The effectiveness of frozenthawed embryo transfer in patients with different transcriptional gene signatures of endometrial receptivity
Aim. To investigate the outcomes of frozen-thawed embryo transfer in patients with different mRNA expression of 15 endometrial genes during the window of implantation.Miroshkina M.I., Korneeva I.E., Burmenskaya O.V., Mishina N.D.
Materials and methods. We examined 36 patients with tubal-peritoneal infertility who underwent pipelle endometrial biopsy in the setting of cyclic hormonal therapy on the 5th day of progesterone administration
(P + 5) in the cycle before embryo transfer. Investigations included a histological and molecular genetic study of endometrial gene expression using RT-PCR. After frozen-thawed embryo transfer, the outcomes of ART were analyzed.
Results. The molecular genetic characteristics of the window of implantation were determined in patients with different embryo transfer outcomes. Based on these findings, interim stages in the receptive endometrium development were identified.
Conclusion. Analysis of the mRNA expression of genes LIF, GPX3, AQP3, NDRG1, GNLY, IMPA2, PAEP, IGFBP1, HABP2, DPP4, TAGLN, IL15, POSTN, HLA-DOB, and MSX1 allows determination of the most favorable day for embryo transfer.
Keywords
Embryo implantation in the human is a complex process that is crucial for a successful pregnancy, but the physiology of implantation remains not fully understood. Successful embryo implantation can only occur during a temporally restricted period of the menstrual cycle called the window of implantation [1]. Embryo implantation can also occur outside the uterine cavity except for the non-receptive endometrium [2]. The study of endometrial receptivity continues to be a challenging problem in modern human reproduction. For the endometrial preparation to harbor the coming blastocyst, hormonal priming with estradiol (E2) and progesterone (P) is essential, which occurs in women with a regular menstrual cycle.
To improve the effectiveness of assisted reproductive technologies (ART), infertile patients with repeat implantation failure often undergo additional cyclic hormonal therapy (CHT). ART technologies' achievements have enabled significant progress in the study of the quality of embryos, vitrification technologies, maturation of gametes, optimization of protocols for controlled ovarian hyperstimulation, and preparation of the endometrium in frozen-thawed embryo transfer cycles. However, one of the significant reasons for embryo implantation failure is impaired endometrial receptivity.
Morphological criteria for endometrial maturation do not differentiate signs of endometrial receptivity for embryo implantation [3]. It is believed that the period of the fertility window coincides with the middle of the secretory phase. Many studies have shown the questionable diagnostic value of this method for determining endometrial receptivity [4]. In recent years, studies addressing this issue have been increasingly focused on molecular genetic biomarkers of endometrial receptivity [5–9]. Besides, molecular genetic research is more promising in determining the fertility window than histological examination [5]. The ERA (endometrial receptivity array) test system is one of the first diagnostic systems developed based on the study of the endometrial expression of 238 genes [9]. The developers of this system have found that every third woman with failed IVF and embryo transfer is at risk of having a shifted window of implantation. Based on this system's results, a personalized embryo transfer algorithm was proposed for the management of patients with repeat implantation failures [10]. Considering that there is no system for identifying molecular genetic predictors of the window of implantation in our country, the development of a Russian analog of this personalized approach offers promising prospects.
This study aimed to compare the outcomes of frozen-thawed embryo transfer with the results of a molecular genetic study of endometrial mRNA gene expression during CHT.
Materials and methods
This pilot study included 36 infertile women who were referred for a frozen-thawed embryo transfer program. All patients from 2–3 days of the menstrual cycle were administered 2 mg estradiol valerate with a gradual increase in the dosage to 6–8 mg/d, followed by 600 mg/d of oral micronized progesterone (P+0). On day 5 of progesterone administration, all patients underwent a Pipelle endometrial biopsy. Endometrial biopsies were obtained using a sterile Pipelle sampler (Pipelle de Cornier; Laboratoire CCD, France). Endometrial biopsy material was divided into samples for histological and molecular genetic analysis.
For histological examination, the obtained material was embedded in paraffin, stained with hematoxylin-eosin, and examined using a light microscope.
Molecular genetic study. Potential biomarkers of the window of implantation were selected using bioinformatic analysis of the freely available results of microarray analysis of samples of normal endometrium in the early and middle secretory phase [11]. As a result of this analysis, 15 genes were selected and studied for mRNA expression: GPX3, PAEP, LIF, DPP4, TAGLN, HABP2, IMPA2, AQP3, HLA-DOB, MSX1, POSTN, IGFBP1, IL15, GNLY, NDRG1 by reverse transcription-polymerase chain reaction (RT-PCR). Endometrial samples were obtained using Pipelle endometrial biopsy on day 5 of P administration in the settings of CHT. Then samples were placed in tubes with transport medium for RNA stabilization (Stor-ex, LLC DNA Technology TS, Russia). Tissue samples were thawed at room temperature, the transport medium was removed, and RNA was extracted using QIAzol Lysis Reagent (Qiagen) in combination with miRNeasy mini kit (Qiagen, Germany).
The reverse transcription reaction was conducted at 40°C for 30 minutes, followed by inactivation of reverse transcriptase at 95°C for 5 minutes.
Amplification was conducted in real-time mode in a volume of 12 μl according to the following program: 15 cycles – 80°С for 5 sec, 94°С for 5 sec; 1 cycle – 94°С 5 min; 50 cycles – 94°С 20 sec, 64°С 20 sec; 10°С – storage. The fluorescence level was measured at each cycle at a temperature of 64°С using the FAM fluorescence channel. To increase PCR's sensitivity and specificity, antibody-based hot-start for fast polymerase activation (TechnoTaq) was used. The enzyme was activated only after heating the amplification mixture at 95°C for 5 minutes. The reaction was set in two repetitions for each point.
Cq cycle values obtained at the end of amplification were entered into MS Excel spreadsheets. Indicator cycles were compared using ΔCq and ΔΔCq methods. The mRNA expression levels of the studied genes were measured in relative units (ru), reflecting the representation of the gene transcript relative to the normalizing factor, calculated based on the mRNA expression level of the reference TBP, B2M, and GUSB genes.
Comparison of transcriptional profiles across all samples was visualized using a heat map. Log transformation and data normalization were performed using the standard z-score method. Based on these indicators, a cluster analysis by the full connection method based on Euclidean distance was performed.
The heat map and related calculations were performed using software development in Python 2.7 and its libraries: Sklearn, Skipy, Pandas, Matplotlib, Seaborn.
All 36 patients received one blastocyst transfer on day 5 of micronized progesterone (P+5) administration in the CHT cycle under ultrasound guidance using a Cook's catheter (Australia). Estradiol valerate and micronized progesterone were administered within 14 days after embryo transfer, followed by measurement of the serum β-chorionic gonadotropin.
A criterion for successful implantation was ultrasound findings on the presence of a viable embryo in the uterine cavity at 5–6 weeks’ gestation.
Statistical analysis
Statistical analysis was performed using Microsoft Excel and IBM SPSS Statistics v22 (USA) software. Quantitative variables showing normal distribution were expressed as means and standard deviation; otherwise, the median and the quartiles Q1 and Q3 were reported. Qualitative variables were summarized as counts and percentages. The statistical significance of between-group differences for continuous variables was tested with the Mann–Whitney test with Bonferroni correction. Cluster analysis was used to identify cluster subgroups depending on changes in gene expression. Fisher's exact test with Bonferroni correction was used for pairwise comparison of cluster groups. Considering the Bonferroni correction, the differences between the obtained data were considered statistically significant at р≤0,008.
Results
The mean age of the patients was 35 (4.5) years, and all patients had a normal body mass index [21.8 (3.4) kg/m2], a regular menstrual cycle lasting 28 (3.5) days. The mean duration of infertility was 5.0 (2.7) years. The incidence of primary and secondary infertility was 38.9% and 61.1%, respectively. A history of failed IVF was reported by 24 women (66.7%). At the time of inclusion into the study, the patients had 2.7 (1.4) cryopreserved embryos of good quality. All patients had preserved ovarian reserve, as evidenced by an AMH level of 2.6 (2.2) IU/L. Serum level of E2 and progesterone on the day of the endometrial biopsy was 1174.1 (451.3) pmol/l and 39.4 (16.0) nmol/l, respectively. The endometrial thickness on the day of the Pipelle biopsy was 8.9 (1.5) mm.
According to histological findings, 66.7% (n=24) and 33.3% (n=12) samples were in the early and middle secretory phase, respectively.
The molecular genetic study results were analyzed using cluster analysis independently of the histological examination and presented in the form of a heat map (Fig. 1).
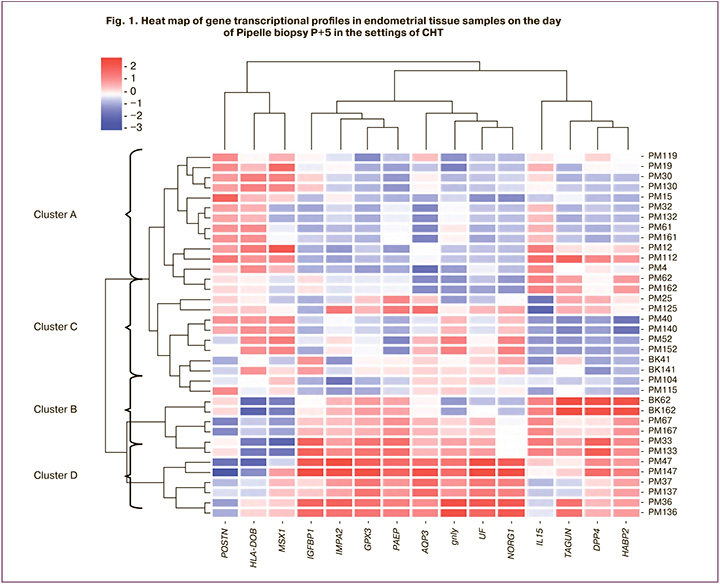
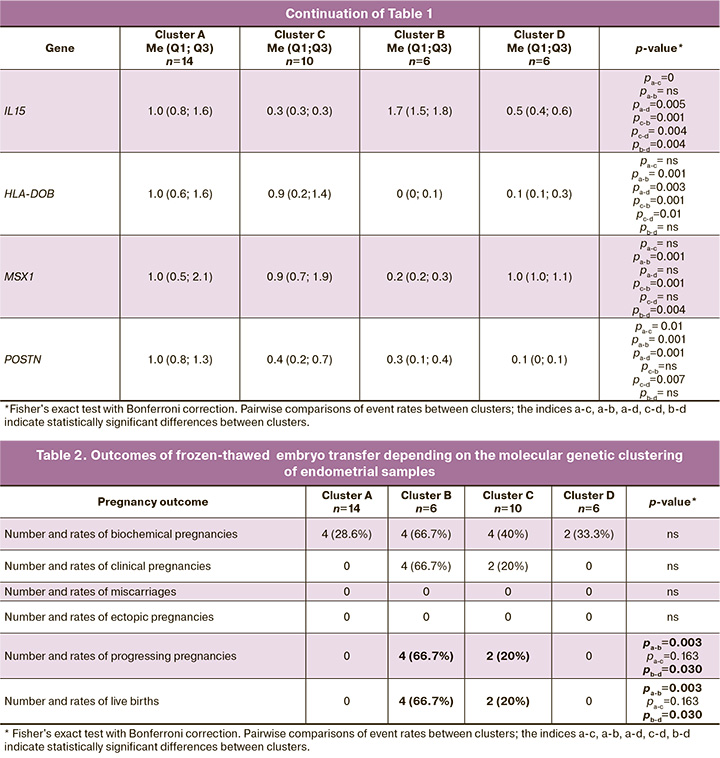
Based on cluster analysis, four stages of endometrial development were identified: cluster A, cluster B, cluster C, and cluster D. Thus, the distinctive features of cluster A (n=14) were the high expression of mRNA of the POSTN, HLA-DOB, and MSX1 genes and low expression of mRNA genes LIF, GPX3, AQP3, NDRG1, GNLY, IMPA2, PAEP, IGFBP1, HABP2, DPP4, and TAGLN. Clusters B (n=6) and C (n=10) were characterized by intermediate expression levels of the studied genes. For cluster D (n = 6), high mRNA expression of genes LIF, GPX3, AQP3, NDRG1, GNLY, IMPA2, PAEP, IGFBP1, HABP2, DPP4, and TAGLN and low expression of genes POSTN, HLA-DOB, and MSX1 were found (Table 1).
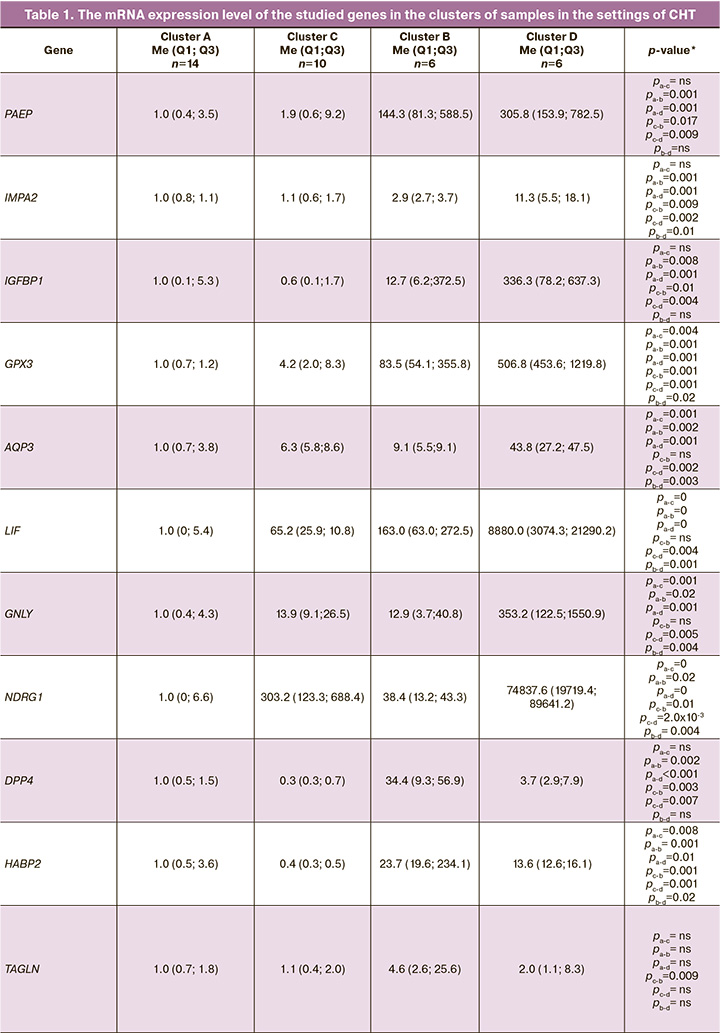
The distribution of endometrial samples by cluster analysis correlated with histological endometrial maturation. In cluster A, 100% of the samples were consistent with the early secretory phase endometrium (14 samples). In cluster C, 80% and 20% of cases (8 samples) corresponded to the early and middle secretory phase endometrium, respectively. Samples of clusters B and D in 66.7% (4 samples) and 100%, respectively, corresponded to the middle secretory phase endometrium. Therefore, cluster staging of the endometrium against the background of CHT reflected the histological maturation of the endometrium (Fig. 2).
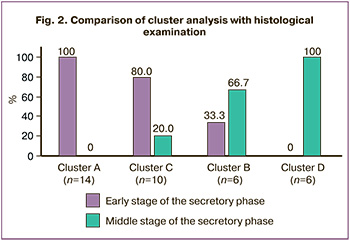 The β-chorionic gonadotropin positivity rate in clusters ranged from 66.7% to 28.6%, but there was no significant difference between clusters. At the same time, there was a statistically significant difference in rates of pregnancy prolongation and live births in cluster B. Pregnancy prolongation and childbirth were also often observed in cluster C. Still, the results were not statistically significant, probably due to the small sample size (Table 2).
The β-chorionic gonadotropin positivity rate in clusters ranged from 66.7% to 28.6%, but there was no significant difference between clusters. At the same time, there was a statistically significant difference in rates of pregnancy prolongation and live births in cluster B. Pregnancy prolongation and childbirth were also often observed in cluster C. Still, the results were not statistically significant, probably due to the small sample size (Table 2).
Discussion
Most of the published research has focused on characteristics of transcriptional profile and the effect on histological changes in the endometrium in the natural cycle [6–8, 11]. A feature of this pilot study is the investigation of the transcriptional profile of genes during the window of implantation in CHT settings. The study's findings suggest a more pronounced heterogeneity of the distribution of endometrial samples compared with a histological study than in previously published studies [12]. Also, new data were obtained that, despite the artificially modeled cycle, due to exogenous effects of E2 and P during the window of implantation, more samples (n=24) were found to be in an early secretory phase, i.e., morphologically immature endometrial structure. Our results are inconsistent with the results of other studies, which showed that histological signs of endometrial maturation did not correlate with the use of CHT [13].
A molecular genetic study showed a gradual increase in the level of mRNA expression of the GPX3, PAEP, LIF, DPP4, TAGLN, HABP2, IMPA2, AQP3, IGFBP1, IL15, GNLY, and NDRG1 genes and a decrease in the level of mRNA expression of the генов HLA-DOB, MSX1, and POSTN genes in cluster D. The study findings are consistent with our earlier work regarding the natural cycle, which proposed a similar cluster staging of endometrial maturation, as in the case of CHT: A → C → B → D [12].
Based on this classification, intermediate stages of endometrial maturation (clusters B and C) have been identified, which correspond to the window of implantation and are optimal for embryo transfer. The highest clinical pregnancy and live birth rates were found among women with mRNA levels in endometrial tissues consistent with intermediate clusters. However, statistically significant differences were found only for cluster B: 4 out of 6 cases belonging to this group. In cluster C, this value was about 20%. Moreover, «attempts at implantation» were detected in clusters A and D. However, further invasion and formation of cytotrophoblasts did not occur, probably due to insufficient maturity or «overmaturity» of the endometrium. Spanish researchers reported similar data of higher clinical pregnancy and childbirth rates among patients with receptive endometrium and unsatisfactory treatment outcomes in patients with a non-receptive endometrium [14].
The main limitation of this study is the small sample size. Further, sufficiently powered studies are required to elucidate the diagnostic value of these findings.
Based on these findings, a personalized algorithm for selecting the day of embryo transfer was proposed for this cohort of patients. The algorithm suggests a molecular genetic analysis of the level of mRNA expression of the genes studied in this work in the cycle before embryo transfer, followed by the determination of the endometrial clustering. If the transcriptional profile in the obtained endometrial sample is consistent with clusters B and C, it is recommended to perform the standard protocol transfer against the background of CHT on day P+5. If the endometrial maturation phase corresponds to clusters A and D, it is recommended to do additional endometrial preparation before embryo transfer. Otherwise, it may be recommended to postpone the embryo transfer 12–24 hours later than the standard approach (for cluster A) in the CHT P+5 cycle and 5–6 or earlier (for cluster D) in the CHT P+4.5 cycle.
Conclusion
Analysis of the mRNA expression of genes LIF, GPX3, AQP3, NDRG1, GNLY, IMPA2, PAEP, IGFBP1, HABP2, DPP4, TAGLN, IL15, POSTN, HLA-DOB, and MSX1 reflects phases of the endometrial cycle and allows optimal timing of embryo transfer.
References
- Ashary N., Tiwari A., Modi D. Embryo implantation: war in times of love. Endocrinology. 2018;159(2): 1188-98. https://dx.doi.org/10.1210/en.2017-03082.
- Edwards R.G. Human implantation: the last barrier in assisted reproduction technologies? Reprod. Biomed. Online. 2006;13(6): 887-904. https://dx.doi.org/ 10.1016/s1472-6483(10)61039-5.
- Noyes R.W., Hertig A.T., Rock J. Dating the endometrial biopsy. Am. J. Obstet. Gynecol. 1975;122(2): 262-3. https://dx.doi.org/10.1016/s0002-9378(16)33500-1.
- Murray M.J., Meyer W.R., Zaino R.J., Lessey B.A., Novotny D.B., Ireland K. et al. A critical analysis of the accuracy, reproducibility, and clinical utility of histologic endometrial dating in fertile women. Fertil. Steril. 2004;81(5): 1333-43. https://dx.doi.org/10.1016/j.fertnstert.2003.11.030.
- Díaz-Gimeno P., Ruiz-Alonso M., Blesa D., Bosch N., Martínez-Conejero A., Alamá P. et al. The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity Fertil. Steril. 2013;99(2): 508-17. https://dx.doi.org/ 10.1016/j.fertnstert.2012.09.046.
- Ponnampalam A.P., Weston G.C., Trajstman A.C., Susil B., Rogers P.A. Molecular classification of human endometrial cycle stages by transcriptional profiling. Mol. Hum. Reprod. 2004;10(12): 879-93. https://dx.doi.org/ 10.1093/molehr/gah121.
- Talbi S., Hamilton .E., Vo K.C., Tulac S., Overgaard M.T., Dosiou C. et al.Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147(3): 1097-121. https://dx.doi.org/10.1210/en.2005-1076.
- Haouzi D., Mahmoud K., Fourar M., Bendhaou K., Dechaud H., De Vos J. et al. Identification of new biomarkers of human endometrial receptivity in the natural cycle Hum. Reprod. 2009;24(1): 198-205. https://dx.doi.org/ 10.1093/humrep/den360.
- Garrido-Gómez T., Ruiz-Alonso M., Blesa D., Diaz-Gimeno P., Vilella F., Simón C. Profiling the gene signature of endometrial receptivity: clinical results. Fertil. Steril. 2013;99(4): 1078-85. https://dx.doi.org/10.1016/j.fertnstert.2012.12.005.
- Ruiz-Alonso M., Galindo N., Pellicer A., Simón C. What a difference two days make: “personalized” embryo transfer (pET) paradigm: a case report and pilot study. Hum. Reprod. 2014;29(6): 1244-7. https://dx.doi.org/ 10.1093/humrep/deu070.
- Burmenskaya O.V., Bozhenko V.K., Smolnikova V.Y., Kalinina E.A., Korneeva I.E., Domnikov A.E. et al. Transcription profile analysis of the endometrium revealed molecular markers of the personalized ‘window of implantation’ during in vitro fertilization. Gynecol. Endocrinol. 2017;33(Suppl. 1): 22-7. https://dx.doi.org/10.1080/09513590.2017.1404236.
- Мирошкина М.И., Корнеева И.Е., Бурменская О.В., Мишина Н.Д. Оценка исходов программ криопереноса в зависимости от транскрипционного профиля генов эндометрия в период предполагаемого «окна имплантации». Акушерство и гинекология. 2020;4: 133-9. https://dx.doi.org/10.18565/aig.2020.4.133-139. [Miroshkina M.I. et al.Evaluation of the outcomes of cryopreservation programs depending on the transcription profile of endometrial genes during the expected “implantation window”. Obstetrics and gynecology. 2020; 4: 133-9.(in Russian)].
- Groll J.M., Usadi R.S., Lessey B.A., Lininger R., Young S.L., Fritz M.A. Effects of variations in serum estradiol concentrations on secretory endometrial development and function in experimentally induced cycles in normal women. Fertil. Steril. 2009;92(6): 2058-61. https://dx.doi.org/ 10.1016/j.fertnstert.2009.06.018.
- Díaz-Gimeno P., Ruiz-Alonso M., Sebastian-Leon P., Pellicer A., Valbuena D., Simón C. Window of implantation transcriptomic stratification reveals different endometrial subsignatures associated with live birth and biochemical pregnancy. Fertil. Steril. 2017;108(4): 703-10. e3. https://dx.doi.org/ 10.1016/j.fertnstert.2017.07.007.
Received 07.09.2020
Accepted 05.10.2020
About the Authors
Maria I. Miroshkina, Postgraduate Student at the F. Paulsen SEC for ART, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.Tel.: +7(985)430-38-33. E-mail: maria_mir18@mail.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Irina E. Korneeva, Dr. Med. Sci., Head of F. Paulsen SEC for ART, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)438-26-22. E-mail: i_korneeva@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Olga V. Burmenskaya, Dr. Biol. Sci., Head of the Laboratory of Oncological Genetics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)438-22-92. E-mail: o_bourmenskaya@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Natalia D. Mishina, Junior Researcher at the Laboratory of Genomic Data Analysis, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(985)217-29-89. E-mail: mis7ha@gmail.com. 117997, Russia, Moscow, Ac. Oparina str., 4.
For citation: Miroshkina M.I., Korneeva I.E., Burmenskaya O.V., Mishina N.D. The effectiveness of frozen-thawed embryo transfer in patients with different transcriptional gene signatures of endometrial receptivity.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 11: 85-92 (in Russian).
https://dx.doi.org/10.18565/aig.2020.11.85-92



