day 30, the number of polynuclear macrophages was reliably lower than that in the PDO + MSC group.
Angiogenesis in the Area of the Prosthesis Implantation
The quantity of blood vessels and their volume density were higher in the PDO+MSC group on days 10 and 30 after transplantation (Fig. 5). It can be asserted that the type of surgical prosthesis which includes MSC culture provides more intensive formation of blood vessels in the transplantation area. It must provide more intensive replacement with one’s own tissues and remodeling of the extracellular matrix. It appears that the angiogenic effect of allogenic MSC is implemented on account of paracrine mechanisms produced by angiogenic factors and polarized by M2 macrophages.

Conclusion
The purpose of the conducted experimental study was the investigation of the safety and effectiveness of the transplantation of the developed combined prosthesis compared with the use only of the reticular PDO construction and with the already registered and widely used, natural fully resorbable surgical prosthesis of Permacol. Thanks to the addition of MSC culture into the components of prosthesis, we managed to reduce significantly the level of the intensity of the inflammatory reaction of the rejection of a foreign body, stimulate angiogenesis, as well as the speed of replacement by the recipient’s own tissues.
References
1. Lak K.L., Goldblatt M.I. Mesh selection in abdominal wall reconstruction. Plast. Reconstr. Surg. 2018; 142(3,Suppl.: Current concepts in abdominal wall reconstruction): 99S-106S.
2. Filisetti C., Costanzo S., Marinoni F., Vella C., Klercy C., Riccipetitoni G. Effectiveness and properties of the biological prosthesis Permacol™ in pediatric surgery: A large single center experience. Ann. Med. Surg. (Lond.). 2016; 7: 48-54.
3. Fatkhudinov T., Tsedik L., Arutyunyan I., Lokhonina A., Makarov A., Korshunov A., Elchaninov A., Kananykhina E., Vasyukova O., Usman N., Uvarova E., Chuprynin V., Eremina I., Degtyarev D., Sukhikh G. Evaluation of resorbable polydioxanone and polyglycolic acid meshes in a rat model of ventral hernia repair. J. Biomed. Mater. Res. B Appl. Biomater. 2018; Aug. 9.
4. Darehzereshki A., Goldfarb M., Zehetner J., Moazzez A., Lipham J.C., Mason R.J., Katkhouda N. Biologic versus nonbiologic mesh in ventral hernia repair: a systematic review and meta-analysis. World J. Surg. 2014; 38(1): 40-50.
5. Majumder A., Winder J.S., Wen Y., Pauli E.M., Belyansky I., Novitsky Y.W. Comparative analysis of biologic versus synthetic mesh outcomes in contaminated hernia repairs. Surgery. 2016; 160(4): 828-38.
6. Liu M., Luo G., Wang Y., He W., Liu T., Zhou D. et al. Optimization and integration of nanosilver on polycaprolactone nanofibrous mesh for bacterial inhibition and wound healing in vitro and in vivo. Int. J. Nanomedicine. 2017; 12: 6827-40.
7. Hallberg H., Lewin R., Elander A., Hansson E. TIGR J. Plast. Surg. Hand Surg. 2018; 52(4): 253-8.
8. Olson M.T., Singhal S., Panchanathan R., Roy S.B., Kang P., Ipsen T. et al. Primary paraesophageal hernia repair with Gore Bio-A tissue reinforcement: long-term outcomes and association of BMI and recurrence. Surg. Endosc. 2018; May 14.
9. Roth J.S., Anthone G.J., Selzer D.J., Poulose B.K., Bittner J.G., Hope W.W., Dunn R.M. Prospective evaluation of poly-4-hydroxybutyrate mesh in CDC class I/high-risk ventral and incisional hernia repair: 18-month follow-up. Surg. Endosc. 2018; 32(4): 1929-36.
10. Kuroda Y., Asada R., So K., Yonezawa A., Nankaku M., Mukai K. et al. A pilot study of regenerative therapy using controlled release of recombinant human fibroblast growth factor for patients with pre-collapse osteonecrosis of the femoral head. Int. Orthop. 2016; 40(8): 1747-54.
11. Zhang Q., Hubenak J., Iyyanki T., Alred E., Turza K.C., Davis G. et al. Engineering vascularized soft tissue flaps in an animal model using human adipose-derived stem cells and VEGF+PLGA/PEG microspheres on a collagen-chitosan scaffold with a flow-through vascular pedicle. Biomaterials. 2015; 73: 198-213.
12. Schon L.C., Gill N., Thorpe M., Davis J., Nadaud J., Kim J. et al. Efficacy of a mesenchymal stem cell loaded surgical mesh for tendon repair in rats. J. Transl. Med. 2014; 12: 110.
13. Арутюнян И.В., Ельчанинов А.В., Фатхудинов Т.Х., Макаров А.В., Кананыхина Е., Большакова Г.Б., Гпинкина В.В., Гольдштейн Д.В., Сухих Г.Т. Элиминация РКН26-меченных ММСК при аллогенной трансплантации. Гены и клетки. 2014; 9(3-1): 45-52.
14. Costa R.G., Lontra M.B., Scalco P., Cavazzola L.T., Gurski R.R. Polylactic acid film versus acellular porcine small intestinal submucosa mesh in peritoneal adhesion formation in rats. Acta Cir. Bras. 2009; 24(2): 128-35.
15. Васюкова О.А., Арутюнян И.В., Цедик Л.В., Коршунов А.А., Ельчанинов А.В., Кананыхина Е.Ю., Лохонина А.В., Макаров А.В., Уварова Е.В., Чупрынин В.Д., Еремина И.З., Фатхудинов Т.Х. Исследование биосовместимости резорбируемых сетчатых протезов для пластики дефектов брюшной стенки и дна малого таза. Акушерство и гинекология. 2016; 12: 96-105.
16. Arutyunyan I., Elchaninov A., Fatkhudinov T., Makarov A., Kananykhina E., Usman N., Bolshakova G., Glinkina V., Goldshtein D., Sukhikh G. Elimination of allogeneic multipotent stromal cells by host macrophages in different models of regeneration. Int. J. Clin. Exp. Pathol. 2015; 8(5): 4469-80.
17. Mills C.D. M1 and M2 macrophages: oracles of health and disease. Crit. Rev. Immunol. 2012; 32(6): 463-88.
Received 07.09.2018
Accepted 21.09.2018
About the Authors
Grinberg, Maria V., assistent of cytology, embryology and histology department, medical faculty, Peoples’ Friendship University of Russia, Moscow, Russia.Adress: 117198, Mikluho-Maklaya st.6, Phone: +7 (495) 434-53-00. E-mail: drmgrinberg@gmail.com. ORCID iD 0000-0002-9159-4232.
Arutyunyan, Irina V., researcher of National medical research center of obstetrics, gynecology and perinatology of Ministry of Healthcare of the Russian Federation,
Moscow, Russia, researcher of Scientific Research Institute of Human Morphology, Moscow, Russia.
Adress: 117997, Russia, Moscow, Akademica Oparina st.4., Phone: 8 (926) 147-44-30. E-mail: labrosta@yandex.ru. ORCID iD 0000-0002-4344-8943.
Tsedik, Larisa V., researcher of State Scientific Institution «Powder Metallurgy Institute», Minsk, Republic of Belarus. Adress: 220071, Minsk, Republic of Belarus,
Platonova st. 41., Phone: +375 (29) 778-75-57. E-mail: tslara@rambler.ru. ORCID iD 0000-0001-8513-1736.
Elchaninov, Andrei V., Ph.D, senior researcher of National medical research center of obstetrics, gynecology and perinatology of Ministry of Healthcare of the Russian Federation, Moscow, Russia, senior researcher of Scientific Research Institute of Human Morphology, Moscow, Russia.
Adress: 117997, Russia, Moscow, Akademica Oparina st.4., Phone: 8 (916) 888-52-92. E-mail: elchandrey@yandex.ru. ORCID iD 0000-0002-2392-4439.
Lokhonina, Anastasia V., junior researcher of National medical research center of obstetrics, gynecology and perinatology of Ministry of Healthcare of the Russian Federation, Moscow, Russia. Adress: 117997, Russia, Moscow, Akademica Oparina st.4., Phone: 8 (910) 431-66-76. E-mail: nastya.serbsky@gmail.com. Orcid ID: 0000-0001-8077-2307.
Makarov, Andrei V., PhD, senior researcher of National medical research center of obstetrics, gynecology and perinatology of Ministry of Healthcare of the Russian Federation, Moscow, Russia, leading researcher of Scientific Research Institute of Human Morphology, Moscow, Russia.
Adress: 117997, Russia, Moscow, Akademica Oparina st.4., Phone: 8 (903) 256-34-04. E-mail: anvitmak@yandex.ru. ORCID iD 0000-0003-2133-2293.
Fatkhudinov, Timur H., Ph.D, chief of National medical research center of obstetrics, gynecology and perinatology of Ministry of Healthcare of the Russian Federation, Moscow, Russia, chief of cytology, embryology and histology department, medical faculty, Peoples’ Friendship University of Russia, Moscow, Russia.
Adress: 117997, Russia, Moscow, Akademica Oparina st.4., Phone: 8 (903) 256-11-57. E-mail: tfat@yandex.ru. ORCID iD 0000-0002-6498-5764.
For citations: Grinberg M.V., Arutyunyan I.V., Tsedik L.V., Makarov A.V., Elchaninov A.V., Lokhonina A.V., Fatkhudinov T.Kh. A tissue-engineered construct based on polydioxanone and multipotent stromal cells for plastic surgery repair of abdominal cavity and pelvic floor defects. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (11): 70-9. (in Russian)
https://dx.doi.org/10.18565/aig.2018.11.70-79



