First experience of autologous platelet rich plasma injection into the endometrium in patients with uterine factor infertility
Objective. To assess the effectiveness of intraendometrial injecting autologous platelet-rich plasma (PRP) in infertile women with thin endometrium which is refractory to treatment. Materials and methods. The study included 42 patients aged 18-38 years (34.9 (3.6) years) with endometrial thickness ≤7 mm during the implantation window. All patients were injected 40 (5) ml of autologous PRP into the endometrium during the proliferative phase of the menstrual cycle, preceding the cycle of embryo transfer into the uterine cavity. Results. After autologous PRP injection into the endometrium in the following menstrual cycle, there was a statistically significant increase (p<0.01) in endometrial thickness during the implantation window in patients receiving estrogen-containing hormone therapy. Clinical pregnancy occurred in 33.3% of women (n=14) after embryo transfer. No cases of allergic reaction or infectious complications were revealed after the intervention. Conclusion. The study has demonstrated, for the first time, a new approach to the treatment of women with infertility caused by thin endometrium which is refractory to the standard treatment. Further research is needed to provide the opportunity for the women with refractory thin endometrium to conceive without surrogacy.Efendieva Z.N., Apolikhina I.А., Kalinina Е.А., Fedorova Т.А., Bakuridze E.М., Belousov D.М., Fathudinov Т.KH., Sukhikh G.Т.
Keywords
One of the most important factors necessary for successful pregnancy is the presence of a functionally active endometrium of adequate thickness and receptivity during the implantation window. According to the literature, endometrial thickness of 7 mm or less in the second phase of the menstrual cycle is associated with a decrease in the pregnancy rate and the development of its adverse outcomes in ovarian stimulation cycles and in vitro fertilization (IVF) programs [1-3]. In addition, in patients with a history of “thin” endometrium, there is often a cancellation of embryo transfer (ET) in frozenthawed oocyte cycles due to the lack of endometrial growth associated with hormone therapy. It should be noted that standard therapy with estrogens and drugs that improve blood microcirculation is inefficient in some patients, and the only opportunity for them to achieve parenthood is surrogacy. However, this method requires significant financial costs and is not acceptable for most women due to moral and ethical aspects. Thus, the research into еру development of new methods for treating infertile women with thin endometrium, which is refractory to standard therapy, is of particular interest to specialists in the field of reproductive medicine.
Nowadays, one of the most promising methods for treating patients with uterine factor of infertility is the infusion of autologous platelet-rich plasma (PRP) into the uterine cavity [4-6]. Autologous PRP is derived from the patient’s whole blood using a double centrifugation, separation of red blood cells and white blood cells, and obtaining an increased concentration of platelets in plasma [7]. The therapeutic effect of using autologous PRP is based on the release of a significant number of growth factors (platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), transforming growth factor (TGF-β), insulin-like growth factor (IGF - 1), hepatocyte growth factor (HGF), etc.), cyto- and chemokines from platelet granules which triggers mechanisms of biological synthesis in tissues [8]. The use of autologous PRP has been shown to stimulate cell proliferation and regeneration, as well as to promote angiogenesis [9]. According to Chang Y. et al. (2019), intrauterine infusions of autologous PRP in women with thin endometrium before ET into the uterine cavity resulted in a statistically significant increase in endometrial thickness, frequency of implantation, and clinical pregnancy, compared to patients who received only hormone therapy [10].
We assumed that injection of autologous PRP into the endometrium of patients could significantly increase the effectiveness of this treatment method. In the modern literature, we have not found any publications whose authors demonstrated injecting autologous PRP into endometrium of infertile women. In order to improve the method and achieve targeted exposure to growth factors in the tissue, we have developed the method for treating infertile women with thin endometrium by injecting autologous PRP with an endoscopic needle directly into the endometrium under the control of hysteroscopy. The research was carried out on the basis of the Department for Obstetrics, Gynecology, Perinatology and Reproduction in Sechenov First Moscow State Medical University and on the basis of the National Medical Research Centre for Obstetrics, Gynecology and Perinatology, Moscow, Russia.
The purpose of this pilot study was to evaluate the effectiveness and safety of injection of autologous PRP in infertile patients with thin endometrium which is refractory to other treatment methods.
Materials and Methods
The prospective study included 42 patients who presented for the treatment of infertility caused by thin endometrium (endometrial thickness ≤7 mm during the window of implantation) in the period from October 2018 to December 2019; all patients signed an informed consent to participate in the study. The study design was reviewed and approved by the Local Ethics Committees of Sechenov First Moscow State Medical University and National Medical Research Centre for Obstetrics, Gynecology and Perinatology, Moscow, Russia.
All the patients met the following inclusion criteria: age from 18 to 38 years, normal karyotype of the patient and her partner, regular ovulatory menstrual cycle, endometrial thickness ≤7 mm during the intended implantation window. Criteria for non-inclusion in the study were as follows: severe pathospermia in the partner, use of donor gametes, premature ovarian insufficiency, abnormalities of internal genitals, systemic blood diseases and coagulopathy, hemoglobin level less than 100 g/L, platelet level less than 100×109/L, platelet abnormalities and dysfunction, antiplatelet and anticoagulant therapy at the time of inclusion in the study.
The patients included in the study underwent clinical and laboratory examination. During the midluteal phase, transvaginal ultrasound examination was performed to assess the thickness and structure of the endometrium, a Doppler study, as well as an aspiration biopsy of the endometrium for further assessment of its receptivity. Ovulation in this cycle was confirmed by tests sensitive to the peak of luteinizing hormone (LH) in the urine and during ultrasound examination.
On the 5-7th day of the menstrual cycle, the patients included in the study were performed sampling of 400 (50) ml whole blood for preparing autologous PRP. Samples were obtained at the Department of Transfusion Medicine and Storage of Blood Components, National Medical Research Centre for Obstetrics, Gynecology and Perinatology, Moscow, Russia.
Before blood donation, patients had the results of blood tests for RW, HIV, hepatitis, blood group and RH factor, clinical and biochemical blood analysis, hemostasiogram and they also had the consultation of transfusion specialist. In the absence of contraindications, patients were allowed to donate blood. Preparation of autologous PRP included the following steps: 1) blood collection in the volume of 400 (50) ml by means of the plastic primary container “Gemakon 500/300” with 63 ml of anticoagulant CPDA; 2) centrifugation of the container with blood in the refrigerator centrifuge “Beckman” for 8 minutes in 1971g mode at a temperature of +22° C; 3) after centrifugation with a plasma extractor, plasma and platelets (upper and middle layers) were moved to the second container. The container with autoerythrocytes was disconnected, and they were reinfused to the patient; 4) repeated centrifugation of the second container with plasma and platelets for 10 minutes with a centrifugal acceleration of 5130 g at a temperature of + 22° C; 5) the upper layer, namely native plasma, was removed to the third container using a plasma extractor; the lower layer, i.e. concentrated platelets suspended in plasma, was moved to a special container for storing platelets LmB Technologie GmbH (Germany), where it could be stored for 5 days at a temperature of +22-24° C.
Following the described method, 40 (5) ml of autologous PRP (thromboconcentrate) containing 0.6-0.7×1011 platelets were obtained from 400 (50) ml of whole blood as a result of two-stage centrifugation. The container was marked and issued when required on the day of the procedure.
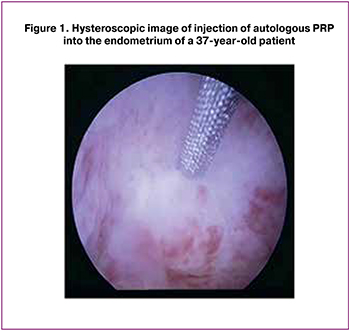 The day after blood donation and receiving PRP (6-8 days of the menstrual cycle), the patients were admitted to a day hospital. After signing the informed consent for medical intervention and participation in the study, patients were injected with 40 (5) ml of autologous PRP into the most atrophic areas of the endometrium using an endoscopic needle “MIT” (manufactured in Russia) with a diameter of 0.6/1.16 mm to a depth of 0.2-0.3 mm under the control of a hysteroscope (Figure 1). The injection was given in an operating room under intravenous anesthesia. The duration of the intervention averaged 15-20 minutes. During one procedure, 6-8 needle injections with autologous PRP were performed on average into the most altered areas of endometrial tissue. In addition to injections into the endometrium, the uterine cavity was infused with 2-3 ml of autologous PRP. After the operation, the patient was monitored for 4 hours in a day hospital room. If the patient’s condition was satisfactory, the patient was discharged home on the same day with recommendations on using barrier contraception during this menstrual cycle. No cases of allergic reactions or infectious complications after the intervention were revealed. ET was not performed during this menstrual cycle.
The day after blood donation and receiving PRP (6-8 days of the menstrual cycle), the patients were admitted to a day hospital. After signing the informed consent for medical intervention and participation in the study, patients were injected with 40 (5) ml of autologous PRP into the most atrophic areas of the endometrium using an endoscopic needle “MIT” (manufactured in Russia) with a diameter of 0.6/1.16 mm to a depth of 0.2-0.3 mm under the control of a hysteroscope (Figure 1). The injection was given in an operating room under intravenous anesthesia. The duration of the intervention averaged 15-20 minutes. During one procedure, 6-8 needle injections with autologous PRP were performed on average into the most altered areas of endometrial tissue. In addition to injections into the endometrium, the uterine cavity was infused with 2-3 ml of autologous PRP. After the operation, the patient was monitored for 4 hours in a day hospital room. If the patient’s condition was satisfactory, the patient was discharged home on the same day with recommendations on using barrier contraception during this menstrual cycle. No cases of allergic reactions or infectious complications after the intervention were revealed. ET was not performed during this menstrual cycle.
In the new menstrual cycle, patients received hormone therapy with estrogens, starting from the 3-4 day. Estrogen preparations were prescribed to patients in the form of tablets of estradiol valerate for oral use and a gel of 0.1% 17-β estradiol for transdermal use. The starting dose was 4 mg of estradiol valerate per day with further adjustment in accordance with the dynamics of endometrial growth. The maximum daily dosage of estrogen preparations was 12 mg. In the middle of the luteal phase of the cycle, the patients underwent a control vaginal ultrasound and a Doppler study to assess the thickness and structure of endometrium, uterine hemodynamic after the intervention. In order to improve the quality of the obtained data, ultrasound was performed to all patients by a single expert before and after the intervention.
When optimal values of endometrial thickness were reached, one good quality defrosted blastocyst-stage embryo was transferred to the uterine cavity and then after ET 600 mg per day of micronized progesterone were administered intravaginally until the results of serum β-chorionic gonadotropin were obtained. The quality of embryos was evaluated according to the morphological criteria of the D. Gardner classification [11]. Before undergoing assisted reproductive technologies (ART), all patients were examined in accordance with Order of Ministry of Health №107n dated 30.08.2012 “On approval of the use of ART, contraindications and limitations to their use” [12].
Biochemical pregnancy was confirmed by an increase in the level of β-chorionic gonadotropin in the blood serum after 14 days, and clinical pregnancy was confirmed by the visualization of the gestational sac in the uterine cavity by ultrasound in three weeks after ET.
Patent request on “Method for the treatment of reproductive-aged patients with thin endometrium using autologous PRP” No. 2020104583 dated 01.02.2020 was submitted.
Statistical analysis was performed using the Statistica 10 software package (USA). All the obtained quantitative parameters were checked for compliance with the normal distribution using the KolmogorovSmirnov criterion. In accordance with the obtained conclusions, quantitative indicators were compared using the Student’s t-test for two independent samples or the nonparametric Mann-Whitney test. To determine the differences in numerical indicators that changed during treatment, a paired Student criterion was used for two dependent samples, in the absence of a normal distribution, we used a nonparametric method – the Wilcoxon T-test.
Numerical parameters that have a normal distribution are presented in the format M (SD), where M is the average value and SD is the standard deviation of the average value. Parameters that have a distribution other than normal are presented in the format Me (Q1; Q3), where Me is the median, and Q1 and Q3 are the upper and lower quartiles. Differences between values at p < 0.05 were considered statistically significant.
Results
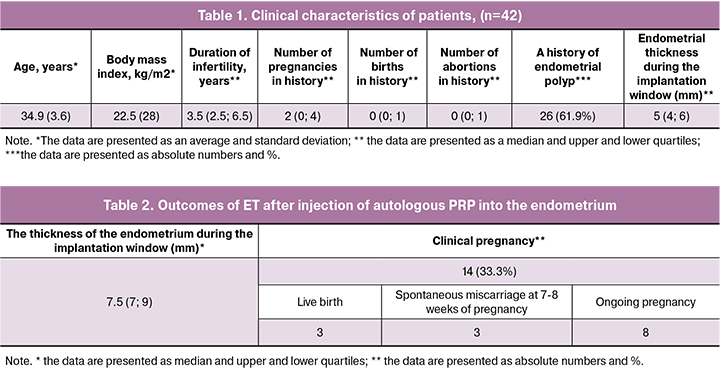
The age of the patients included in the study ranged from 18 to 38 years and averaged 34.9 (3.6) years. The duration of infertility in patients ranged from 1 year to 15 years and averaged 3.5 (2.5-6.5) years. At the same time, primary infertility was found in 26.1% (n = 11) of patients, and secondary infertility was found in 73.9% (n = 31). The study population of patients was homogeneous in terms of body mass index, levels of LH, follicle-stimulating hormone (FSH), estradiol in the blood, and the frequency of general somatic morbidity. More than half of women (71.4% (n = 30)) had a history of ET cancellation in frozen-thawed oocyte cycles due to inadequate growth of the endometrium caused by the therapy with estrogens and drugs improving blood circulation. The thickness of the endometrium during the implantation window in the patients included in the study averaged 5.4 (4; 6) mm during the ultrasound examination. Clinical characteristics and anamnestic data of the patients included in the study are presented in Table 1.
When taking the patients’ history, the women with thin endometrium often mentioned scanty menstruation, 69% (n = 29) of them associated a change in menstrual secretions with a specific intrauterine intervention in the anamnesis, accompanied by scraping the walls of the uterine cavity. After injecting autologous PRP into the endometrium under the control of hysteroscopy, 88.1% of patients (n = 37) noted a subjective improvement in the quality of menstruation, an increase in the volume of menstrual blood in the following menstrual cycle.
In the history of patients taking part in the study, there were the conditions that led to intrauterine interventions with curettage, namely: endometrial polyp - 61.9% (n = 26), curettage of the walls of the uterus for non – developing pregnancy – 52.3% (n = 22), diagnostic curettage of the walls of the uterus – 76.1% (n = 32), abortions - 28.5% (n = 12).
All patients included in the study, in addition to hormonal therapy, previously received additional therapy for improving the structure and achieving optimal indicators of endometrial thickness. In particular, 95.2% (n = 40) of patients underwent physiotherapy, 90.5% (n = 38) of women had leech therapy, 85.7% took sildenafil citrate (n = 36), 28.5% - granulocyte colonystimulating growth factor (n = 12), and 16.6% - human placenta hydrolysate (n = 7).
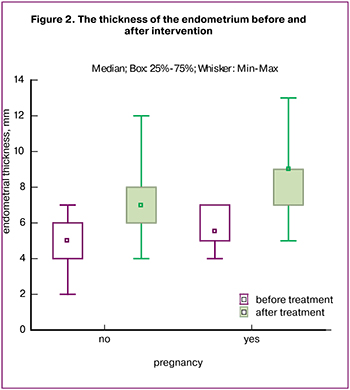 The primary endpoint was endometrial thickness after intraendometrial injection of autologous PRP, and the secondary endpoint was the onset of clinical pregnancy (Table 2).
The primary endpoint was endometrial thickness after intraendometrial injection of autologous PRP, and the secondary endpoint was the onset of clinical pregnancy (Table 2).
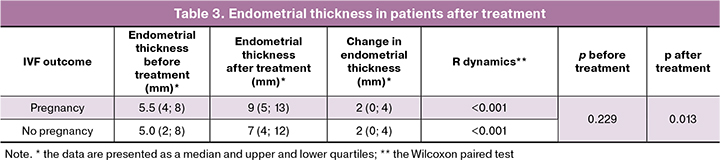
After the intervention, the thickness of the endometrium during the implantation window when the patients were administered hormone therapy increased significantly, compared with the initial values, Me = 7.5 (7; 9) (p < 0.001) (Fig. 2). After ET pregnancy occurred in 33.3% of patients (n = 14). To date, three pregnancies have ended with the live birth, three pregnancies ended with spontaneous miscarriage at 7-8 weeks, and eight pregnancies develop normally in accordance with the term.
The patients were divided into 2 groups: patients with pregnancy (n = 14) and patients who did not become pregnant (n = 28). Despite the fact that when comparing the increase in endometrial thickness after treatment in patients, we did not get a statistically significant difference between the groups (p = 0.12 (Mann-Whitney criterion)), which may be due to small samples, there was a tendency to a greater increase in endometrial thickness in women who became pregnant (Table 3).
Discussion
In this article, we described for the first time our method of injecting autologous PRP to patients with thin endometrium which is refractory to the therapy. Currently, patients with uterine factor infertility belong to a special category, since there is no single effective approach to their treatment. The literature presents data on the influence of endometrial thickness and structure on the probability of pregnancy and live birth in IVF programs. Liu K.E. et al. (2018) when analyzing more than 40,000 ET cycles, have shown that pregnancy rate and live birth decrease with each millimeter for endometrial thickness less than 8 mm for ET in fresh IVF cycles and less than 7 mm for ET in frozen-thawed oocyte cycles, respectively [13].
Among the main causes that lead to endometrial thinning, repeated mechanical damage during intrauterine interventions is particularly highlighted. The data obtained by us are consistent with those presented in the literature [14]. The lack of effect from traditional therapy with estrogen drugs led to the cancellation of ET in frozen-thawed oocyte cycles in patients with thin endometrium due to an inadequate response to the therapy. In addition, the lack of effect from the treatment led to the administration of doses of estrogens that exceed the recommended ones for endometrial growth.
The use of autologous PRP before hormone therapy with estrogens led to a significant increase in the thickness of the endometrium, which allowed ET to be performed in the study group of patients.
Nowadays, the literature presents the results of several studies to assess the effectiveness of intrauterine infusions of autologous PRP in the treatment of women with infertility and thin endometrium [4-6, 15, 16]. The results of the first study on the use of PRP in the treatment of uterine factor infertility were presented in 2015 [17].
In the study of Molina A. et al. (2017) (n = 19), intrauterine infusions of autologous PRP were performed on the 10th and 12th days of hormonal replacement therapy in patients with thin endometrium before ET. According to the presented data, the pregnancy rate was 73.7% (n = 15), and the live birth rate was 26.3% (n = 5) [6]. Other authors presented the results of their study (n = 10), in which intrauterine infusions of 0.5 ml of autologous PRP were performed on days 11-12 and 13-14 of hormonal replacement therapy before ET into the uterine cavity in patients with endometrial thickness <7 mm during the implantation window in previous cycles. Pregnancy occurred in 50% (n = 5) of patients [5]. Kim H. et al. (2019) in their study performed 2-3 procedures of intrauterine infusion of 0.7-1.0 ml of autologous PRP with hormonal replacement therapy in patients with thin endometrium before ET. According to the presented data, ET was performed in 22 patients, 2 of them discontinued participation in the study, the pregnancy rate was 30% (n = 6), the live birth rate was 20% (n = 4). The thickness of the endometrium on average increased by 0.6 mm compared to previous cycles, which was not statistically significant. It should be noted that patients in this study were transferred 2-3 embryos [15].
The limitations of published works on evaluating the effectiveness of autologous PRP on uterine factor infertility include small samples of patients, the lack of a single algorithm for preparing and applying PRP, as well as the lack of information about the concentration of platelets and the presence or absence of white blood cells in the collected plasma, which significantly complicated the comparative analysis of the results presented.
Injection of autologous PRP into the endometrium of patients provides the effect of growth factors directly in the most hypotrophic areas of endometrial tissue. In addition, it is possible to inject the volume several times higher than that of the infusion and repeated procedures for achieving the desired therapeutic effect are not necessary. Moreover, adverse reactions and infectious complications can be minimal due to the fact that PRP is obtained from the patient’s own blood.
In the time of active development of regenerative medicine, research on the possibilities of using PRP-therapy in the reproductive sphere has become particularly relevant. The literature shows that the use of autologous PRP stimulates the growth, migration, and adhesion of endometrial mesenchymal stem cells in vitro experiments [18].
Nowadays, the use of PRP is widespread in many areas of medicine and it can be considered as an additional method in IVF programs [19]; but there is no single standard for preparing PRP. It has been shown that platelet concentration of at least 1,000,000 per microliter in PRP is necessary to obtain a therapeutic effect [20].
It is important to emphasize that the injection of autologous PRP into the endometrium according to the described method was performed by us to eight patients older than 38 years, whose average age was 44.6 (4.8) years; however, they were not included in the statistical analysis. Pregnancy occurred in three patients after ET, two of them ended with the live birth of full-term children, one pregnancy was terminated at 18-19 weeks.
Conclusion
Despite the development of modern science, today there is no single effective therapy for uterine factor infertility, so for women with a refractory thin endometrium, the only opportunity to realize their reproductive potential is surrogacy. This article presents our preliminary data. At the moment, the study continues and the dynamics of indicators of uterine hemodynamic and endometrial receptivity in patients with thin endometrium after therapy are evaluated. The method we developed allows us to target the most altered areas of the endometrium, making it possible to improve its growth and structure, which is probably due to the development of angiogenesis and cell proliferation under the influence of released growth factors directly in the endometrial tissue.
References
- Richter K.S., Bugge K.R., Bromer J.G., Levy M.J. Relationship between endometrial thickness and embryo implantation, based on 1,294 cycles of in vitro fertilization with transfer of two blastocyst-stage embryos. Fertil. Steril. 2007; 87(1): 53-9. https://dx.doi.org/10.1016/j.fertnstert.2006.05.064.
- Kasius A., Smit J.G., Torrance H.L., Eijkemans M.J., Mol B.W., Opmeer B.C. et al. Endometrial thickness and pregnancy rates after IVF: A systematic review and meta-analysis. Hum. Reprod. Update. 2014; 20(4): 530-41. https://dx.doi.org/10.1093/humupd/dmu011.
- Liu K.E., Hartman M., Hartman A., Luo Z.C., Mahutte N. The impact of a thin endometrial lining on fresh and frozen-thaw IVF outcomes: an analysis of over 40 000 embryo transfers. Hum. Reprod. 2018; 33(10): 1883-8. https://dx.doi.org/10.1093/humrep/dey281.
- Tandulwadkar S.R., Naralkar M.V., Surana A.D., Selvakarthick M., Kharat A.H.Autologous intrauterine platelet-rich plasma instillation for suboptimal endometrium in frozen embryo transfer cycles: A pilot study. J. Hum. Reprod. Sci. 2017; 10(3): 208-12. https://dx.doi.org/10.4103/jhrs.JHRS_28_17.
- Zadehmodarres S., Salehpour S., Saharkhiz N., Nazari L. Treatment of thin endometrium with autologous platelet-rich plasma: a pilot study. JBRA Assist. Reprod. 2017; 21(1): 54-6. https://dx.doi.org/10.5935/1518-0557.20170013.
- Molina A., Sánchez J., Sánchez W., Vielma V. Platelet-rich plasma as an adjuvant in the endometrial preparation of patients with refractory endometrium. JBRA Assist. Reprod. 2018; 22(1): 42-8. https://dx.doi.org/10.5935/1518-0557.20180009.
- Amable P.R., Carias R.B.V., Teixeira M.V.T., da Cruz Pacheco Í., do Amaral R.J.F.C., Granjeiro J.M. et al. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res. Ther. 2013; 4(3): 67. https://dx.doi.org/10.1186/scrt218.
- Aghajanova L., Houshdaran S., Balayan S., Manvelyan E., Irwin J.C., Huddleston H.G., Giudice L.C. In vitro evidence that platelet-rich plasma stimulates cellular processes involved in endometrial regeneration. J. Assist. Reprod. Genet. 2018; 35(5): 757-70. https://dx.doi.org/10.1007/s10815-018-1130-8.
- Nazari L., Salehpour S., Hoseini S., Zadehmodarres S., Ajori L. Effects of autologous platelet-rich plasma on implantation and pregnancy in repeated implantation failure: A pilot study. Int. J. Reprod. Biomed. 2016; 14(10): 625-8.
- Chang Y., Li J., Wei L.N., Pang J., Chen J., Liang X. Autologous platelet-rich plasma infusion improves clinical pregnancy rate in frozen embryo transfer cycles for women with thin endometrium. Medicine (Baltimore). 2019; 98(3): e14062. https://dx.doi.org/10.1097/MD.0000000000014062.
- Gardner D.K., Schoolcraft W.B. Culture and transfer of human blastocysts. Curr. Opin. Obstet. Gynecol. 1999; 11(3): 307-11. https://dx.doi.org/10.1097/00001703-199906000-00013.
- Приказ Минздрава России №107н от 30 августа 2012г «О порядке использования вспомогательных репродуктивных технологий, противопоказаниях и ограничениях к их применению». [Prikaz Minzdrava Rossii No.107n on 30 August 2012 «O poryadke ispol’zovaniya vspomogatel’nykh reproduktivnykh tekhnologii, protivopokazaniyakh i ogranicheniyakh k ikh primeneniyu». (in Russian).]
- Liu K.E., Hartman M., Hartman A., Luo Z.C., Mahutte N. The impact of a thin endometrial lining on fresh and frozen-thaw IVF outcomes: an analysis of over 40 000 embryo transfers. Hum. Reprod. 2018; 33(10): 1883-8. https://dx.doi.org/10.1093/humrep/dey281.
- Абдурахманова Н.Ф., Гвоздева А.Д., Зиганшина М.М., Долгушина Н.В. Результаты программ вспомогательных репродуктивных технологий у пациенток с «тонким» эндометрием. Гинекология. 2019; 21(1): 23-7. [Abdurakhmanova N.F., Gvozdeva A.D., Ziganshina M.M., Dolgushina N.V. The results of assisted reproductive technology programs in patients with “thin” endometrium. Ginecology/Ginekologiya. 2019; 21(1): 23-7. (in Russian).] https://dx.doi.org/10.26442/20795696.2019.1.190232.
- Kim H., Shin J.E., Koo H.S., Kwon H., Choi D.H., Kim J.H. Effect of autologous platelet-rich plasma treatment on refractory thin endometrium during the frozen embryo transfer cycle: A pilot study. Front. Endocrinol. (Lausanne). 2019; 10: 61. https://dx.doi.org/10.3389/fendo.2019.00061.
- Nazari L., Salehpour S., Hoseini S., Zadehmodarres Sh., Azargashb E. Effects of autologous platelet-rich plasma on endometrial expansion in patients undergoing frozen-thawed embryo transfer: A double-blind RCT. Int. J. Reprod. Biomed. 2019; 17: 443-8. https://dx.doi.org/10.18502/ijrm.v17i6.4816.
- Chang Y., Li J., Chen Y., Wei L., Yang X., Shi Y., Liang X. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int. J. Clin. Exp. Med. 2015; 8(1): 1286-90.
- Wang X., Liu L., Mou Sh., Zhao H., Fang J., Xiang Y. et al. Investigation of platelet-rich plasma in increasing proliferation and migration of endometrial mesenchymal stem cells and improving pregnancy outcome of patients with thin endometrium. J. Cell. Biochem. 2018; Dec. 3. https://dx.doi.org/10.1002/jcb.28014.
- Urman B., Boza A., Balaban B. Platelet-rich plasma another add-on treatment getting out of hand? How can clinicians preserve the best interest of their patients? Hum. Reprod. 2019; 34(11): 2099-103. https://dx.doi.org/10.1093/humrep/dez190.
- Marx R.E. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001; 10(4): 225-8. https://dx.doi.org/10.1097/00008505-200110000-00002.
Received 05.02.2020
Accepted 07.02.2020
About the Authors
Zulfiya N. Efendieva, postgraduate at the Department of Obstetrics, Gynecology, Perinatology and Reproductology, Institute of Professional Education of the I.M. Sechenov First MSMU of Ministry of Health of Russia (Sechenov University). E-mail: efendievaz@yandex.ru.2-4, Bolshaya Pirogovskaya str., Moscow, 119991, Russian Federation.
Inna A. Apolikhina, MD, professor, Head of Department of Aesthetic Gynecology and Rehabilitation of National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. Professor of the Department of Obstetrics, Gynecology, Perinatology and Reproductology, Institute of Professional Education of the I.M. Sechenov First MSMU of Ministry of Health of Russia (Sechenov University). E-mail: i_apolikhina@oparina4.ru.
4, Oparina str., Moscow, 117997, Russian Federation.
Elena A. Kalinina, MD, professor, Head of Department of Assistive technologies in infertility treatment of National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. E-mail: e_kalinina@oparina4.ru.
4, Oparina str., Moscow, 117997, Russian Federation.
Tatiana A. Fedorova, MD, professor, Head of Transfusional Department of National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. E-mail: t_fyodorova@oparina4.ru.
4, Oparina str., Moscow, 117997, Russian Federation.
Eteri M. Bakuridze, transfusiologist, Head of the Department of Extracorporal methods of treatment and detoxication of National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. Е-mail: e_bakuridze@oparina4.ru.
4, Oparina str., Moscow, 117997, Russian Federation.
Dmitrii M. Belousov, PhD, doctor of the Department of the Functional diagnosis of National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. E-mail: d_belousov@oparina4.ru.
4, Oparina str., Moscow, 117997, Russian Federation.
Timur K, Fathudinov, PhD, MD, leading researcher, Laboratory of regenerative medicine of National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. Deputy Director for Scientific development of FSBI «Research Institute of Human Morphology».
E-mail: tfat@yandex.ru. 4, Oparina str., Moscow, 117997, Russian Federation.
Gennady T. Sukhikh, MD, PhD, professor, Academician of Russian Academy of Sciences, Director of National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. E-mail: gtsukhih@mail.ru.
4, Oparina str., Moscow, 117997, Russian Federation.
For citation: Efendieva Z.N., Apolikhina I.А., Kalinina Е.А., Fedorova Т.А., Bakuridze E.М., Belousov D.М., Fathudinov Т.KH., Sukhikh G.Т. First experience of autologous platelet rich plasma injection into the endometrium in patients with uterine factor infertility. Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 4: 82-89. (In Russian).
https://dx.doi.org/10.18565/aig.2020.4.82-89



