Effectiveness of IVF in patients with normogonadotropic anovulation depending on ovarian aromatase activity
Objective. To investigate the outcomes of IVF/ICSI in patients with anovulatory infertility depending on ovarian aromatase activity. Materials and methods. This prospective cohort study comprised 98 patients with anovulatory infertility. Depending on ovarian aromatase activity, patients were divided into Group I (low activity, n = 60), Group II (normal activity, n = 24), and Group III (high activity, n = 14). The outcomes of IVF/ICSI were analyzed. Results. The proportion of mature oocytes was significantly higher in the Group II than in Group I [89.0% (80–100) vs. 78.5% (69–86), p <0.05] and did not differ from Group III [89.0% (67–100)]. The fertilization rate in the Group II was significantly higher than in Group I [(73% (56–86) vs. 59% (49–75), p = 0.04] and did not differ from Group III [71% (43–80)]. The biochemical and clinical pregnancy rates and live birth rate per embryo transfer in Group II were higher than in Group I [45.5% vs 10.0% (p = 0.005); 40.9% vs 10.0% (p = 0.01), and 31.8% vs 10.0% (p = 0.05), respectively], and did not differ from Group III , where these rates were 35.7%; 35.7% and 28.6%, respectively. Hyperergic response to ovarian stimulation was observed in 55%, 25%, and none of the patients in Group I, Group II, and Group III, respectively. Conclusion. In patients with normogonadotropic anovulation with low ovarian aromatase activity, embryo transfer in fresh cycles is associated with a high risk of ovarian hyperstimulation syndrome and results in a low pregnancy rate.Yakovlev P.P, Kogan I.Yu., Tarasova M.A., Mekina I.D., Niauri D.A. , Gzgzyan A.M.
Keywords
Currently, infertility is estimated to affect about one in seven married couples. Among them, in a quarter of couples the cause of infertility is attributed to ovulatory disorders [1].
Normogonadotropic anovulation, also classified as World Health Organization class 2 (WHO2), is the most frequent cause of endocrine infertility. In this category of patients, anovulation may be caused by both central regulatory disorders and ovarian factors, including changes in ovarian aromatase activity [2]. The aromatase enzyme P450 is responsible for a key step in the biosynthesis of estradiol (E2) in the antral follicle’s granulosa cells and plays an important role in the regulation of folliculogenesis, preparing the endometrium for embryo implantation, both under physiological conditions and controlled ovarian stimulation (COS) with gonadotropin. The activity of ovarian aromatase is one of the key factors for successful oocyte maturation [3]. There is evidence of the significant role of ovarian aromatase in the pathogenesis of anovulation; a decrease in its activity may lead to the insufficient synthesis of E2 triggering positive feedback [4].
Recently, various techniques for estimating ovarian aromatase activity either by obtaining ovarian tissue or granulosa cells or indirectly have been developed. One of them is the determination of the coefficient of activity of ovarian follicle aromatase using the ratio of E2 and anti-Muller hormone (AMH), reflecting the number of antral follicles on day 2 of the menstrual cycle [5]. The strong correlation between aromatase expression in the antral follicle’s granulosa cells obtained by a transvaginal puncture in IVF protocols and empirically calculated ovarian aromatase activity [6] allowed us to use the proposed method to evaluate ovarian aromatase activity without invasive methods. Previous studies have reported an association between ovarian aromatase activity, determined by the presence of a polymorphism in the СУР19 gene, and COS parameters in women undergoing IVF [7, 8].
This study aimed to assess the effect of ovarian aromatase activity on the effectiveness of IVF/ICSI protocols in patients with normogonadotropic anovulation.
Materials and methods
This prospective cohort study comprised 98 patients with anovulatory infertility. Inclusion criteria for study patients were age 20-38 years, infertility, requiring IVF (IVF/ICSI), AMH level of more than 1.0 ng/ml, and having an anovulatory cycle. The exclusion criteria were body mass index less than 18 kg/m2 and more than 35 kg/m2, stage III/IV external genital endometriosis, type 1 diabetes, contraindications for IVF, according to the order of the Ministry of Health of 30.08.2012 N 107n (update of 11.06.2015). Chronic anovulation was diagnosed by the presence of amenorrhea or opsomenorrhea, taking into account ultrasound findings (absence of corpus luteum) in combination with the blood progesterone level less than 16 nmol/l in phase II of the menstrual cycle, and /or the absence of the LH surge in the middle of the menstrual cycle, determined using urine strip test [9]. Before induction of superovulation (on day 2 of the menstrual cycle), all patients were tested for serum levels of E2 and AMH. The activity of ovarian aromatase was determined by calculating the coefficient using the following equation: CA = E2/AMG, where CA is the coefficient of activity of ovarian follicle aromatase; E2 – the basal blood estradiol level on cycle day 2 (nmol/l); AMH - the blood level of anti-Mullerian hormone on cycle day 2 (ng/ml). CA was classified as low, normal, and high in the ranges > 37.8, from 37.8 to 90.7, and > 90.7, respectively [5].
Depending on the activity of ovarian follicle aromatase, all patients were divided into Group I (low activity, n = 60, 61.2%), Group II (normal activity, n = 24, 24.5%), and Group III (high activity, n = 14, 14.3%).
The controlled ovarian hyperstimulation protocol consisted of gonadotropin-releasing hormone (GnRH) antagonists.
The recombinant FSH (r-FSH) (Puregon, Organon, Netherlands, Gonal-F, Merck Serono, Italy) was administered daily starting from day 2-3 of the menstrual cycle. On day 5 of controlled ovarian hyperstimulation, the dose of gonadotropins was adjusted, and the decision on the use of GnRH antagonists (Cetrotide, Merck Serono, Switzerland) was made to prevent a premature LH surge when one or more of the leading follicles reached 14 mm in size. Triggering the final oocyte maturation was decided once three leading follicles reached 17 mm in diameter. A human recombinant human chorionic gonadotropin (hCG) preparation Ovitrelle (Merc Serono, Italy) at a dose of 250µg or a GnRH agonist Diphereline (Ipsen Pharma, France) at a dose of 0.2 mg were used as triggers for ovulation.
Thirty-six hours after injecting the ovulation trigger, the patients underwent transvaginal follicular puncture (TFP) and oocyte aspiration. Fertilization was carried out by IVF or ICSI using the standard technique. Normal fertilization was confirmed by the presence of two pronuclei 17-18 h following IVF or ICSI. The quality of embryos was assessed on day 4 [10]. All patients received the luteal phase support according to a standard protocol using natural vaginal micronized progesterone 600 mg/day from the day of TFP. Triggering final follicular maturation with a GnRH agonist was followed by a more intensive luteal support with estrogens [11]. Transvaginal ultrasound-guided embryo transfer of no more than two embryos was performed on days 4-5 of cultivation using a Cook soft catheter.
The effectiveness of IVF was assessed using the following indicators: the initial dose of gonadotropins; duration of ovarian stimulation with gonadotropins; total dose of gonadotropins; the number of retrieved oocyte-cumulus complexes by TFP; oocyte fertilization rate; the percentage of mature oocytes; the number of good quality embryos on day 4 of development; effective dose of gonadotropins; the number of cryopreserved embryos; the frequency of hyperergic response (more than 16 oocytes); endometrial thickness at the time of embryo transfer. The effective dose was determined by the amount of gonadotropin used to obtain one good quality oocyte/embryo. The fertilization rate was defined as the ratio of diploid zygotes to the total number of oocyte-cumulus complexes. The proportion of mature oocytes was determined by the ratio of oocytes at the MII stage to the total number of oocytes retrieved. Biochemical pregnancy was defined as pregnancy detected by a positive βhCG blood test 14 days after embryo transfer. Clinical pregnancy was confirmed by the presence of a gestational sac with a fetal heartbeat on transvaginal ultrasonography on day 21 after embryo transfer. The pregnancy rate and live birth rate were calculated based on the number of embryos transferred.
Statistical analysis was performed using Statistica 10 (USA) statistical software package. Descriptive statistics for variables showing normal distribution were presented as mean (M) and the standard error of the mean (m). Differences between groups were assessed by univariate analysis of variance. For quantitative variables showing a distribution other than normal, the median (Me), the 25th and 75th percentiles (L-H) were calculated. Differences between two groups were analyzed using the Mann-Whitney U test; to compare more than two independent groups the Kruskal-Wallis ANOVA was used. Statistical analysis of qualitative nominal data was performed using the chi-square test (χ2) for 2×2 contingency tables. Differences between the groups were considered statistically significant at p < 0.05.
Results
Patients with low ovarian aromatase activity constituted the highest proportion (61.2%) of the study cohort, followed by women with normal (24.5%) and high (14.3%) activity. Among patients with low ovarian aromatase activity the leading factor of infertility was PCOS; in the other groups, the most common cause of infertility was a tubal-peritoneal factor. Comparison of the clinical and medical history characteristics showed that the groups were comparable in body mass index and duration of infertility. Patients with ovarian aromatase activity were older than other study participants. The basal level of FSH did not differ between study groups, while the level of AMH was the highest in Group I and the lowest in Group III (Table 1).
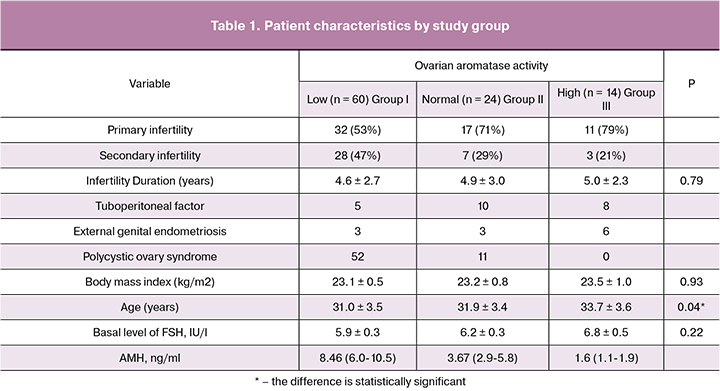
The starting dose of gonadotropins in Group III was significantly higher than in Groups I and II. The total dose of gonadotropins increased with an increase in ovarian aromatase activity and was greatest in Group III. The number of retrieved oocyte-cumulus complexes the greatest in the group with low ovarian aromatase activity and was significantly higher than in Groups II and III. HCG as a trigger for the final oocyte maturation was used in 18%, 42% and most of the protocols in Group I, Group II, and Group III, respectively. Hyperergic response to COS was observed in 55%, 25%, and none of the patients in Group I, Group II, and Group III, respectively (Table 2).
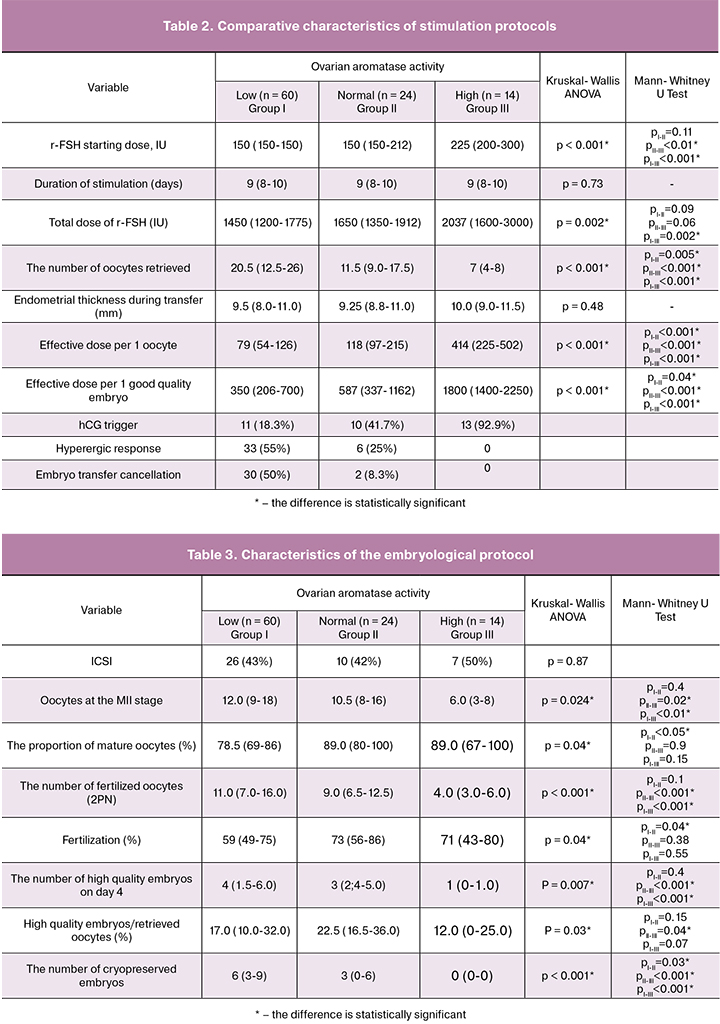
Forty-three patients underwent fertilization with ICSI; ICSI rates did not differ between the study groups. The number of mature oocytes in the groups with low and normal ovarian aromatase activity was significantly greater than in the group with high ovarian aromatase activity. The highest proportion of mature oocytes as a percentage of the total number of retrieved oocyte-cumulus complexes was found in the group with normal and high ovarian aromatase activity. In Group II, the proportion of mature oocytes was significantly higher than among patients in Group I. A similar pattern was observed regarding the number of fertilized oocytes (2PN) and the fertilization rate. Thus, the number of diploid zygotes in the groups with low and normal ovarian aromatase activity was significantly greater than in Group III. However, patients in Groups II and III had the highest fertilization rates, yet the fertilization rate in Group II was significantly higher than in Group I. The highest and lowest number of high-quality embryos and cryopreserved embryos were observed in Group I and III, respectively. The proportion of high-quality embryos as a percentage of the total number of retrieved oocytes was the highest in Group II and significantly exceeded that in Group III (Table 3).
Discussion
The group of patients with low ovarian aromatase activity had the highest embryo transfer cancellation rate (50% of protocols).
In most cases, the cancellation of embryo transfer in this group was associated with a high risk of developing ovarian hyperstimulation syndrome. In total, embryos were transferred in 67.3% of cases. Evaluation of the clinical results of IVF protocols regarding clinical pregnancy rate per embryo transfer in fresh cycles showed that the overall pregnancy rate per embryo transfer was 28.1%, and the live birth rate was 21.2%. There were significant differences in pregnancy rates between the study groups categorized by ovarian aromatase activity. Thus, the lowest (10%) rate of biochemical and clinical pregnancies and live births was observed in the group of patients with low ovarian aromatase activity. These parameters were the highest in the group with normal ovarian aromatase activity and amounted to 45.5%, 40.9%, and 31.8%, respectively. The clinical outcomes in this group were significantly better than the group with low ovarian aromatase activity. In Group III, these figures were not statistically significantly different from Group II, where these rates were 35.7%; 35.7% and 28.6%, respectively.
In Group II, one patient had an ectopic pregnancy, and one underwent termination of pregnancy before 12 weeks’ gestation. One patient in Group III had a spontaneous miscarriage before 12 weeks’ gestation.
The proportion of patients with reduced ovarian aromatase activity was higher among women with anovulatory infertility (61.2%). Given the high rate of low ovarian aromatase activity among patients with normogonadotropic anovulation, a possible role of low activity of this enzyme in the pathogenesis of anovulation may be assumed. The decrease in aromatase P450 activity may be caused by genetic factors, like CYP19 gene polymorphism, and endocrine factors. Thus, an elevated level of AMH, observed in the group with reduced ovarian aromatase activity, can inhibit the catalytic activity of FSH dependent aromatase through inhibition of the CYP19 gene in granulosa cells [12].
The patients in the group with high ovarian aromatase activity were significantly older than women in the groups with low and normal ovarian aromatase activity, which is consistent with the results of other authors [13]. It can be assumed that the age-related increase in aromatase P450 activity compensates for the physiological decline in the ovarian reserve and allows a sufficient level of E2 to be maintained. At the same time, a higher starting and total dose of gonadotropins in the group with a high ovarian aromatase activity indicates a lower sensitivity of the ovaries to the stimulating action of gonadotropins.
The highest proportion of high-quality embryos was in the group with normal ovarian aromatase activity, which may be due to the large number of mature oocytes, higher fertilization rate, and the direct influence of the aromatase P450 activity on the quality of embryos. This assumption was confirmed by the study of Carpintero N.L. et al. in 2014, where the relationship between the intrafollicular level of E2 and the quality of embryos was investigated [14].
The dose of gonadotropins needed to retrieve a good quality oocyte/embryo was the lowest in the group with low ovarian aromatase activity, which is explained by the high incidence of the hyperergic response and, as a consequence, high risk of ovarian hyperstimulation syndrome. In this group, in 81.3% of cases, the trigger was replaced for a GnRH agonist, followed by more intensive luteal phase support. The number of cryopreserved embryos in this group was significantly higher than in the groups with normal and high ovarian aromatase activity. Differences in the number of cryopreserved embryos between Group I and Group II can be explained not only by a higher ovarian reserve in Group I but also by a higher embryo transfer cancellation rate, which reached 50% compared with 8% in Group II. The high risk of ovarian hyperstimulation syndrome in the group with low P450 aromatase activity may be due to low enzyme activity leading to an increase in intrafollicular androgens that can increase the effect of FSH on granulosa cells by improving the FSH receptor expression [15].
Among patients with normal ovarian aromatase activity, a large proportion of mature oocytes and the highest fertilization rate were observed. This observation can be explained by the fact that the intrafollicular concentration of E2 determines the differentiation and maturation of oocytes [16]. Job Lamb J.D. et al. 2010 also reported a positive correlation between the intrafollicular E2 level and the number of mature oocytes and fertilization rate [17]. In earlier studies, Mendoza C. et al. showed that a higher level of E2 in the follicular fluid was associated with the degree of oocyte maturity [18].
The best pregnancy outcomes were achieved in the groups with normal and high ovarian aromatase activity. Similar results were reported by Neal M.S. et al. in 2004, who showed that the aromatase activity of granulosa cells retrieved in the IVF protocol in patients who achieved pregnancy was significantly higher than in those who did not become pregnant after transferring the same number of embryos [19]. Besides, a higher level of E2 in the follicular fluid, a high ratio of E2 to testosterone, and the maximum volume of follicles during puncture are associated with the onset of pregnancy in IVF protocols [14, 20, 21].
Conclusion
Given low effectiveness of IVF protocols and a high risk of ovarian hyperstimulation syndrome in patients with low ovarian aromatase activity, cycle segmentation with subsequent transfer of cryopreserved embryos may be advisable in this patient category. Before introducing such an approach to broad clinical practice, further prospective, controlled, randomized trials are needed.
References
1. Royal College of Obstetricians and Gynaecologists. Fertility: assessment and treatment for people with fertility problems. National Collaborating Centre for Women’s and Children’s Health Commissioned by the National Institute for Health and Clinical Excellence. February 2013.
2. Савина В.А. Овариальная ароматаза р450 при нормогонадотропной недостаточности яичников. Журнал акушерства и женских болезней. 2012; 61(1): 84-9. [Savina VA. Ovarian aromatase р450 and normo gonadotropic ovarian deficiency. Journal of Obstetrics and Women´s Diseases. 2012; 61(1):84-89. (in Russian)]
3. Яковлев П.П. Активность ароматазы Р450 яичников в естественном менструальном цикле и при стимуляции суперовуляции. Журнал акушерства и женских болезней. 2017; 66(5): 46-55. [P.P. Yakovlev. Aromatase P450 activity in the natural menstrual cycle and during controlled ovarian stimulation. Journal of Obstetrics and Women’s Diseases. 2017; 5: 46-55. (in Russian)]
4. Самойлович Я.А., Потин В.В., Тарасова М.А., Ярмолинская М.И., Швед Н.Ю., Николаенков И.П., Ткаченко Н.Н., Тимофеева Е.М. Дефицит овариальной ароматазы как причина нормогонадотропной ановуляции. Российский вестник акушера-гинеколога. 2015; 15(2): 25-30. [Samojlovich JaA, Potin VV, Tarasova MA, Yarmolinskaya M.I., Shved N.Yu., Nikolaenkov I.P. et al. Ovarian aromatase deficiency as the cause of anovulation normogonadotropic. Rossijskij vestnik akushera-ginekologa. 2015;2:25-31. (in Russian).]
5. Потин В.В., Тарасова М.А., Ярмолинская М.И., Мишарина Е.В., Николаенков И.П., Самойловuч Я.А. и др. Способ оценки ароматазной активности антральных фолликулов яичников. Пат. № 2619345; опубл. 15.05.2017, Бюл. № 14. [Potin VV, Tarasova MA, Jarmolinskaja MI, Misharina EV., Nikolaenkov IP., Samojlovich JaA. et al. Method for ovaries antral follicles aromatase activity assessment. Pat. № 2619345 opubl. 15.05.2017, Bjul. № 14. (in Russian).] Уточн., я не нашла этот патент
6. Яковлев П.П., Крылова Ю.С., Мекина И.Д., Полякова В.О., Кветной И.М., Тарасова М.А., Коган И.Ю., Гзгзян А.М., Родичкина В.Р. Активность овариальной ароматазы: методы оценки и клиническое значение в протоколах ЭКО. Журнал акушерства и женских болезней. 2018; 67(2): 61-9. [Yakovlev P.P., Krylova Ju.S. , Mekina I.D., Polyakova V.O., Kvetnoy I. M., Tarasova M.A. et al. Ovarian aromatase activity: evaluation methods and the clinical significance in IVF protocols. Journal of Obstetrics and Women’s Diseases. 2018; 2: 56-62. (in Russian)]
7. Lazaros L., Hatzi E., Xita N., Makrydimas G., Kaponis A., Takenaka A. et al. Aromatase (CYP19) gene variants influence ovarian response to standard gonadotrophin stimulation. J. Assist. Reprod. Genet. 2012; 29(2): 203-9. doi: 10.1007/s10815-011-9673-y.
8. Altmae S., Haller K., Peters M., Saare M., Hovatta O., Stavreus-Evers A. et al. Aromatase gene (CYP19A1) variants, female infertility and ovarian stimulation outcome: a preliminary report. Reprod. Biomed. Online. 2009; 18(5): 651-7. doi: https://doi.org/10.1016/S1472-6483(10)60009-0.
9. Lynch K.E., Mumford S.L., Schliep K.C., Whitcomb B.W., Zarek S.M., Pollack A.Z. et al. Assessment of anovulation in eumenorrheic women: comparison of ovulation detection algorithms. Fertil. Steril. 2014; 102(2): 511-8. e2. doi: 10.1016/j.fertnstert.2014.04.035
10. Tao J., Tamis R., Fink K., Williams B., Nelson-White T., Craig R. The neglected morula/compact stage embryo transfer. Hum. Reprod. 2002; 17(6): 1513-8.
11. Коган И.Ю., Геркулов Д.А. Актуальные научно-практические направления в стратегии гормональной терапии в посттрансферном периоде. Проблемы репродукции. 2016; 22(3): 20-7. [Kogan I.Yu., Gerkulov D.A. Current scientific and practical luteal phase support strategies. Problemy reprodukcii. 2016; 3: 20-27. (in Russian).]
12. Chang H.M., Klausen C., Leung P.C. Antimüllerian hormone inhibits follicle-stimulating hormone-induced adenylyl cyclase activation, aromatase expression, and estradiol production in human granulosa-lutein cells. Fertil. Steril. 2013; 100(2): 585-92. e1. doi: 10.1016/j.fertnstert.2013.04.019.
13. Shaw N.D., Srouji S.S., Welt C.K., Cox K.H., Fox J.H., Adams J.A. et al. Compensatory increase in ovarian aromatase in older regularly cycling women. J. Clin. Endocrinol. Metab. 2015; 100(9): 3539-47. doi: 10.1210/JC.2015-2191.
14. Carpintero N.L., Suárez O.A., Mangas C.C., Varea C.G., Rioja R.G. Follicular steroid hormones as markers of oocyte quality and oocyte development potential. J. Hum. Reprod. Sci. 2014; 7(3): 187-93. doi: 10.4103/0974-1208.142479
15. Luo W., Wiltbank M.C. Distinct regulation by steroids of messenger RNAs for FSHR and CYP19A1 in bovine granulosa cells. Biol. Reprod. 2006; 75(2): 217-25. doi: 10.1095/biolreprod.105.047407
16. Makabe S., Naguro T., Stallone T. Oocyte-follicle cell interactions during ovarian follicle development, as seen by high resolution scanning and transmission electron microscopy in humans. Microsc. Res. Tech. 2006; 69(6): 436-49. doi: 10.1002/jemt.20303
17. Lamb J.D., Zamah A.M., Shen S., McCulloch C., Cedars M.I., Rosen M.P. Follicular fluid steroid hormone levels are associated with fertilization outcome after intracytoplasmic sperm injection. Fertil. Steril. 2010; 94(3): 952-7. doi: 10.1016/j.fertnstert.2009.04.010
18. Mendoza C., Cremades N., Ruiz-Requena E., Martinez F., Ortega E., Bernabeu S. et al. Relationship between fertilization results after intracytoplasmic sperm injection, and intrafollicular steroid, pituitary hormone and cytokine concentrations. Hum. Reprod. 1999; 14(3): 628-35.
19. Neal M.S., Younglai E.V., Holloway A.C., Foster W.G. Aromatase activity in granulosa cells as a predictor of pregnancy potential. International Congress Series: 2004. Elsevier B.V.; 2004: 139-42. doi: 10.1016/j.ics.2004.05.022.
20. Andersen C.Y. Characteristics of human follicular fluid associated with successful conception after in vitro fertilization. J. Clin. Endocrinol. Metab. 1993; 77(5): 1227-34. doi: 10.1210/jcem.77.5.7521343
21. Mendoza C., Ruiz-Requena E., Ortega E., Cremades N., Martinez F., Bernabeu R. et al. Follicular fluid markers of oocyte developmental potential. Hum. Reprod. 2002; 17(4): 1017-22.
Received 02.04.2018
Accepted 20.04.2018
About the Authors
Yakovlev, Pavel P., PhD student, FSBSI “The Research Institute of Obstetrics, Gynecology and Reproductology named after D.O. Ott”.199034, Russia, St. Petersburg, Mendeleevskaya line str. 3. Tel.: +79500008220. E-mail: iakovlevpp@gmail.com.
Kogan, Igor Yu., PhD, AM RAM, Acting Head of the FSBSI “The Research Institute of Obstetrics, Gynecology and Reproductology named after D.O. Ott”.
199034, Russia, St. Petersburg, Mendeleevskaya line str. 3. Tel.: +78123289833. E-mail: ikogan@mail.ru.
Tarasova, Marina A., MD, PhD, professor. FSBSI “The Research Institute of Obstetrics, Gynecology and Reproductology named after D.O. Ott”.
199034, Russia, St. Petersburg, Mendeleevskaya line str. 3. Tel.: +78123289889. E-mail: tarasova@ott.ru.
Mekina, Irina D., Candidate of Biological Sciences, research fellow of Department Reproductive Technologies. The Research Institute of Obstetrics, Gynecology and Reproductology named after D.O. Ott.
199034, Russia, St. Petersburg, Mendeleevskaya line str. 3. Tel.: +78123289822. E-mail: iagmail@ott.ru
Niauri, Dariko, A., MD, Head of the Department of Obstetrics, Gynecology and Reproduction of the Faculty of Medicine, St. Petersburg State University.
199034, Russia, St. Petersburg, Univercity Coast str. 7-9. Tel.: +78123289833. E-mail: d.niauri@ mail.ru.
Gzgzyan, Alexander M., MD, Head of Department Reproductive Technologies. The Research Institute of Obstetrics, Gynecology and Reproductology named
after D.O. Ott. 199034, Russia, St. Petersburg, Mendeleevskaya line str. 3. Tel.: +78123289822. E-mail: iagmail@ott.ru.
For citations: Yakovlev P.P, Kogan I.Yu., Tarasova M.A., Mekina I.D., Niauri D.A., Gzgzyan A.M. Effectiveness of IVF in patients with normogonadotropic anovulation depending on ovarian aromatase activity. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (12): 56-62. (in Russian)
https://dx.doi.org/10.18565/aig.2018.12.56-62



