Use of granulocyte colony-stimulating factor and platelet-rich plasma in patients with “thin” endometrium in frozen embryo transfer programs
Objective. To compare the effectiveness of granulocyte colony-stimulating factor (G-CSF) and autologous platelet-rich plasma (PRP) in patients with “thin” endometrium in frozen embryo transfer programs.Dzhincharadze L.G., Abubakirov A.N., Mishieva N.G., Fedorova T.A., Bakuridze E.M., Bystrykh O.A.
Materials and methods. We conducted a prospective cohort study, which included 58 patients with “thin” endometrium. The group with PRP included 37 patients, G-CSF group included 21 patients. All patients received hormone replacement therapy (HRT). Patients in PRP group in addition to HRT were given an intrauterine injection of autologous PRP on the 8–9th, 10–11th, and 12–13th days of the menstrual cycle; patients in G-CSF group in addition to HRT were given an intrauterine injection of recombinant G-CSF on the 5-6th and 12-13th days of the menstrual cycle. The primary outcome was an increase in endometrial thickness greater than 7 mm on the day of embryo transfer, the secondary outcome was pregnancy rates.
Results. Endometrial thickness greater than 7 mm on the day of embryo transfer in PRP group was observed in 26 (70.27%) patients, in G-CSF group - in 13 (61.9%) patients, the differences were not statistically significant (p=0.515). The average endometrial thickness on the day of embryo transfer in PRP group was 7.79 (1.42) mm, in G-CSF group - 7.21 (1.42) mm, the difference was not statistically significant (p=0.146). In PRP group embryo transfer was performed in 31 (83.78%) patients, and in G-CSF group in 12 (57.14%) patients, the difference was statistically significant (p=0.026). Pregnancy occurred in 16 (51.61%) patients in PRP group and in 4 (33.33%) patients in G-CSF group; the difference was not statistically significant (p=0.282).
Conclusion. In our study we did not find statistically significant differences in either an increase in endometrial thickness or in the pregnancy rates between the two groups. Subsequent studies should be conducted to identify differences in the effectiveness of these treatment methods.
Keywords
Endometrial thickness at the end of the follicular phase and on the day of embryo transfer plays a crucial role in the assessment of possible achieving and maintaining pregnancy. In the clinical practice endometrium thickness of 7 mm is considered to be the minimal thickness of the endometrium by the end of the follicular phase which can be associated with the higher pregnancy rate [1]. Pregnancy rate increases if the endometrium thickness is more than 7 mm on the day of embryo transfer [2].
ROC analysis of two studies determined threshold value of endometrium thickness associated with success- ful implantation in the programs of assisted reproductive technologies (ART). Endometrium thickness should be equal to 8 mm on the day when human chorionic gonad- otropin (hCG) is administered [3, 4]. In spite of the fact that endometrium thickness is assumed to be an indirect assessment method of its receptivity, the researchers in one study did not find any statistically significant differ- ences in receptivity of «thin» and normal endometrium [5]. In their work they studied the expression of estrogen receptors α (ER), progesterone receptors (PR) and leu- kemia inhibitory factor (LIF) in the glandular epithe- lium and stroma of the normal and thin endometrium.
The infertile women showing an inadequate growth of the endometrium undergo changes in the standard scheme of estradiol treatment with the subsequent increase in the dosage of the administered preparations. Estrogen contributes to the endometrial proliferation due to the contraction of spiral arteries and decrease in oxygen tension in the functional layer that may facilitate embryo implantation [6, 7].
A great number of new methods for increasing endo- metrial thickness have been recently suggested including the use of low doses of aspirin, vasodilators, administra- tion of vitamin E intravaginally, L-arginine, sildenafil citrate, physiotherapy, intrauterine administration of stem and progenitor cells [8]. None of the above-men- tioned methods can solve the problem of thin endome- trium and the rate of cancelling embryo transfer cycles remains very high in this condition.
Granulocyte colony-stimulating factor
Granulocyte colony-stimulating factor (G-CSF) is a glycoprotein belonging to the group of colony– stimulating factors. It stimulates the development of granulocyte colonies. G-CSF is an amino acid polypeptide and it is produced by numerous cells including endothelial monocytes and endometrial cells [9]. G-CSF plays a vital role in endometrial decidualization, trophoblast development and placental metabolism. G-CSF contributes to the mobilization, migration, and differentiation of stem cells. It also facilitates endometrial regeneration by stimulating angiogenesis and reducing the apoptotic activity of endometrial cells. Moreover, G-CSF influences embryo implantation and course of pregnancy due to the temporary suppression of the immune response which is associated with its effect on lymphocytes, macrophages and type 2 T-helper cells [10].
Recombinant G-CSF has been used in clinical practice for more than 20 years. It has been used in treating neutropenia that occurs during chemotherapy in patients with cancer [11,12].
For the first time, G-CSF as a preparation was used to increase endometrial thickness by N. Gleicher et al. in 2011 [13]. Intrauterine perfusion of recombinant G-CSF at a dose of 300 micrograms was used in the study. The researchers reported on an increase in endometrial thickness to 7 mm or more in 48 hours after administration of the drug in four patients in ART program. Pregnancy was achieved in all four patients, but ectopic pregnancy was detected in one case which according to the authors was not associated with the administration of G-CSF. In the subsequent numerous studies there were attempts to determine the effectiveness of G-CSF for increasing endometrial thickness in patients with refractory thin endometrium in ART programs [14–21]. The obtained results were controversial: a statistically significant increase in endometrial thickness was frequently revealed by the researchers, however, there was no statistically significant increase in the pregnancy rate and live birth.
Platelet-rich plasma
Intrauterine administration of autologous platelet- rich plasma (PRP) has become a new approach to the treatment of thin endometrium. After activation of platelets in PRP, they become bioactive; cytokines and growth factors such as vascular endothelial growth factor (VEGF), transformative growth factor (TGF), platelet growth factor (PDGF), and epidermal growth factor (EGF) are released [22]. They are presumed to regulate cell migration, proliferation, differentiation and contribute to the accumulation of extracellular matrix [23].
Nowadays, PRP is widely used in various branches of medicine, such as orthopedics and ophthalmology; it can be used in the treatment of wounds for the improvement of tissue regeneration [24].
In 2014 Yajie Changl et al. were the first to suggest using this preparation to increase endometrial thickness in patients in ART programs [25]. The study included five patients with thin endometrium (<7 mm) undergoing ART treatment. In addition to cyclic hormone replacement therapy (HRT), patients were given 0.5–1 ml of intrauterine PRP on the 10th day of cyclic HRT administration. The researchers stated that all patients had an increase in endometrial thickness of more than 7 mm and achieved pregnancy after therapy. This research was followed by various other studies evaluating the effectiveness of this preparation in patients with refractory thin endometrium in ART programs [26– 30]. The results of the study were also controversial, though some authors revealed a statistically significant increase in endometrial thickness and pregnancy rate in comparison with the control group.
In view of the above, the aim of our study was to evaluate the effectiveness of G-CSF and autologous PRP in patients with thin endometrium in frozen embryo transfer programs.
Materials and Methods
The prospective cohort study included 58 patients who in previous cycles of ART had a cancellation of embryo transfer into the uterine cavity due to thin endometrium (<7mm). In this cycle, all patients received cyclic HRT from the 4–5th day of the menstrual cycle: estradiol valerate (Bayer Schering Pharma, France) treatment was started with an injection of 6 mg per day, the dose of the drug increased depending on the response of the endometrium to therapy. The maximum daily dose was 12 mg per day. In the second phase of menstrual cycle, the patients were given injections of 400 mg of micronized progesterone (CYNDEA PHARMA, S. L., Spain) in the vagina and 40 mg of dydrogesterone (Solvay Pharmaceuticals, the Netherlands) orally.
The first group consisted of 37 patients who were administered cyclic HRT and autologous PRP at a dose of 5–7 ml on days 8–9, 10–11 and 12–13 of the menstrual cycle. In the second group, there were 21 patients receiving cyclic HRT and recombinant G-CSF (Filgrastim) at a dose of 300 mcg (Leucostim, Biocad, Russia) on days 5–6 and 12–13 of the menstrual cycle. Both medical preparations were given as intrauterine injections using an insemination catheter (Smiths Medical International Limited).
The primary outcome of the study was an increase in endometrial thickness of more than 7 mm on the day of embryo transfer, and the secondary outcome was pregnancy rate. Endometrial thickness was measured using ultrasonography (GE Voluson E8) on the 13–15th day of the menstrual cycle before administering progestin and on the 20–22nd day of the menstrual cycle, that is on the day of embryo transfer into the uterus. The endometrium was considered to be thin if its thickness was 7 mm or less.
To diagnose pregnancy, patients were measured the amount of hCG present in the blood on the 14th day after embryo transfer. If hCG result was positive, pelvic ultrasound was performed on the 21st day after embryo transfer. Visualization of the gestational sac in the uterine cavity under ultrasound guidance was considered as the clinical pregnancy; if these indicators were absent, the pregnancy was biochemical. In 5–6 weeks after embryo transfer, pelvic ultrasound was performed to determine the fetal heartbeat.
The participants of the study met the following inclusion criteria:
- age 20–42 years;
- regular ovulatory menstrual cycle (25–34 days);
- body mass index: 18–30 kg/m2;
- infertility caused by tubal and/or male factor and/or external genital endometriosis;
- unexplained infertility;
- a history of canceling embryo transfer due to the presence of thin endometrium;
- absence of intrauterine pathology confirmed by hysteroscopy;
- presence of at least three vitrified blastocysts: 1) excellent (≥3AA); 2) good (3, 4, 5, 6, AB and BA) and/or average (3, 4, 5, 6 BB, AC and CA) (according to blastocyst scoring system of Gardner and Schoolcraft (1999));
- absence of individual intolerance to G-CSF and contraindications according to the instructions to this medical preparation;
- informed consent to participate in the study.
The patients were excluded from the study if they had a history of cancer, severe external genital endometriosis or adenomyosis, intrauterine synechiae, polyps, submucous uterine fibroids or congenital malformations of the uterus before surgery.
The study was approved by the Local Ethics Committee of the National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia. It should be noted that G-CSF is an off lable medication and according to the instructions, it is not intended for intrauterine administration.
PRP injection preparation
On the 8th day of the menstrual cycle, autologous blood in the volume of 400.0 ml was collected in the first container of the built-in Blood Bag System (JMS Singapore Ltd.) with 63 ml of anticoagulant CPDA. Then it was followed by centrifugation of the container with blood in the refrigerator centrifuge "Beckman" for 8 minutes in 1971g mode at a temperature of +22°C. After centrifugation with a plasma extractor, plasma and platelets (upper and middle layers) were moved to the second container. The container with autoerythrocytes was disconnected, and they were reinfused to the patient. The repeated centrifugation of the second container with plasma and platelets was performed for 10 minutes with a centrifugal acceleration of 5130 g at a temperature of + 22°C. The upper layer, namely native plasma, was removed to the third container using a plasma extractor; the lower layer, i.e. concentrated platelets suspended in plasma, was moved to a special container for storing platelets (JMS Singapore Ltd.). The container with autologous PRP was labeled, then it was left for one hour for platelet disaggregation, after that it was transferred to a climate chamber for mixing platelets LmB Technologie GmbH (Germany), where it could be stored for five days at a temperature of +22–24°C. Following the described method, 40±5 ml of autologous PRP containing 0.6–0.7 x 1011 platelets were obtained from 400±50 ml of whole blood as a result of two-stage centrifugation. In sterile conditions, autologous PRP was subsequently divided into three doses in platelet storage containers which were given to the treatment room on the days of manipulation. The patients were given 5–7 ml of intrauterine injections according to the above-mentioned scheme using a catheter for insemination.
Statistical analysis
Statistical analysis was performed using STATISTICA software package (StatSoft Inc.). Constant variables that have a normal distribution were described using the arithmetic mean and standard deviation, M (SD). They werecomparedinthegroupswithanormaldistributionand equality of variances by means of Student's t-test. Discrete variables were compared using the Chi-square criterion, their results are presented as percentages. At a significance level of p<0.05 the results were considered statistically significant. The effect size for continuous data was calculated as the difference between the average values with a 95% confidence interval (CI).
Results
The characteristics of the patients included in the study are presented in Table 1.
The average increase in endometrial thickness compared to the previous cycle in RPR group was 0.47 mm (p=0.085) and in G-CSF group it was 0.42 mm (p=0.329), the differences were not statistically significant. Endometrial thickness greater than 7 mm on the day of embryo transfer in PRP group was observed in 26 (70.27%) patients, in G-CSF group – in 13 (61.9%) patients, the differences were not statistically significant (p=0.515). The average endometrial thickness on the day of embryo transfer in PRP group was 7.79 (1.42) mm, in G-CSF group – 7.21 (1.42) mm, the difference was not statistically significant (p = 0.146, the difference in mean values is 0.58 with 95% CI [-0.1972, 1.3572]).
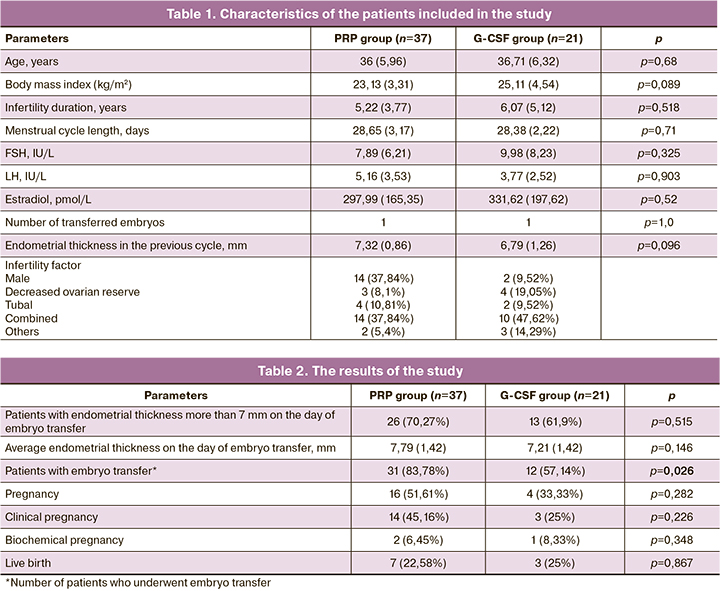
In PRP group embryo transfer was performed in 31 (83.78%) patients, and in G-CSF group in 12 (57.14%) patients, the difference was statistically significant (p=0.026). Pregnancy occurred in 16 (51.61%) patients in PRP group and in 4 (33.33%) patients in G-CSF group; the difference was not statistically significant (p=0.282). Among them, 14 (45.16%) patients achieved clinical pregnancy in PRP group and 3 (25%) patients – in G-CSF group, the difference is not statistically significant (p=0.226). There was not any statistically significant difference in the live birth rate between two groups: 7 births in PRP group (22.58%) and 3 births in G-CSF group (25%) (p=0.867).
It should be noted that our study did not identify any side effects after the use of PRP and G-CSF.
The results of the study are presented in Table 2.
Discussion
According to some studies, endometrial thickness is one of the possible indicators of endometrial receptivity. Nowadays, there are no effective methods to increase its thickness. Therefore, this study was aimed at comparing two new methods of preparing patients with thin endometrium refractory to standard methods of treatment for the transfer cycle of thawed embryo. In our work we chose autologous PRP and recombinant G-CSF. The results of our study showed that the difference in the increase in endometrial thickness after the injection of the above medications compared with the previous cycle where patients took only cyclic HRT was not statistically significant. There were no statistically significant differences in endometrial thickness on the day of embryo transfer between the two groups either. The number of patients whose endometrial thickness was more than 7mm on the day of embryo transfer did not differ between the groups significantly. Only the number of patients who underwent embryo transfer into the uterine cavity was statistically different: it was higher in RTR group, 31 (83.78%) vs. 12 (57.14%), respectively, p=0.026. This may be due to the presence of a better endometrial structure in PRP group, but this indicator was not evaluated in our study. There were no statistically significant differences in pregnancy rate, including clinical pregnancy and the frequency of live birth.
Conclusion
PRP has more advantages due to the fact that it is made from autologous blood which excludes infections and allergic reactions, while G-CSF is an off lable medication and according to the instructions, it is used to treat neutropenia in patients after chemotherapy and is injected subcutaneously or intravenously. However, in our study we did not notice any side effects after intrauterine administration of this drug. As a result of our research, we cannot say whether any medication can be preferred in preparing patients with thin endometrium for embryo transfer. Further randomized trials should be conducted on a larger sample of patients.
References
- Khalifa E., Brzyski R.G., Oehninger S., Acosta A.A., Muasher S.J. Sonographic appearance of the endometrium: the predictive value for the outcome of in vitro fertilization in stimulated cycles. Hum. Reprod. 1992; 7(5): 677-80. https://dx.doi.org/10.1093/oxfordjournals.humrep.a137718.
- Zhang X., Chen C.H., Confino E., Barnes R., Milad M., Kazer R.R.. Increased endometrial thickness is associated with improved treatment outcome for selected patients undergoing in vitro fertilization-embryo transfer. Fertil. Steril. 2005; 83(2): 336-40. https://dx.doi.org/10.1016/j.fertnstert.2004.09.020.
- McWilliams G.D., Frattarelli J.L. Changes in measured endometrial thickness predict in vitro fertilization success. Fertil. Steril 2007; 88: 74-81. https://dx.doi.org/10.1016/j.fertnstert.2006.11.089.
- Basir G.S., O W.S., So W.W., Ng E.H., Ho P.C. Evaluation of cycle-to-cycle variation of endometrial responsiveness using transvaginal sonography in women undergoing assisted reproduction. Ultrasound Obstet. Gynecol. 2002;19(5): 484-89. https://dx.doi.org/10.1046/j.1469-0705.2002.00685.x.
- Куликова Г.В., Абдурахманова Н.Ф., Файзуллина Н.М., Асатурова А.В., Щеголев А.И., Зиганшина М.М., Долгушина Н.В. Рецептивность «тонкого» эндометрия у пациенток в программах вспомогательных репродуктивных технологий. Акушерство и гинекология. 2019; 10: 100-7. [Kulikova G.V., Abdurakhmanova N.F., Fayzullina N.M., Asaturova A.V., Schegolev A.I., Ziganshina M.M., Dolgushina N.V. The receptivity of thin endometrium in patients in the assisted reproductive technologies programs. Obstetrics and Gynecology/Akusherstvo i ginekologiya. 2019; (10): 100-7. (in Russian).] https://dx.doi.org/10.18565/aig.2019.10.100-107.
- Yang J.H., Chen M.J., Che C.D., Chen S.U., Ho H.N., Yang Y.S. Optimal waiting period for subsequent fertility treatment after hysteroscopic various surgeries. Fertil. Steril. 2013; 99(7): 2092-6. https://dx.doi.org/10.1016/j.fertnstert.2013.01.137.
- Casper R.F. It’s time to pay attention to the endometrium. Fertil. Steril. 2011; 96(3): 519-21. https://dx.doi.org/10.1016/j.fertnstert.2011.07.1096.
- Sher G., Fisch J.D. Vaginal sildenafil (Viagra): a preliminary report of a novel method to improve uterine artery blood flow and endometrial development in patients undergoing IVF. Hum. Reprod. 2000; 15(4): 806-9. https://dx.doi.org/10.1093/humrep/15.4.806.
- Barash A., Dekel N., Fieldust S., Segal I., Schechtman E., Granot I. Local injury to the endometrium doubles the incidence of successful pregnancies in patients undergoing in vitro fertilization. Fertil. Steril. 2003; 79(6): 1317-22. https://dx.doi.org/10.1016/s0015-0282(03)00345-5.
- Thomas J., Liu F., Link D.C. Mechanisms of mobilization of hematopoietic progenitors with granulocyte colony-stimulating factor. Curr. Opin. Hematol. 2002; 9(3): 183-9. https://dx.doi.org/10.1097/00062752-200205000-00002.
- Dale D.C., Cottle T.E., Fier C.J., Bolyard A.A., Bonilla M.A., Boxer L.A. et al. Severe chronic neutropenia: treatment and follow-up of patients in the Severe Chronic Neutropenia International Registry. Am. J. Hematol. 2003; 72: 82-93. https://dx.doi.org/10.1002/ajh.10255.
- Gómez Raposo C., Pinto Marín A., González Barón M. Colony-stimulating factors: clinical evidence for treatment and prophylaxis of chemotherapyinduced febrile neutropenia. Clin. Transl. Oncol. 2006; 8(10): 729-34. https://dx.doi.org/10.1007/s12094-006-0119-4.
- Gleicher N., Vidali A., Barad D.H. Successful treatment of unresponsive thin endometrium. Fertil. Steril. 2011; 95(6): 2123. e13-7. https://dx.doi.org/10.1016/j.fertnstert.2011.01.143.
- Gleicher N., Kim A., Michaeli T., Lee H.J., Shohat-Tal A., .Lazzaroni E., Barad D.H.A pilot cohort study of granulocyte colony-stimulating factor in the treatment of unresponsive thin endometrium resistant to standard therapies. Hum. Reprod. 2013; 28(1): 172-7. https://dx.doi.org/10.1093/humrep/des370.
- Kunicki M., Łukaszuk K., Liss J., Skowrońska P., Szczyptańska J. Granulocyte colony stimulating factor treatment of resistant thin endometrium in women with frozen-thawed blastocyst transfer. Syst. Biol. Reprod. Med. 2017; 63(1): 49-57. https://dx.doi.org/10.1080/19396368.2016.1251505.
- Xu B., Zhang Q., Hao J., Xu D., Li Y. Two protocols to treat thin endometrium with granulocyte colony-stimulating factor during frozen embryo transfer cycles. Reprod. Biomed. Online. 2015; 30(4): 349-58. https://dx.doi.org/10.1016/j.rbmo.2014.12.006.
- Barad D.H., Yu Y., Kushnir V.A., Shohat-Tal A., Lazzaroni E., Lee H.J.,Gleicher N. A randomized clinical trial of endometrial perfusion with granulocyte colony-stimulating factor in in vitro fertilization cycles:impact on endometrial thickness and clinical pregnancy rates. Fertil. Steril. 2014; 101(3): 710-5. https://dx.doi.org/10.1016/j.fertnstert.2013.12.016.
- Tehraninejad E., Davari Tanha F., Asadi E., Kamali K., Aziminikoo E., Rezayof E.G-CSF intrauterine for thin endometrium, and pregnancy outcome. J. Fam. Reprod. Health. 2015; 9(3): 107-12.
- Li Y., Pan P., Chen X., Li L., Li Y., Yang D. Granulocyte colony-stimulating factor administration for infertile women with thin endometrium in frozen embryo transfer program. Reprod. Sci. 2014 21(3): 381-5. https://dx.doi.org/10.1177/1933719113497286.
- Maryam Eftekhar, Mozhgan Sayadi, Farideh Arabjahvani. Transvaginal perfusion of G-CSF for infertile women with thin endometrium in frozen ET program: A nonrandomized clinical trial. Iran. J. Reprod. Med. 2014;12(10): 661-6.
- Mishra V., Choudhary S., Sharma U., Aggarwal R., Agarwa R., Gandhi K., Goraniya N. Effects of Granulocyte Colony-Stimulating Factor (GCSF) on persistent thin endometrium in frozen embryo transfer (FET) cycles. J. Obstet. Gynecol. India. 2016; 66(Suppl. 1): S407-11. https://dx.doi.org/10.1007/s13224-015-0775-9.
- Lee J.W., Kwon O.H., Kim T.K., Cho Y.K., Choi K.Y., Chung H.Y. et al. Plateletrich plasma: quantitative assessment of growth factor levels and comparative analysis of activated and inactivated groups. Arch. Plast. Surg. 2013; 40(5): 530-5. https://dx.doi.org/10.5999/aps.2013.40.5.530.
- Grageda E., Lozada J.L., Boyne P.J., Caplanis N., McMillan P.J. Bone formation in the maxillary sinus by using platelet-rich plasma: an experimental study in sheep. J. Oral Implantol. 2005; 31(1): 2-17. https://dx.doi.org/10.1563/0-692.1.
- Dhillon R.S., Schwarz E.M., Maloney M.D. Platelet-rich plasma therapy - future or trend? Arthritis Res. Ther. 2012; 14(4): 219. https://dx.doi.org/10.1186/ar3914.
- Chang Y., Li J., Chen Y., Wei L., Yang X., Shi Y., Liang X. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int. J. Clin. Exp. Med. 2015; 8(1): 1286-90.
- Tandulwadkar S.R., Naralkar M.V., Surana A.D., Selvakarthick M., Kharat A.H.Autologous intrauterine platelet-rich plasma instillation for suboptimal endometrium in frozen embryo transfer cycles: A pilot study. J. Hum. Reprod. Sci. 2017; 10(3): 208-12. https://dx.doi.org/10.4103/jhrs.JHRS_28_17.
- Molina A., Sánchez J., Sánchez W., Vielma V. Platelet-rich plasma as an adjuvant in the endometrial preparation of patients with refractory endometrium. JBRA Assist. Reprod. 2018; 22(1): 42-8. https://dx.doi.org/10.5935/1518-0557.20180009.
- Eftekhar M., Neghab N., Naghshineh E., Khani P. Can autologous platelet rich plasma expand endometrial thickness and improve pregnancy rate during frozen-thawed embryo transfer cycle? A randomized clinical trial. Taiwan. J. Obstet. Gynecol. 2018; 57(6): 810-3. https://dx.doi.org/10.1016/j.tjog.2018.10.007.
- Chang Y., Li J., Wei L.N., Pang J., Chen J., Liang X. Autologous platelet-rich plasma infusion improves clinical pregnancy rate in frozen embryo transfer cycles for women with thin endometrium. Medicine (Baltimore). 2019; 98(3): e14062. https://dx.doi.org/10.1097/MD.0000000000014062.
- Zadehmodarres S., Salehpour S., Saharkhiz N., Nazari L. Treatment of thin endometrium with autologous platelet-rich plasma: a pilot study. JBRA Assist. Reprod. 2017; 21(1): 54-6. https://dx.doi.org/10.5935/1518-0557.20170013.
Received 03.02.2020
Accepted 07.02.2020
About the Authors
Lana G. Dzhincharadze, graduate student of 1st gynecology department, Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.Tel.: +7 (926) 0737773. E-mail: lanachka@list.ru.
117997 Russia, Moscow, Ac. Oparina str. 4.
Aydar N. Abubakirov, PhD, head of 1st gynecology department, Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Tel.: +7 (495) 4382622. E-mail: nondoc555@yahoo.com.
117997 Russia, Moscow, Ac. Oparina str. 4.
Nona G. Mishieva, PhD, Senior researcher of 1st gynecology department, Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Tel.: +7 (495) 4382622. E-mail: nondoc555@mail.ru.
117997 Russia, Moscow, Ac. Oparina str. 4.
Tatyana A. Fedorova, Phd, professor, Head of department of transfusiology and extracorporeal hemocorrection, Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7 (495) 438-71-35. E-mail: t_fyodorova@oparina4.ru.
117997 Russia, Moscow, Ac. Oparina str. 4.
Eteri M. Bakuridze, PhD, head of clinical work of department of transfusiology and extracorporeal hemocorrection, Research Center of Obstetrics,
Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7 (903) 1037244. E-mail: eteri.bakuridze@mail.ru
117997 Russia, Moscow, Ac. Oparina str. 4.
Oksana A. Bystrikh, PhD, head of department of transfusion immunology and the preparation of blood components. Research Center of Obstetrics,
Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7 (903) 1511031. E-mail: O_bystrikh@oparina4.ru
117997 Russia, Moscow, Ac. Oparina str. 4.
For citation: Dzhincharadze L.G., Abubakirov A.N., Mishieva N.G., Fedorova T.A., Bakuridze E.M., Bystrykh O.A. Use of granulocyte colony-stimulating factor and platelet-rich plasma in patients with “thin” endometrium in frozen embryo transfer programs.
Akusherstvo i Ginekologiya/Obstetrics and gynecology. 2020; 4: 90-96. (In Russian).
https://dx.doi.org/10.18565/aig.2020.4.90-96



