Receptivity of thin endometrium in patients undergoing assisted reproduction
Aim. To investigate the receptivity of the thin endometrium in patients with infertility conceiving and not conceiving in assisted reproductive technology (ART) programs.Kulikova G.V., Abdurakhmanova N.F., Faizullina N.M., Asaturova A.V., Shchegolev A.I., Ziganshina M.M., Dolgushina N.V.
Materials and methods. This prospective study comprised 32 patients who sought ART treatment for infertility. Patients were stratified into groups by whether they had conceived or fail to conceive, and depending on the endometrial thickness. An endometrial thickness threshold was 8 mm. During the implantation window in the cycle preceding the cycle of ovarian stimulation and embryo transfer, all patients underwent a pelvic ultrasound examination to measure the endometrial thickness and pipelle endometrial biopsy. We studied the expression of estrogen receptors α (ER), progesterone receptors (PR), and leukemia inhibitory factor (LIF) in the endometrial superficial and glandular epithelium, and endometrial stroma.
Results. Patients with normal endometrial thickness had an increased ER and decreased PR expressions in all endometrial tissues resulting in a lower PR/ER ratio in the endometrial glands and stroma. The expression of receptivity markers was not statistically significantly associated with the endometrial thickness and the likelihood of pregnancy.
Conclusion. There is no significant difference between normal and thin endometrium in the expression of ER, PR, and LIF in the endometrial superficial and glandular epithelium, and endometrial stroma.
Keywords
It is generally accepted that the quality of the euploid embryo and endometrial receptivity with a high implantation potential are critical to a successful pregnancy. Insufficient thickness the endometrium is thought to be associated with a probability of not achieving pregnancy and a lower implantation rate in assisted reproductive technologies (ART) programs [1 - 3]. For the first time, an endometrial thickness threshold as a prognostic criterion for pregnancy was proposed by Gonen et al. in 1989 [4]. Since that time, the concept of minimal endometrial thickness has been widely used in clinical practice. At the same time, the so-called thin endometrium was considered the endometrium less than 6-8 mm during the implantation window [2, 3, 5]. However, there is no hard evidence that the thin endometrium reduces the likelihood of pregnancy, both in the natural cycle and in ART programs. Data on the prognostic role of this estimate are still contradictory, as are data on impaired receptivity of the thin endometrium, which can be the cause of implantation failures [6 - 10]. The difficulty in assessing the receptivity of the endometrium lies in that it was investigated in a cycle different from the cycle of conception. In this case, the thickness of the endometrium and its receptivity can vary from cycle to cycle.
This study aimed to investigate the receptivity of the thin endometrium in patients with infertility conceiving and not conceiving in ART programs.
Materials and methods
The prospective study comprised 32 patients who sought ART treatment for infertility from 2016 to 2018, had no contraindications to ART programs, and signed informed consent to participate in the study. The inclusion criteria were as follows: the normal karyotype of the patient and her partner, patients’ age from 18 to 40 years, body mass index (BMI) from 18 to 29.9 kg/m2. The exclusion criteria were contraindications to ART, pronounced pathospermia of the partner, the use of donor gametes or surrogacy, a “poor” response to ovarian stimulation, the absence of good quality blastocysts, and the development of ART complications in the study cycle.
Patients were stratified into groups by whether they had conceived or fail to conceive, and depending on the endometrial thickness. Group 1 (n = 12) included patients who had a normal endometrial thickness and became pregnant; group 2 (n = 6) included patients with normal endometrial thickness and failed to get pregnant; patients with thin endometrium, who were unable to achieve a pregnancy made up group 3 (n = 14). An endometrial thickness threshold was 8 mm, according to a previous study [11].
Before being included in the ART program, all women were examined according to the Order of the Ministry of Health of Russia dated 30.08.2012 No. 107n “On the Procedure for Using Assisted Reproductive Technologies, Contraindications and Restrictions on Their Use” [12].
In the cycle preceding the cycle of ovarian stimulation and embryo transfer, during the implantation window (on days 6-8 after ovulation), all patients underwent a pelvic ultrasound examination to measure the endometrial thickness and pipelle endometrial biopsy from the uterine bottom. Ovulation was confirmed by ultrasound and ovulation tests measuring the peak urinary luteinizing hormone (LH) level.
The obtained endometrial samples were processed according to a standard method to obtain paraffin blocks. A histological examination of the endometrium was performed to determine the expression of estrogen receptors α (ER), progesterone receptors (PR), and leukemia inhibitory factor (LIF) in the endometrial superficial and glandular epithelium, and endometrial stroma by immunohistochemical method (IHC) on dewaxed 3-4 µm-thick sections. The expression level of steroid receptors was determined using mouse monoclonal antibodies to ER (clone 1D5 RTU “DAKO”, Denmark) and PR (clone 636 RTU “DAKO”, Denmark); the expression of LIF was detected using mouse monoclonal antibodies (clone 9824, 1: 100, R&D Systems, USA).
Results of IHC reactions for ER and PR were analyzed taking into account the number of stained cells and the color intensity in the endometrial epithelium and stroma using the histochemical scoring (H-Score) according to equation: HS = 1a + 2b + 3c, where a is the percentage of weakly stained cells, b - the percentage of moderately stained cells, and s is the percentage of strongly stained cells; 1, 2, and 3 are staining intensity, expressed in points. The final score of expression of severity for ER and PR was categorized as 0-10 (no expression), 11-100 (weak expression), 101-200 (moderate expression), and 201-300 (strong expression).
The results of the IHC reaction for LIF were evaluated by a semi-quantitative method according to the generally accepted scoring system: no immunostained cells (-) - 0 points; less than 20% of immunostained cells (+)1 point; from 20 to 40% of stained cells (++)2 points; and more than 40% of stained cells (+++) 3 points.
In the ART program, ovarian stimulation was performed using recombinant follicle-stimulating hormone (rFSH) alone or in combination with LH, and the administration of gonadotropin-releasing hormone antagonists. The ovulation trigger was administered as soon as ≥1 follicle reached ≥17 mm in diameter. A chorionic gonadotropin (CG) at a dose of 8,000-10,000 IU was used as a trigger. Transvaginal ovarian puncture (TVP) was performed 36 hours after the ovulation Oocytes were fertilized by intracytoplasmic sperm injection (ICSI). Indications for ICSI included subfertile sperm in the partner and a low fertilization rate in the previous in vitro fertilization (IVF) program. Morphological evaluation of embryos was performed according to the classification of Gardner et al. [13] on day 5 after TVP.
The embryo was transferred into the uterine cavity on day 5 after TVP in the “fresh” cycle. One best-quality blastocysts were transferred. The post-transfer period was managed according to a unified protocol.
Biochemical pregnancy was defined as a rise in serum ß-CG level on day 14 days after the embryo transfer; clinical pregnancy was detected by ultrasound imaging of the intrauterine fetal sac on day 21 days after the embryo transfer.
Statistical analysis was performed using the Statistica 10 (USA) statistical software package. Categorical variables were compared by the χ2 test; Student’s t-test, ANOVA, or the Mann-Whitney/Kruskal–Wallis test were used for comparing continuous data between three and three groups. An association measure for comparing binary data was the odds ratio adjusted for confounders (ORadj), calculated by the logistic regression. Correlation analysis was performed using the Spearman nonparametric rank correlation test. Differences between the groups were considered statistically significant at p<0.05.
The study was approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Results
The mean age and BMI of patients did not differ between the groups (p > 0.05). There were no differences between the groups in menstrual function, number of pregnancies, parity, hormonal parameters, and somatic comorbidity. A significant proportion of patients, who failed to get pregnant (groups 2 and 3) had a history of gynecological diseases such as external genital endometriosis, uterine fibroids, and endometrial polyps. They also had a longer history of infertility.
When assessing the features of ovarian stimulation in the ICSI program, no differences were found between the groups in the total gonadotropin dose, duration of ovarian stimulation, or the type of medication for ovarian stimulation (Table 1).

In the ICSI program, patients who achieved a pregnancy had a higher blastulation rate and, as a result, yielded a greater number of excellent quality blastocysts (Table 2).
Ultrasound evaluation did not reveal significant differences in the uterine size, ovarian volume, and the antral follicle count, which is a marker of ovarian reserve. The patients who conceived after embryo transfer had a significantly greater endometrial thickness (9.6 ± 1.1 mm), than those who did not (7.0 ± 1.2 mm) (p < 0.0001). In patients with an endometrial thickness ≥8 mm, pregnancy occurred in 66.7% of cases (in 12 out of 18 patients), while patients with a thin endometrium failed to conceive (0 out of 14 people) (p = 0.0001). It should be noted that such factors (confounders) as endometriosis and the number of excellent quality blastocysts, which had a statistically significant relationship with the onset of pregnancy in a univariate analysis, could influence the ability to conceive. The odds ratio for pregnancy based on multivariate analysis using logistic regression, depending on the presence of a thin endometrium and taking into account endometriosis, was 1.47 (95% CI = 1.05; 2.5). In multivariate analysis, the quality of blastocysts lost statistical significance.
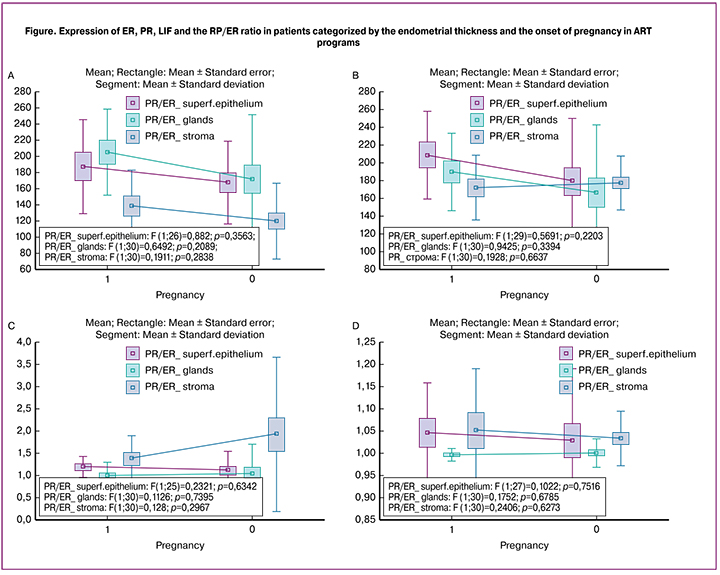
Histological examination showed that in all cases, the endometrium was in the middle secretory phase. The IHC analysis did not detect statistically significant differences in the studied receptivity parameters depending on the onset of pregnancy and endometrial thickness (p > 0.05). The ER expression was observed in all endometrial layers; it was maximal in the endometrial glands, minimal in the endometrial stroma, and ranged from 15 to 275. The highest ER expression level in all endometrial tissues was observed in group 1 (Figure a). The PR expression was also observed in all endometrial layers, was maximal in the superficial epithelium, less in the endometrial glands and stroma, and ranged from 25 to 300. The highest level of PR expression in endometrial superficial and glandular epithelium was observed in group 1 and did not differ between groups in the endometrial stroma (figure, b). The PR/ER ratio ranged from 0.21 to 9.0 and was highest in the stroma and minimal in the endometrial glands. There was a slightly higher PR/ER ratio in endometrial superficial and glandular epithelium in the pregnancy group (group 1); in the glands it was the same regardless of pregnancy. In the stroma, the highest PR/ER ratio was observed in patients who failed to get pregnant (groups 2 and 3) (Figure c). LIF expression was observed in all endometrial tissues; mostly, it was weakly expressed, maximal in the stroma, minimal in the endometrial glands, and ranged from 0.65 to 1.55 points. The highest level of LIF expression was observed both in the endometrial epithelium and stroma in patients who achieved a pregnancy (figure, d).
The correlation analysis between endometrial thickness and the expression of the studied markers demonstrated a number of correlations including a weak positive correlation with the expression of ER in all endometrial tissues; weak positive correlation with the expression of PR in the superficial epithelium; weak negative correlation with the expression of PR in the endometrial glands and stroma; weak negative correlation with PR/ER in all endometrial tissues; weak positive correlation with LIF expression in superficial epithelium; weak negative correlation with the expression of LIF in the endometrial glands and stroma. All correlations were not statistically significant.
Discussion
The findings of the first stage of our study showed that the endometrial thickness affected pregnancy rates in ART programs. The patients with endometrial thickness <8 mm, measured by ultrasound during the implantation window, had 1.47-fold lower odds of pregnancy. Many studies have reported that endometrial thickness was associated with infertility, miscarriage, and later with the effectiveness of ART programs [14–16]. Also, according to various studies, the endometrial thickness from 6 to 8 mm has been considered as specific threshold defining “normal” and thinendometrial linings [17–19].
In determining the threshold endometrial thickness, we relied on our data that were reported earlier, in which this value was 8 mm [11]. Our data are consistent with a study by Miwa et al. (2009), and other researchers, in which pregnancy rates in ART programs in patients with endometrium ≤8 mm was significantly lower than in patients with endometrial thickness> 8 mm (5.9% compared to 22.4%) [ 20, 21]. And yet, despite a plethora of studies providing compelling evidence of the negative effect of a thin endometrium on the outcomes of ART programs, there is conflicting evidence regarding the impact of endometrial thickness and pregnancy chances [8].
During the 2nd stage of the study, we investigated the association of endometrial receptivity with the endometrial thickness and the onset of pregnancy in ART cycles. Our findings showed mixed results. It is known that with compromised endometrium (in the presence of external genital endometriosis, a history of multiple failed ART cycles, etc.) is associated with an increase in the expression of ER and PR, and these changes are mainly noted in the endometrial superficial epithelium, which first “enters into dialogue” with the blastocyst [22, 23]. It is believed that high ER expression is associated with endometrial proliferation and the changes in gene expression resulting in a reduction in the capacity of endometrial implantation [24]. There are opposite data, in which, on the contrary, patients with external genital endometriosis were found to have a decreased, rather than increased, the endometrial expression of ER and PR [25]. In our study, we also observed, although not significant, an increase in ER expression in patients who were able to conceive and those with normal endometrial thickness. The lowest levels of ER expression were found in patients with thin endometrium who failed to achieve a pregnancy. This trend was found in both the epithelial and stromal components of the endometrium.
As for PR expression, according to the literature, patients with external genital endometriosis and infertility had an abnormal PR-A and PR-B ratio, and PR-B expression was not detected at all in endometriotic heterotopies, which indicates the development of progesterone resistance [26, 27]. In our study, a higher PR expression was observed in the endometrial superficial and glandular epithelium in patients who were able to conceive and those with normal endometrial thickness.
An important indicator is the PR/ER ratio, which normally ranges between 2 and 4 [22]. In superficial epithelium and glands, a slightly higher PR/ER ratio was observed at the onset of pregnancy, while in the stroma, the highest PR/ER ratio was noted in patients who failed to conceive. Moreover, in our study, this indicator was quite low; in the superficial epithelium it was on average (1.18 ± 0.22) among patients who became pregnant and (1.11 ± 0.39) among those who did not; in the stroma it was (1.37 ± 0.52) and (1.92 ± 1.14), respectively. This tendency indicates the importance of the relative prevalence of PR over ER in the endometrial superficial epithelium that contacts with the blastocyst.
As for the most important, according to the literature, receptor marker LIF [28, 29], in our study, it was weakly expressed and did not differ significantly between the study groups. It had a trend to higher levels in patients with the onset of pregnancy and normal endometrial thickness, both in the endometrial epithelium and stroma. Our data are consistent with the literature reporting that women with infertility, prior failed attempts at ART programs, endometriosis, and recurrent miscarriage show a decrease in LIF expression during the implantation window [30–32]. Moreover, in the endometrial stroma, there was a tendency to a higher expression of LIF than in the epithelium, which differs from data reported by other researchers [33].
Conclusion
The thin endometrium is associated with poor ART outcomes, reducing the chance of pregnancy by 1.47 times. Moreover, the expression of receptivity markers during the implantation window in the cycle preceding ovarian stimulation and embryo transfer was not related to the likelihood of pregnancy in the ART cycle. Also, it was not statistically significantly associated with the endometrial thickness. These observations may be explained by insufficient diagnostic accuracy of the studied receptivity markers. This suggests a need for further research to identify more sensitive and specific test systems for assessing endometrial receptivity outside the conception cycle.
References
- Kumbak B., Erden H.F., Tosun S., Akbas H, Ulug U., Bahçeci M. Outcome of assisted reproduction treatment in patients with endometrial thickness less than 7 mm. Reprod Biomed Online. 2009; 18(1): 79-84. DOI: 10.1016/s1472-6483(10)60428-2
- Zhang T., Li Z., Ren X., Huang B., Zhu G., Yang W., Jin L. Endometrial thickness as a predictor of the reproductive outcomes in fresh and frozen embryo transfer cycles: A retrospective cohort study of 1512 IVF cycles with morphologically good-quality blastocyst. Medicine (Baltimore). 2018; 97(4): e9689. doi:10.1097/MD.0000000000009689.
- Liu K.E., Hartman M., Hartman A., Luo Z.-C., Mahutte N. The impact of a thin endometrial lining on fresh and frozen-thaw IVF outcomes: an analysis of over 40 000 embryo transfers. Hum Reprod. 2018; 33(10): 1883-8. doi:10.1093/humrep/dey281.
- Gonen Y., Casper R.F., Jacobson W., Blankier J. Endometrial thickness and growth during ovarian stimulation: a possible predictor of implantation in in vitro fertilization. Fertil Steril. 1989; 52(3): 446-50. 4 DOI: 10.1016/s0015-0282(16)60916-0
- Oliveira J.B., Baruffi R.L., Mauri A.L., Petersen C.G., Borges M.C., Franco J.G. Endometrial ultrasonography as a predictor of pregnancy in an in-vitro fertilization programme after ovarian stimulation and gonadotrophin-releasing hormone and gonadotrophins. Hum Reprod. 1997; 12(11): 2515-8. DOI: 10.1093/humrep/12.11.2515
- Khalifa E., Brzyski R.G., Oehninger S., Acosta A.A., Muasher S.J. Sonographic appearance of the endometrium: the predictive value for the outcome of in-vitro fertilization in stimulated cycles. Hum Reprod. 1992; 7(5): 677-80. DOI: 10.1093/oxfordjournals.humrep.a137718
- Serafini P., Batzofin J., Nelson J., Olive D. Sonographic uterine predictors of pregnancy in women undergoing ovulation induction for assisted reproductive treatments. Fertil Steril. 1994; 62(4): 815-22. DOI: 10.1016/s0015-0282(16)57010-1
- Weiss N.S., van Vliet M.N., Limpens J., Hompes P.G.A, Lambalk C.B., Mochtar M.H., van der Veen F., Mol B.W.J, van Wely M. Endometrial thickness in women undergoing IUI with ovarian stimulation. How thick is too thin? A systematic review and meta-analysis. Hum Reprod. 2017;32(5):1009–18. doi:10.1093/humrep/dex035.
- Sher G., Herbert C., Maassarani G., Jacobs M.H. Assessment of the late proliferative phase endometrium by ultrasonography in patients undergoing in-vitro fertilization and embryo transfer (IVF/ET). Hum Reprod. 1991; 6(2): 232-7. DOI: 10.1093/oxfordjournals.humrep.a137312
- Check J.H., Lurie D., Dietterich C., Callan C., Baker A. Adverse effect of a homogeneous hyperechogenic endometrial sonographic pattern, despite adequate endometrial thickness on pregnancy rates following in-vitro fertilization. Hum Reprod. 1993; 8(8): 1293-6. DOI: 10.1016/j.fertnstert.2009.12.025
- Абдурахманова Н.Ф., Гвоздева А.Д., Зиганшина М.М., Долгушина Н.В. Результаты программ ВРТ у пациенток с «тонким» эндометрием. Гинекология. 2019; 21(1): 23-27. [Abdurakhmanova N.F., Gvozdeva A.D., Ziganshina M.M., Dolgushina N.V. The results of assisted reproductive technology programs in patients with “thin” endometrium. Ginekologija. Gynicology 2019; 21(1): 23-27. (In Russian)] DOI: 10.26442/20795696.2019.1.190232
- Приказ Минздрава России №107н от 30 августа 2012 г «О порядке использования вспомогательных репродуктивных технологий, противопоказаниях и ограничениях к их применению». 2012. [Prikaz Minzdrava Rossii №107n ot 30 avgusta 2012 g “O porjadke ispol’zovanija vspomogatel’nyh reproduktivnyh tehnologij, protivopokazanijah i ogranichenijah k ih primeneniju.” 2012. (In Russian)]. www.garant.ru
- Gardner D.K., Schoolcraft W.B. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999; 11(3): 307-11. PMID: 10369209
- Noyes N., Liu H.C., Sultan K., Schattman G., Rosenwaks Z. Endometrial thickness appears to be a significant factor in embryo implantation in in-vitro fertilization. Hum Reprod. 1995; 10(4): 919-22. DOI: 10.1093/oxfordjournals.humrep.a136061
- Rinaldi L., Lisi F., Floccari A., Lisi R., Pepe G., Fishel S. Endometrial thickness as a predictor of pregnancy after in-vitro fertilization but not after intracytoplasmic sperm injection. Hum Reprod. 1996; 11(7): 1538-41. DOI: 10.1093/oxfordjournals.humrep.a019434
- Weissman A., Gotlieb L., Casper R.F. The detrimental effect of increased endometrial thickness on implantation and pregnancy rates and outcome in an in vitro fertilization program. Fertil Steril. 1999; 71(1): 147-9.
- Bassil S. Changes in endometrial thickness, width, length and pattern in predicting pregnancy outcome during ovarian stimulation in in vitro fertilization. Ultrasound Obstet Gynecol. 2001; 18(3): 258. doi:10.1046/j.1469-0705.2001.00502.x.
- Kovacs P., Matyas S., Boda K., Kaali S.G. The effect of endometrial thickness on IVF/ICSI outcome. Hum Reprod. 2003; 18(11): 2337-41. DOI: 10.1093/humrep/deg461
- Dain L., Bider D., Levron J., Zinchenko V., Westler S., Dirnfeld M. Thin endometrium in donor oocyte recipients: enigma or obstacle for implantation? Fertil Steril. 2013; 100(5): 1289-1295.e2. doi:10.1016/j.fertnstert.2013.07.1966.
- Miwa I., Tamura H., Takasaki A., Yamagata Y., Shimamura K., Sugino N. Pathophysiologic features of «thin» endometrium. Fertil Steril. 2009;91(4):998–1004. doi:10.1016/j.fertnstert.2008.01.029.
- Takasaki A., Tamura H., Taketani T., Shimamura K., Morioka H., Sugino N. A pilot study to prevent a thin endometrium in patients undergoing clomiphene citrate treatment. J Ovarian Res. BioMed Central; 2013; 6(1): 94. doi:10.1186/1757-2215-6-94.
- Левиашвили М.М., Демура Т.А., Мишиева Н.Г., Файзуллина Н.М., Назаренко Т.А., Калинина Е.А. Оценка рецептивности эндометрия у пациенток с безуспешными программами экстракорпорального оплодотворения в анамнезе. Акушерство и гинекология. 2012; 4 (1): 65-9.[Leviashvili M.M., Demura T.A., Mishiyeva N.G., Faizullina N.M., Nazarenko T.A., Kogan E.A. Evaluation of endometrial receptivity in patients with a history of failed in vitro fertilization programs. Akusherstvo i ginekologija/Obstetrics and Gynegology. 2012; 4/1: 65-9. (In Russian)]
- Коган Е.А., Калинина Е.А., Колотовкина А.В., Файзуллина Н.М., Адамян Л.В. Морфологический и молекулярный субстрат нарушения рецептивности эндометрия у бесплодных пациенток с наружным генитальным эндометриозом. Акушерство и гинекология. 2014; (8): 47-51.[Kogan E.A., Kalinina E.A., Kolotovkina A.V., Faizullina N.M., Adamyan L.V. The morphological and molecular substrate of impaired endometrial receptivity in infertile patients with external genital endometriosis who enter an assisted reproductive technology program. Akusherstvo i ginekologija/Obstetrics and Gynegology. 2014; (8): 47-51. (In Russian)]
- Moberg C. Levels of oestrogen receptor, progesterone receptor and αB-crystallin in eutopic endometrium in relation to pregnancy in women with endometriosis. Hum Fertil. 2014; (19): 1-8. doi: 10.3109/14647273.2014.922705.
- Денисова В.М., Ярмолинская М.И., Полякова В.О., Рулев В.В., Дурнова А.О. Особенности экспрессии рецепторов половых стероидных гормонов при наружном генитальном эндометриозе. Молекулярная медицина. [Denisova V.M., Yarmolinskaya M.I., Polyakova V.O., Rulev V.V., Durnova A.O. Features of expression of sex steroid hormones receptors in pelvic endometriosis. Molekuljarnaja medicina/Molecular medicine. 2014; (5): 29-32.(In Russian)]. eLIBRARY ID: 22307911
- Leach R.E., Jessmon P., Coutifaris C., Kruger M., Myers E.R., Ali-Fehmi R., Carson S.A., Legro R.S., Schlaff W.D., Carr B.R., Steinkampf M.P., Silva S., Leppert P.C., Giudice L., Diamond M.P. High throughput, cell type-specific analysis of key proteins in human endometrial biopsies of women from fertile and infertile couples. Hum Reprod. 2012; 27(3): 814-28. doi: 10.1093/humrep/der436.
- Bulun S.E., Cheng Y.H., Pavone M.E., Xue Q., Attar E., Trukhacheva E., Tokunaga H., Utsunomiya H., Yin P., Luo X., Lin Z., Imir G., Thung S., Su E.J., Kim J.J. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010; 28(1): 36-43.doi: 10.1055/s-0029-1242991.
- Dimitriadis E., Menkhorst E., Salamonsen L.A., Paiva P. Review: LIF and IL11 in trophoblast-endometrial interactions during the establishment of pregnancy. Placenta. 2010; 31 Suppl: S99-104. doi:10.1016/j.placenta.2009.12.027.
- Marwood M., Visser K., Salamonsen L.A., Dimitriadis E. Interleukin-11 and leukemia inhibitory factor regulate the adhesion of endometrial epithelial cells: implications in fertility regulation. Endocrinology. 2009; 150(6): 2915-23. doi:10.1210/en.2008-1538.
- Dimitriadis E., Sharkey A.M., Tan Y.L., Salamonsen L.A., Sherwin J.R.A. Immunolocalisation of phosphorylated STAT3, interleukin 11 and leukaemia inhibitory factor in endometrium of women with unexplained infertility during the implantation window. Reprod Biol Endocrinol. 2007; 5: 44. doi:10.1186/1477-7827-5-44.
- Dimitriadis E., Stoikos C., Stafford-Bell M, Clark I., Paiva P., Kovacs G., Salamonsen L.A. Interleukin-11, IL-11 receptoralpha and leukemia inhibitory factor are dysregulated in endometrium of infertile women with endometriosis during the implantation window. J Reprod Immunol. 2006; 69(1): 53-64. doi:10.1016/j.jri.2005.07.004.
- Tsai H.D., Chang C.C., Hseih Y.Y., Lo HY. LIF expression in different endometrial locations between fertile and infertile women throughout different menstrual phases. JARG. 2000; 17(8): 415-8. DOI: 10.1023/a:1009457016871
- Ниаури Д.А., Гзгзян А.М., Кветной И.М., Коган И.Ю., Джемлиханова Л.Х., Крихели И.О., Федорова И.Д., Лесик Е.А., Шарфи Ю.Н., Крылова Ю.С., Шильникова Е.М. Иммуногистохимическая характеристика рецептивности эндометрия в циклах ЭКО. Акушерство и гинекология. 2014; (9): 44-9. [Niauri D.A., Gzgzyan A.M., Kvetnoy I.M., Kogan I.Yu., Dzhemlikhanova L.Kh.,Krikheli I.O., Fedorova I.D., Lesik E.A., Sharfi Yu.N., Krylova Yu.S., Shilnikova E.M. Immunohistochemical characteristics of endometrial receptivity in IVF cycles. Akusherstvo i ginekologija/Obstetrics and Gynegology. 2014; (9): 44–9. (In Russian)].
Received 16.05.2019
Accepted 21.06.2019
About the Authors
Kulikova Galina Victorovna, PhD., Senior Research Associate, Pathology Department Kulakov NMRC OGP, Ministry of Healthcare of the Russian Federation. 4 Oparin str, 117997, Moscow, RussiaAbdurakhmanova Nigora Farukhovna, post-graduate student of the 3rd year of the Department of Auxiliary Technologies in Infertility Treatment, Kulakov NMRC OGP, Ministry of Healthcare of the Russian Federation. 4 Oparin str, 117997, Moscow, Russia
Fayzullina Nafisa Mynavarovna, PhD., Senior Research Associate, Pathology Department Kulakov NMRC OGP, Ministry of Healthcare of the Russian Federation.4 Oparin str, 117997, Moscow, Russia
Asaturova Aleksandra Vyacheslavovna M.D., Ph.D. senior scientific researcher, Kulakov NMRC OGP, Ministry of Healthcare of the Russian Federation. 4 Oparin str,
117997, Moscow, Russia
Shchegolev Aleksandr Ivanovich, MD, professor, head of Pathology Department Kulakov NMRC OGP, Ministry of Healthcare of the Russian Federation. 4 Oparin str,
117997, Moscow, Russia
Ziganshina Marina Mihajlovna, PhD., Senior Research Associate, Laboratory of Clinical Immunology, Kulakov NMRC OGP, Ministry of Healthcare of the Russian Federation. 4 Oparin str, 117997, Moscow, Russia
Dolgushina Nataliya Vitalievna, M.D., Ph.D., M.P.H., Head of R&D Department Kulakov NMRC OGP, Ministry of Healthcare of the Russian Federation. 4 Oparin str,
117997, Moscow, Russia
For citation: Kulikova G.V., Abdurakhmanova N.F., Faizullina N.M., Asaturova A.V., Shchegolev A.I., Ziganshina M.M., Dolgushina N.V. Receptivity of thin endometrium in patients undergoing assisted reproduction.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2019; 10:100-7. (In Russian).
https://dx.doi.org/10.18565/aig.2019.10.100-107



