Пациентки, проходящие лечение в программах вспомогательных репродуктивных технологий (ВРТ), очень гетерогенная группа по уровню ответа на овариальную стимуляцию и шансам наступления беременности. Основными маркерами прогноза исхода протокола экстракорпорального оплодотворения (ЭКО) являются возраст и овариальный резерв.
Пациентки до 35 лет с высоким овариальным резервом в целом имеют хорошие шансы наступления беременности [1, 2]. Но эта тенденция несколько омрачается высокими рисками развития синдрома гиперстимуляции яичников, необходимостью криоконсервации всех эмбрионов, что влечет за собой увеличение времени до наступления беременности и родов, а также рост стоимости лечения. В литературе имеются данные, свидетельствующие, что не только доза, но также и тип гонадотропина могут влиять на ответ яичников и риски развития синдрома гиперстимуляции яичников. Несколько ретроспективных исследований показали, что применение менопаузальных гонадотропинов в протоколах ЭКО по сравнению с препаратами рекомбинантного фолликулостимулирующего гормона (ФСГ) имеет ряд преимуществ. Анализ циклов у пациентов с потенциально высоким ответом яичников на стимуляцию показал, что использование менотропинов ассоциировано с меньшим средним количеством полученных ооцитов, значительно меньшим гиперответом яичников (>15 ооцитов), более редкой необходимостью терапии синдрома гиперстимуляции яичников (СГЯ) и увеличением частоты живорождений в циклах с агонистами и антагонистами гонадотропин-рилизинг-гормона (ГнРГ) [3, 4].
У пациенток старшего репродуктивного возраста снижение результативности ЭКО в основном связывают с повышенными рисками хромосомной аномалии эмбриона [5, 6]. С этим связаны снижение частоты имплантации и повышение частоты прерывания беременности в раннем сроке. Однако стоит отметить выявленную значительную гетерогенность в частоте анеуплоидий эмбрионов у пациентов одних возрастных групп. Также в исследованиях отмечена тенденция к снижению частоты прерывания беременности на раннем сроке при использовании менопаузальных гонадотропинов для стимуляции суперовуляции в протоколах ЭКО [7, 8].
Значительная часть общего числа протоколов ЭКО, которые проводятся в России, финансируется за счет средств фонда обязательного медицинского страхования. Важными аспектами в таком случае будут являться сокращение времени до родов здоровым ребенком и оптимизация затрат на лечение. Повышение частоты родов на начатый цикл и снижение частоты осложнений в таком случае являются приоритетными задачами.
Цель исследования: оценить клиническую эффективность и безопасность применения высокоочищенных человеческих менопаузальных гонадотропинов (ВО-ЧМГ) в протоколах ЭКО в существующей клинической практике в протоколах с агонистами и антагонистами ГнРГ в Северо-Западном регионе России.
Материалы и методы
Открытое мультицентровое наблюдательное исследование было проведено на базе 13 центров Северо-Западного региона России в течение 3 лет – с 2017 по 2020 гг. В исследование были включены 1504 пациента в возрасте от 20 до 43 лет, удовлетворяющие показаниям к проведению ЭКО в соответствии с приказом МЗ РФ № 107н. Все пациенты были разделены на 4 группы в зависимости от возраста. Распределение по группам и гормональные показатели указаны в таблице 1.
Всем пациенткам были назначены препараты ВО-ЧМГ для ежедневного введения. Стимуляция суперовуляции проводилась по стандартной схеме с агонистами или антагонистами ГнРГ с применением препарата «Менопур Мультидоза» 1200 МЕ с или без дополнительного применения препарата «Менопур» 75 МЕ. Стартовая доза препарата выбиралась индивидуально в зависимости от овариального резерва и составляла от 150 до 300 МЕ. Антагонист ГнРГ назначали при достижении лидирующим фолликулом диаметра 14 мм. Критерием введения триггера финального созревания фолликулов было достижение 3 фолликулами (при развитии 3 и более фолликулов) или 1 фолликулом (при развитии менее 3 фолликулов) диаметра 17 мм. В качестве триггера использовался рекомбинантный хорионический гонадотропин человека и хорионический гонадотропин человека. В случае гиперответа яичников на стимуляцию (>20 фолликулов более 12 мм в диаметре) в протоколах с антагонистами ГнРГ в качестве триггера использовались агонисты ГнРГ (трипторелина ацетат 0,1). Оплодотворение ооцитов осуществлялось с помощью стандартной процедуры инсеминации ооцитов спермой супруга или с применением ИКСИ (интрацитоплазматическая инъекция сперматозоида в яйцеклетку).
Перенос эмбрионов осуществлялся на 5-й день культивирования. Максимальное количество переносимых эмбрионов не превышало 2. Эффективность протокола оценивали с помощью показателя частоты имплантации (с помощью оценки концентрации β-субъединицы хорионического гонадотропина человека (ХГЧ) в крови на 12–14-й день после переноса эмбрионов), частоты клинической беременности (визуализация плодного яйца в полости матки), частоты родов.
Статистический анализ
Статистическая обработка данных выполнена с помощью электронных таблиц Microsoft Excel и пакета прикладных программ Statistica (StatSoft Inc., США).
Результаты
Овариальный резерв пациенток снижался от более молодой группы исследования (20–24 года) к более возрастной (40–43 года), подробная характеристика представлена в таблице 1. Длительность стимуляции гонадотропинами незначительно изменялась в зависимости от возраста и не имела достоверных различий среди групп. Протоколы с агонистами ГнРГ имели тенденцию к более длительной стимуляции по сравнению с протоколами с антагонистами ГнРГ. Общая курсовая доза гонадотропинов возрастала с увеличением возраста исследуемых, но не достигла статистической значимости (табл. 2).
Значимых различий среди возрастных групп в доле зрелых ооцитов, частоте оплодотворения и дробления, а также частоте бластуляции и доле бластоцист хорошего и отличного качества обнаружено не было. Эмбриологические характеристики протоколов ЭКО в исследуемых группах представлены в таблице 3.
Из 1504 пациенток, вступивших в протокол ЭКО, у 1423 был выполнен перенос эмбриона. Доля отмененных по различным причинам переносов составила 5,39% (81/1504). Высокие риски развития синдрома гиперстимуляции яичников послужили причиной отмены переноса лишь в 21/1504 (1,4%) случае. Среди других причин отмены переноса эмбриона чаще всего встречались неудовлетворительное качество эмбриона – 39/81 (48,2%) и патология эндометрия – 18/81 (22,2%). Неудовлетворительное качество ооцитов и аномальное оплодотворение отмечены в 2/81 (2,5%) и 1/81 (1,2%) случаях соответственно.
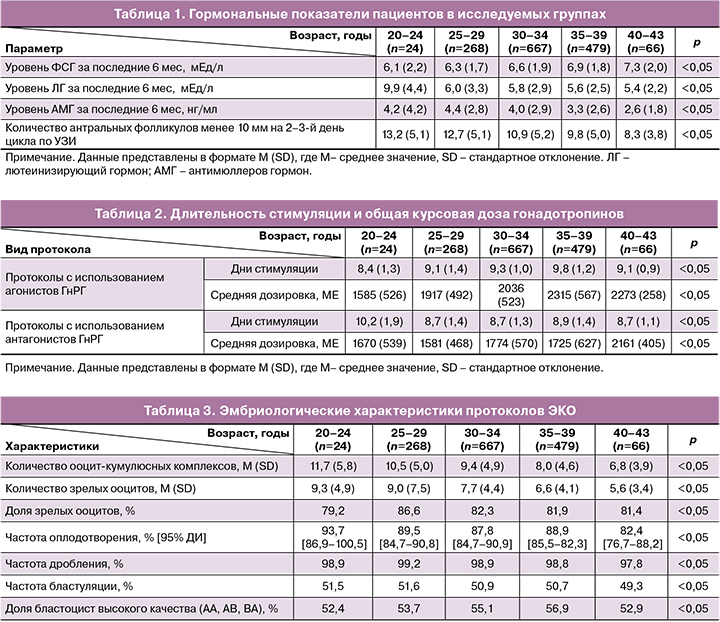
Частота наступления клинической беременности на перенос составила 52,9% (752/1423). Частота родов на перенос эмбриона составила 43,1% (613/1423). Исходы циклов ЭКО в зависимости от группы исследования представлены в рисунке.
Количество одноплодных беременностей составило 631/752 (83,91%). В результате лечения родились 619 здоровых детей со средним весом 3188 (676) г, ростом 50,6 (4,2) см со средней оценкой по шкале Апгар 7 (7; 8)/8 (8; 9) баллов. Ранние преждевременные роды отмечены в 7 случаях. В 5 случаях выявлены тяжелые пороки развития плодов, несовместимые с жизнью (врожденный порок сердца, диафрагмальная грыжа, отсутствие желчного пузыря, множественные пороки развития в 2 случаях).
Обсуждение
В проведенном многоцентровом наблюдательном исследовании отмечена высокая частота наступления клинической беременности на фоне применения препаратов ВО-ЧМГ для стимуляции суперовуляции в протоколе ЭКО.
Несмотря на отсутствие различий в эмбриологических характеристиках протоколов ЭКО (табл. 3), имеются значительное снижение частоты наступления беременности и родов, а также повышение частоты невынашивания у пациентов старших возрастных групп (рисунок). Полученные данные можно объяснить отсутствием корреляции между морфокинетическими параметрами развития эмбриона и частотой анеуплоидий эмбрионов [6, 9].
Существует много способов оценки эффективности протокола ЭКО, наиболее широко используемыми являются частота наступления клинической беременности и частота живорождений. Наиболее объективным методом оценки эффективности протокола ЭКО следует считать частоту живорождений на начатый цикл лечения, поскольку он наиболее полно отражает выполнение поставленных целей лечения и позволяет произвести экономический анализ эффективности протоколов. В современной литературе представлено несколько исследований, описывающих эффективность применения менотропинов в клинической практике. Наиболее крупное исследование, проведенное у пациентов общей популяции, в котором изучалась эффективность применения ВО-ЧМГ, было опубликовано в 2012 г. [3]. Возраст, овариальный резерв, индекс массы тела пациентов в исследовании MEGASET [3] были сопоставимы с таковыми в настоящем наблюдательном исследовании. Частота родов на начатый цикл у пациентов 21–34 лет в исследовании MEGASET составила 29% против 45,1% в данном исследовании. Последнее опубликованное исследование по сравнению эффективности применения ВО-ЧМГ и рекомбинантного ФСГ в протоколах ЭКО у пациенток до 35 лет – MEGASET-HR [10]. Основную группу исследования составляли пациенты с высоким овариальным резервом и предполагаемым гиперответом на стимуляцию. Учитывая высокие риски развития СГЯ и отмены переноса в связи с угрозой развития СГЯ, целесообразно сравнить частоту наступления беременности на перенос эмбрионов, которая составила 52,2% против 45,1% в наблюдательном исследовании. Разница в частоте родов может быть обусловлена меньшим количеством полученных ооцитов, а следовательно, меньшим выбором эмбрионов на перенос. Частота прерываний беременности на раннем сроке для двух исследований практически не отличалась и составила 14,0 и 13,8% для MEGASET-HR и наблюдательного исследования соответственно.
Высокая частота наступления беременности на перенос при использовании менотропинов может быть объяснена лучшим гормональным профилем циклов [11, 12]. В нескольких крупных исследованиях подтверждено негативное влияние повышенного прогестерона в день введения триггера финального созревания ооцитов на частоту наступления беременности в свежих переносах [13, 14]. Применение ВО-ЧМГ ассоциировано с более низкими концентрациями прогестерона сыворотки в день введения триггера, более высокими концентрациями эстрадиола и ХГЧ, что может явиться причиной повышения частоты наступления беременности на перенос эмбрионов [12, 15].
В группах отмечена тенденция к снижению числа антральных фолликулов и увеличению базального уровня ФСГ плазмы крови с увеличением возраста исследуемых пациентов. Отмечено не только снижение частоты наступления беременности на перенос эмбрионов в цикле ЭКО у пациентов старшего репродуктивного возраста, но и увеличение расхода гонадотропинов (табл. 1, 2). Частота живорождений в группе пациентов старшего репродуктивного возраста снижается за счет не только низкой частоты имплантации, но и более высокой, чем в молодом возрасте, частоты невынашивания беременности. В исследовании израильских коллег EISG [8] проанализирована эффективность применения ВО-ЧМГ у пациентов старшего репродуктивного возраста. При сравнении основных показателей эффективности протоколов ЭКО выявлены значительно большая частота наступления клинической беременности (39,5% против 29,6%) и более низкая частота прерывания беременности на ранних сроках (22,9% против 25,4%) для наблюдательного исследования и исследования ESIG соответственно. В исследовании 2011 г. отмечена сравнимая частота наступления беременности при использовании мочевых гонадотропинов у пациенток от 35 до 39 лет (44,4%), что в значительной степени было выше, чем в контрольной группе с рекомбинантным ФСГ (29,7%) [8].
В исследовании MEGASET-HR также отмечено значительное преимущество ВО-ЧМГ перед р-ФСГ в снижении частоты невынашивания беременности [10]. При переносе в свежем цикле эта разница может быть объяснена отличиями в стимуляции и гормональном профиле циклов: снижением пиковых значений эстрадиола, снижением уровня прогестерона в день введения триггера финального созревания фолликулов и, следовательно, снижением негативного влияния высокого уровня прогестерона на эндометрий в протоколах с ВО-ЧМГ [11, 15]. В то же время положительный эффект от применения менотропинов распространяется и на криопротоколы, где влияния стимуляции на эндометрий нет. В сравниваемых группах различий в морфологии и плоидности эмбрионов выявлено не было, следовательно, снижение рисков невынашивания связано с другими параметрами качества ооцитов, которые в данный момент выявить не удалось.
Перенос эмбрионов был отменен в 81 случае, в том числе в 21 случае по причине развития СГЯ, что составило 1,4% общего числа циклов (81/1504). При сравнении с мировыми данными отмечено, что риски гиперстимуляции яичников в общей популяции составляют около 5% всех начатых циклов, и можно отметить высокую безопасность использования ВО-ЧМГ у пациентов.
Заключение
Учитывая высокую эффективность и безопасность ВО-ЧМГ, выявленную в открытом мультицентровом наблюдательном исследовании, можно рекомендовать использование таковых как препаратов первой линии в общей популяции при стимуляции суперовуляции в протоколах ЭКО.



