Effectiveness of intrauterine administration of autologous platelet-rich plasma for the preparation of the "thin" endometrium for the program of defrosted embryo transfer
Purpose. To identify the effectiveness of platelet-rich plasma (PRP) use for preparation of "thin" endometrium for cryopreservation programs. Materials and methods. A prospective study was conducted; the main group included 37 patients with "thin" endometrium, among whom embryo transfer cycles were repeatedly canceled due to insufficient endometrial thickness. Against the background of receiving cyclic hormone therapy (CGT), patients were administered intrauterine PRP on the 8–9, 10–11, and 12–13 days of the menstrual cycle. The control group included 17 patients with "thin" endometrium who received only CGT. The thickness of endometrium was assessed by ultrasound examination of pelvic organs. The endometrial thickness over 7 mm was considered optimal for embryo transfer. Results. After the use of PRP, the endometrial thickness of more than 7 mm was in 18 (48.65%) patients, in the control group – in 8 patients (p=0.92). The average endometrial thickness in the main group on the day of embryo transfer was 7.79 mm, in the control group – 6.89 mm (p=0.02). The embryo transfer cycle was cancelled in 6 patients (16.22%) in the main group and in 13 (76.47%) in the control group (p<0.001). Embryo transfer was performed in 32 (86.49%) patients in the main group and in 4 (23.53%) in the control group. Pregnancy occurred in 16 patients (50%) in the main group and in none of the patients in the control group (p=0.58). Among these, 14 (43.75%) patients in the main group had a clinical pregnancy; 4 (28.57%) patients had spontaneous miscarriage, and in 2 (14.29%) patients live births were documented. Conclusion. With intrauterine administration of PRP in addition to CGT, there is a statistically significant increase in the thickness of the endometrium, the frequency of termination of the embryo transfer cycle decreases; nominally, pregnancy rate is higher in the main group, but the difference is not statistically significant.Dzhincharadze L.G., Abubakirov A.N., Mishieva N.G., Bakuridze E.M., Bystrykh O.A.
Keywords
The success of assisted reproductive technologies (ART) primarily depends on the ability of a genetically healthy embryo to be implanted in the receptive endometrium. Endometrium is one of the main factors that affects the optimal outcomes of programs.
The uterus becomes receptive in the middle of the luteal phase of the menstrual cycle (on day 19–23); this period is known as the «implantation window» [1]. The first steps in the implantation process are attachment of the blastocyst to the epithelial layer of the endometrium, followed by the invasion of the trophoblast between the epithelial cells. According to available data, from half to 2/3 of all implantation failures are associated with insufficient endometrial receptivity [2].
In clinical practice, the following indirect research methods are used to assess the receptivity of the endometrium: measurement of the thickness of the endometrium, assessment of its structure and volume, and Doppler examination of the uterine vessels. Endometrial thickness of 7 mm or less is considered suboptimal and associated with a lower pregnancy rate. Endometrial receptivity disorder also can influence habitual miscarriage. Optimal endometrial thickness is a necessary condition for the onset and development of pregnancy in ART programs [4–8].
However, there is currently no consensus what thickness of endometrium is sufficient for successful implantation. Moreover, it is likely that in cycles with ovarian stimulation and cycles with endometrial preparation in oocyte donation programs, surrogacy, and cryoprotocols, this optimal thickness may be different [9]. In stimulated cycles of standard in vitro fertilization (IVF) programs, endometrial thickness on the day of ovulation trigger introduction in presence of at least one normally developing follicle, is on average 8-12 mm [10].
Shapiro et al. found that the minimum 6 mm endometrium is necessary for successful implantation. [11]. Two studies were conducted using ROC analysis, where it was determined that the threshold value of endometrial thickness associated with successful implantation is equal to 8 mm on the day of ovulation trigger administration [8, 12]. In other studies, the minimum endometrial thickness at the end of the follicular phase, associated with higher pregnancy rate, is 7 mm [6, 13].
According to Kasius et al. the incidence of "thin" endometrium is low, equal to 2.4% [14], but this problem still remains unsolved.
With poor endometrial growth in patients with infertility, the first step is to change the standard scheme for prescribing estradiol drugs. Estrogens contribute to proliferation of endometrium with reduction of spiral arteries and, consequently, of oxygen tension in the functional layer; this, in turn, is supportive for embryo implantation [15].
Many other therapies have been proposed to increase endometrial thickness, including the use of low-dose aspirin, vasodilators, intravaginal administration of vitamin E, L-arginine, and sildenafil citrate, and intrauterine administration of stem and progenitor cells [16]. There is evidence of increased endometrial thickness among postmenopausal women who have taken such antihypertensive medications as beta-blocker drugs – atenolol (Tenormin) [17, 18], however today no studies have been conducted on this drug usage aimed to increase the thickness of the endometrium in ART programs.
In 2011, Gleicher et al. used granulocyte colony-stimulating factor (G-CSF) to treat “thin" endometrium in patients in ART programs [19]; more studies followed to assess effectiveness of this drug to achieve optimal endometrial thickness [20–26]. In most studies, the authors noted an increase in endometrial thickness when prescribing G-CSF, but this did not always lead to an increase in the frequency of pregnancy.
Another new approach of treatment of "thin" endometrium is intrauterine administration of autologous plasma enriched with platelets (PRP) [27]. By activating platelets in PRP, cytokines and growth factors such as vascular endothelial growth factor (VEGF), transforming growth factor (TGF), platelet growth factor (PDGF) and epidermal growth factor (EGF) become bioactive [28].
Apparently, these growth factors are released after platelets’ degranulation and contribute to implementation of the mechanisms necessary for process of tissue regeneration.
Currently, PRP is widely used in various industries, such as orthopedics and ophthalmology, and for improvement of tissue regeneration [29].
In 2014, Chang et al. [30] conducted the first study where this drug was used to increase endometrium thickness in patients in ART programs. The study included 5 patients with "thin" endometrium (<7 mm). In the treatment cycles in addition to the cyclic hormone therapy (CHT) intrauterine administration of PRP in the amount of 0.5–1 ml was performed on the 10th day of taking CHT. If no endometrial growth was noted for 72 hours, the drug was re-administered. The authors reported an increase in endometrium thickness to 7 mm or more and conception in all patients after therapy. Subsequent studies [31, 32] also revealed a positive effect of the use of PRP – an increase in endometrial thickness of endometrium, refractory to standard methods of treatment.
In connection with the above, we decided to conduct our own a study to determine the effectiveness of use of PRP for the preparation of "thin" endometrium in the program of thawed embryos.
Materials and methods
A prospective study was conducted, which was approved by the Ethics Committee of Academician V. I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. The main group included 37 patients with "thin" endometrium, in whom several cycles have been cancelled due to insufficient endometrial thickness. The control group included 17 patients with "thin" endometrium. The criteria for inclusion in the study were: age 20–42 years; regular menstrual cycle (25–34 days); body mass index: 18–30 kg/m2; infertility due to tubal and/or male factor and/or external genital endometriosis; idiopathic infertility; the history of embryo transfer cancellation due to "thin" endometrium; normal uterine cavity confirmed by hysteroscopy; at least 3 vitrified blastocysts: 1) excellent quality; 2) good quality and/or average quality (according to Gardner and Schoolcraft classification, 1999); informed consent to participate in the study.
Patients were excluded from the study if they had a history of cancer, severe external genital endometriosis, intrauterine pathology or congenital malformations of the uterus before surgical treatment. Endometrial thickness was measured using ultrasound machine GE Voluson E8 on the 13–15th day of the menstrual cycle until the appointment of progesterone and on the 20–22nd day of the menstrual cycle, the day of embryo transfer into the uterus. endometrium with a thickness of 7 mm or less was considered “thin”.
All patients in both groups received CHT from the 4th–5th day of the menstrual cycle: estradiol valerate (Bayer Schering Pharma, France) was administered orally 6–12 mg per day, the dose of the drug increased depending on the "response" of the endometrium to therapy. Patients in the main group, in addition to CHT, were administered intrauterine PRP in a volume of 5–7 ml on days 8–9, 10–11, and 12–13 of the menstrual cycle using an insemination catheter (Smiths Medical International Ltd.). In the luteal phase of the cycle, the patients received 400 mg of micronized progesterone (CYNDEA PHARMA, S. L., Spain) in the vagina and 40 mg of didrogesterone (Solvay Pharmaceuticals, the Netherlands) orally. A blood test for the beta subunit of chorionic gonadotropin was performed on the 14th day after embryo transfer. Pelvic ultrasound was performed on the 21st day after embryo transfer. When the fetal egg was detected by ultrasound, the pregnancy was regarded as clinical.
The primary end point of the study was an increase in the endometrial thickness of more than 7 mm on the day of embryo transfer, and the secondary end point was documentation of clinical pregnancy.
Statistical analysis
Statistical analysis was performed in the program STATISTICA (StatSoft Inc.). To describe the constant variables with a normal distribution, the arithmetic mean and standard deviation – M (SD) were used and they were compared by the Student's t-test between groups with a normal distribution of features and equality of variances in the compared groups. To compare the discrete variables, the criterion χ2 was applied; the results are presented as percentages. The p<0.05 value was evaluated as statistically significant. For binary outcomes, the effect size was calculated as the risk ratio (RR) and absolute risk with a 95% confidence interval (CI), and for continuous data, as the difference between the mean values with 95% CI.
The technology of PRP preparation
On the 8th day of the menstrual cycle, autologous blood was collected in the volume of 400.0 ml in the first container of the integrated "Blood bag" system (JMS Singapure) with 63 ml of the anticoagulant CPDA. Next, the blood container was centrifuged in a Becman refrigerated centrifuge for 8 minutes in the 1971g mode at a temperature of + 22°C. After centrifugation with a plasma extractor, the plasma and platelets (upper and middle layer) were moved to the second container. The container with autoerythrocytes was detached, and they were reinfused to the patient. The second container with plasma and platelets was re-centrifuged for 10 minutes with a centrifugal acceleration of 5130g at a temperature of + 22°C. After that, using a plasma extractor, the upper layer – native plasma-was removed to the third container, and the lower layer, concentrated platelets suspended in plasma, was moved to a special container for platelets storage (JMS, Singapure). The container with PTP was transferred to the climatic chamber for mixing platelets of LmB Technologie GmbH (Germany), where it can be stored for 5 days at a temperature of +22–24°C.
Results
The study included 37 patients in the main group and 17 patients in the control group who met the inclusion criteria. The main characteristics of the patients are presented in the table.
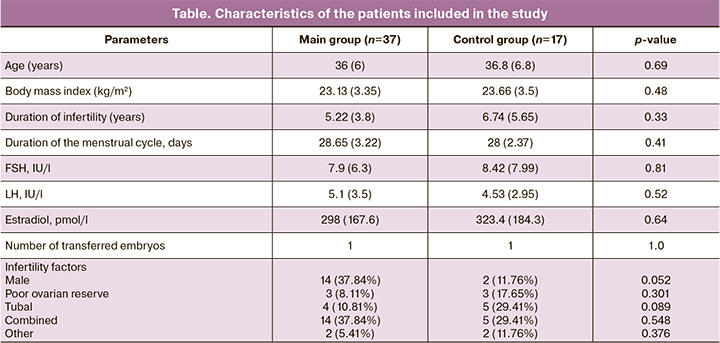
After administration of PRP, the endometrial thickness was more than 7 mm in 18 (48.65%) patients, and in the control group – in 8 (47.06%) patients, the difference was not statistically significant (p=0.92, HR – 1.034 with 95% CI, absolute risk in the PTP group – 0.486, in the control group – 0.471). The average value of the increase in endometrial thickness, compared with previous cycles in the group with PRP was 0.5 mm. The average endometrial thickness in the main group on the day of embryo transfer was 7.79 (1.42) mm, and in the control group – 6.89 (1.15) mm, the differences were statistically significant (p=0.02, the difference in mean values was 0.9 with 95% CI [0.1105, 1.6895]. Cancellation of the embryo transfer cycle was observed in 6 patients (16.22%) in the main group and in 13 (76.47%) patients in the control group, the differences were statistically significant (p<0.001, HR – 0.212 with 95% CI). Embryo transfer was performed in 32 (86.49%) patients in the main group and in 4 (23.53%) patients in the control group. Pregnancy occurred in 16 patients (50%) in the main group and in none of the patients in the control group (p=0.58), the differences were not statistically significant. Among them, clinical pregnancy occured in 14 (43.75%) patients from the main group; spontaneous miscarriage – in 4 (28.57%) patients; live birth – in 2 (14.29%) patients. None of the patients had any side effects after the administration of PTP.
Discussion
We investigated the effectiveness of the use of PTP to increase the thickness of the endometrium in patients with "thin" endometrium. The endometrium of the patients included in our study did not respond to the use of standard methods of treatment of "thin" endometrium, i.e. large doses of estrogen drugs, which led to repeated cancellation of embryo transfer cycles. The combined administration of PRP in combination with CHT made it possible to achieve optimal endometrial thickness in most patients for embryo transfer; the difference in endometrial thickness between the main group and the control group was statistically significant; the difference in the frequency of cancellation of embryo transfer cycles was also statistically significant. Nominally, the pregnancy rate was higher in the main group compared to the control group, but the value was not statistically significant.
Based on our results, it can be assumed that the use of PRP improves the outcomes of ART programs in patients with "thin" endometrium, since this cohort of patients is very complex and there are no effective methods for preparing the "thin" endometrium for embryo transfer. Importantly, PRP is made from autologous blood, which excludes allergic reactions and the transmission of infectious diseases.
Conclusion
With intrauterine administration of PRP in addition to CHT, there is a statistically significant increase in the thickness of the endometrium, and the frequency of cancellation of the embryo transfer cycle decreases. The pregnancy rate is nominally higher in patients treated with PRP, but it is not statistically significant. Further studies on a larger patients cohorts are necessary.
References
- Senturk L.M., Erel C.T. Thin endometrium in assisted reproductive technology. Curr. Opin. Obstet. Gynecol. 2008; 20(3): 221-8. https://dx.doi.org/10.1097/GCO.0b013e328302143c.
- Simon C., Moreno C., Remohi J., Pellicer A. Molecular interactions between embryo and uterus in the adhesion phase of human implantation. Hum. Reprod. 1998; 13(Suppl. 3): 219-32; discussion 233-6. https://dx.doi.org/10.1093/humrep/13.suppl_3.219.
- Edwards R.G. Human implantation: the last barrier in assisted reproduction technologies? Reprod. Biomed. Online. 2006; 13(6): 887-904. https://dx.doi.org/10.1016/s1472-6483(10)61039-5.
- Paria B.C., Song H., Dey S.K. Molecular signaling in uterine receptivity for implantation. Semin. Cell. Dev. Biol. 2000; 11(2): 67-76. https://dx.doi.org/10.1006/scdb.2000.0153.
- Zenke U., Chetkowski R.J. Transfer and uterine factors are the major recipient related determinants of success with donor eggs. Fertil. Steril. 2004; 82(4): 850-6. https://dx.doi.org/10.1016/j.fertnstert.2004.03.057.
- Zhang X., Chen C.H., Confino E., Barnes R., Milad M., Kazer R.R. Increased endometrial thickness is associated with improved treatment outcome for selected patients undergoing in vitro fertilization-embryo transfer. Fertil. Steril. 2005; 83(2): 336-40. https://dx.doi.org/10.1016/j.fertnstert.2004.09.020.
- Esmailzadeh S., Faramarzi M. Endometrial thickness and pregnancy outcome after intrauterine insemination. Fertil. Steril. 2007; 88: 432-7. https://dx.doi.org/10.1016/j.fertnstert.2006.12.010.
- McWilliams G.D., Frattarelli J.L. Changes in measured endometrial thickness predict in vitro fertilization success. Fertil. Steril. 2007; 88(1): 74-81. https://dx.doi.org/10.1016/j.fertnstert.2006.11.089.
- Боярский К.Ю., Гайдуков С.Н., Пальченко Н.А. Современный взгляд на проблему рецептивности и тонкого эндометрия в программах ВРТ (обзор литературы). Проблемы репродукции. 2013; 19(4): 51-60. [Boyarsky K.Yu., Gaidukov S.N., Palchenko N.A. Modern view on the problem of receptivity and thin endometrium in ART programs (literature review). Problems of reproduction. 2013; 19(4): 51-60. (in Russian)].
- Корсак В.С., Каменецкий Б.А., Михайлов А.В. Значимость толщины и ультразвуковой структуры эндометрия в программе ЭКО. Проблемы репродукции. 2001; 7(3): 36-8. [Korsak V.S., Kamenetsky B.A., Mikhailov A.V. The significance of the thickness and ultrasound structure of the endometrium in the IVF program. Problems of reproduction. 2001; 7(3): 36-8. (in Russian)].
- Shapiro H., Cowell C., Casper R.F. The use of vaginal ultrasound for monitoring endometrial preparation in a donor oocyte program. Fertil. Steril. 1993; 59(5): 1055-8. https://dx.doi.org/10.1016/s0015-0282(16)55927-5.
- Basir G.S., O W.S., So W.W., Ng E.H., Ho P.C. Evaluation of cycle-to-cycle variation of endometrial responsiveness using transvaginal sonography in women undergoing assisted reproduction. Ultrasound Obstet. Gynecol. 2002; 19(5): 484-9. https://dx.doi.org/10.1046/j.1469-0705.2002.00685.x.
- Khalifa E., Brzyski R.G., Oehninger S., Acosta A.A., Muasher S.J. Sonographic appearance of the endometrium: the predictive value for the outcome of in vitro fertilization in stimulated cycles. Hum. Reprod. 1992; 7(5): 677-80. https://dx.doi.org/10.1093/oxfordjournals.humrep.a137718.
- Kasius A., Smit J.G., Torrance H.L., Eijkemans M.J., Mol B.W., Opmeer B.C., Broekmans F.J. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum. Reprod. Update. 2014; 20(4): 530-41. https://dx.doi.org/10.1093/humupd/dmu011.
- Yang J.H., Chen M.J., Chen C.D., Chen S.U., Ho H.N., Yang Y.S. Optimal waiting period for subsequent fertility treatment after hysteroscopic various surgeries. Fertil. Steril. 2013; 99(7): 2092-6. https://dx.doi.org/10.1016/j.fertnstert.2013.01.137.
- Sher G., Fisch J.D. Vaginal sildenafil (Viagra): a preliminary report of a novel method to improve uterine artery blood flow and endometrial development in patients undergoing IVF. Hum. Reprod. 2000; 15(4): 806-9. https://dx.doi.org/10.1093/humrep/15.4.806.
- Okman-Kilic T., Kucuk M. The effects of antihypertensive agents on endometrial thickness in asymptomatic, hypertensive, postmenopausal women. Menopause. 2003; 10(4): 362-5. https://dx.doi.org/10.1097/01.GME.0000051508.69832.BA.
- Martinez-Rubio M.P., Alcazar J.L. Ultrasonographic and pathological endometrial findings in asymptomatic postmenopausal women taking antihypertensive drugs. Maturitas. 2003; 46(1): 27-32. https://dx.doi.org/10.1016/s0378-5122(03)00160-9.
- Gleicher N., Vidali A., Barad D.H. Successful treatment of unresponsive thin endometrium. Fertil Steril. 2011; 95(6): 2123. e13-7. https://dx.doi.org/10.1016/j.fertnstert.2011.01.143.
- Gleicher N., Kim A., Michaeli T., Lee H.-J., Shohat-Tal A., Lazzaroni E., Barad D.H. A pilot cohort study of granulocyte colony-stimulating factor in the treatment of unresponsive thin endometrium resistant to standard therapies. Hum. Reprod. 2013; 28(1): 172-7. https://dx.doi.org/10.1093/humrep/des370.
- Kunicki M., Łukaszuk K., Liss J., Skowrońska P., Szczyptańska J. Granulocyte colony stimulating factor treatment of resistant thin endometrium in women with frozen-thawed blastocyst transfer. Syst. Biol. Reprod. Med. 2017; 63(1): 49-57. https://dx.doi.org/10.1080/19396368.2016.1251505.
- Xu B., Zhang Q., Hao J., Xu D., Li Y. Two protocols to treat thin endometrium with granulocyte colony-stimulating factor during frozen embryo transfer cycles. Reprod. Biomed. Online. 2015; 30(4): 349-58. https://dx.doi.org/10.1016/j.rbmo.2014.12.006.
- Tehraninejad E., Tanha F.D., Asadi E., Kamali K., Aziminikoo E., Rezayof E. G-CSF intrauterine for thin endometrium, and pregnancy outcome. J. Family Reprod. Health. 2015; 9(3): 107-12.
- Li Y., Pan P., Chen X., Li L., Li Y., Yang D. Granulocyte colony-stimulating factor administration for infertile women with thin endometrium in frozen embryo transfer program. Reprod. Sci. 2014; 21(3): 381-5. https://dx.doi.org/10.1177/1933719113497286.
- Eftekhar M., Sayadi M., Arabjahvani F. Transvaginal perfusion of G-CSF for infertile women with thin endometrium in frozen ET program: A non--randomized clinical trial. Iran. J. Reprod. Med. 2014; 12(10): 661-6.
- Mishra V.V., Choudhary S., Sharma U., Aggarwal R., Agarwal R., Gandhi K., Goraniya N. Effects of granulocyte colony-stimulating factor (GCSF) on persistent thin endometrium in frozen embryo transfer (FET) cycles. J. Obstet. Gynecol. India. 2016; 66(Suppl. 1): 407-11. https://dx.doi.org/10.1007/s13224-015-0775-9.
- Amable P.R., Carias R.B., Teixeira M.V., da Cruz Pacheco I., Correa do Amaral R.J., Granjeiro J.M., Borojevic R. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res. Ther. 2013; 4: 67. https://dx.doi.org/10.1186/scrt218.
- Lee J.W., Kwon O.H., Kim T.K., Cho Y.K., Choi K.Y., Chung H.Y. et al. Platelet-rich plasma: quantitative assessment of growth factor levels and comparative analysis of activated and inactivated groups. Arch. Plast. Surg. 2013; 40(5): 530-5. https://dx.doi.org/10.5999/aps.2013.40.5.530.
- Dhillon R.S., Schwarz E.M., Maloney M.D. Platelet-rich plasma therapy – future or trend? Arthritis Res. Ther. 2012; 14(4): 219. https://dx.doi.org/10.1186/ar3914.
- Chang Y., Li J., Chen Y., Wei L., Yang X., Shi Y., Liang X. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int. J. Clin. Exp. Med. 2015; 8(1):1286-90.
- Tandulwadkar S.R., Naralkar M.V., Surana A.D., Selvakarthick M., Kharat A.H.Autologous intrauterine platelet-rich plasma instillation for suboptimal endometrium in frozen embryo transfer cycles: A pilot study. J. Hum. Reprod. Sci. 2017; 10(3): 208-12. https://dx.doi.org/10.4103/jhrs.JHRS_28_17.
- Molina A., Sánchez J., Sánchez W., Vielma V. Platelet-rich plasma as an adjuvant in the endometrial preparation of patients with refractory endometrium. JBRA Assist. Reprod. 2018; 22(1): 42-8. https://dx.doi.org/10.5935/1518-0557.20180009.
Received 13.01.2020
Accepted 10.07.2020
About the Authors
Lana G. Dzhincharadze, graduate student of 1st Gynecology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(926)073-77-73. E-mail: lanachka@list.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.Aydar N. Abubakirov, PhD, Head of 1st Gynecology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(495)438-26-22. E-mail: nondoc555@yahoo.com. 117997, Russia, Moscow, Ac. Oparina str., 4.
Nona G. Mishieva, PhD, Senior researcher of 1st Gynecology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(495)438-26-22. E-mail: nondoc555@mail.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Eteri M. Bakuridze, PhD, Head of clinical work, Department of Transfusiology and Extracorporeal Hemocorrection, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(903)103-72-44. E-mail: eteri.bakuridze@mail.ru.
117997, Russia, Moscow, Ac. Oparina str., 4.
Oksana A. Bystrykh, PhD, Head of Department of Transfusion Immunology and the Preparation of Blood Components, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(903)151-10-31. E-mail: O_bystrikh@oparina4.ru.
117997, Russia, Moscow, Ac. Oparina str., 4.
For citation: Dzhincharadze L.G., Abubakirov A.N., Mishieva N.G., Bakuridze E.M., Bystrykh O.A. Effectiveness of intrauterine administration of autologous platelet-rich plasma for the preparation of the "thin" endometrium for the program of defrosted embryo transfer.
Obstetrics and gynecology. 2021; 2: 90-95 (in Russian)
https://dx.doi.org/10.18565/aig.2021.2.90-95



