Early- and late-onset fetal growth restriction: differential diagnosis based on pro- and antioxidant system markers
Objective. To investigate pro- and antioxidant system parameters in early- and late-onset fetal growth restriction.Ganichkina M.B., Vysokikh M.Yu., Tyutyunnik V.L., Chagovets V.V., Kan N.E.
Materials and methods. The study included 82 patients: the main group consisted of 41 women whose pregnancies were complicated by fetal growth restriction and the control group included 41 women without fetal growth restriction. The main group was divided into two subgroups. Subgroup Ia consisted of 20 pregnant women with early-onset fetal growth restriction (manifested before 34 weeks’ gestation), subgroup Ib included 21 patients with late-onset fetal growth restriction (manifested after 34 weeks’ gestation). Maternal plasma pro- and antioxidant system markers were evaluated using spectrophotometry, polarography and Western blot analysis.
Results. A significant increase in oxidative stress level was observed in early-onset fetal growth restriction subgroup, whereas there was a decrease in late-onset fetal growth restriction subgroup compared to the controls. Logistic regression model was developed to differentiate early- and late-onset fetal growth restriction using maternal plasma pro- and antioxidant system parameters.
Conclusion. Maternal plasma pro- and antioxidant system markers can be used in differential diagnosis of early- and late-onset fetal growth restriction after 34 weeks’ gestation.
Keywords
Fetal growth restriction (FGR) is an actual problem in modern obstetrics and one of the leading contributors to perinatal morbidity and mortality. Along with infectious causes and congenital malformations of the fetus, FGR significantly influences perinatal mortality [1]. FGR affects not only the fetal condition, but also the subsequent development of the child, determining his lifelong health [2–4]. The incidence of FGR varies in different countries and has an inverse relationship with a decrease in the gestational age. FGR occurs more frequently in countries with limited resources. Approximately 10% of full-term infants in developed countries are small to gestational age children. While in developing countries, their rate is 23% [5]. Currently, this pathology occurs in 5–18% of cases on the territory of the Russian Federation [1, 6].
Early-onset FGR occurs in 20–30% of cases; it is associated with early-onset preeclampsia, severe placental insufficiency and chronic fetal hypoxia in 50% of cases. Late-onset FGR is identified in 70–80% of cases of FGR and is associated with preeclampsia in 10% of cases [7]. Both early- and late-onset FGR are likely to be associated with impaired placental function, but the question of whether these changes are similar in the case of early- and late-onset FGR is still unknown. Placental insufficiency in early-onset FGR is a consequence of early implantation disorders [8]. It is not entirely clear whether late-onset FGR is the result of early implantation disorders or the outcome of impaired placental function in the second half of pregnancy. The latter statement is supported by deviations in the Doppler parameters of the uterine arteries in the third trimester of pregnancy with normal indicators at an earlier time [9].
It is important for clinicians to determine the time of FGR manifestations, due to the different pathogenetic pathways and different severity of perinatal outcomes for newborns with early-onset and late-onset FGR. In some cases, early-onset FGR is diagnosed at a later stage since prenatal diagnosis is not accurate enough; this fact increases the probability of antenatal fetal death, as well as the risk of adverse perinatal outcomes [10]. Therefore, it is necessary to search for markers which could differentiate between two pathogenetically different forms of this pregnancy complication and thus reduce the risks for newborns.
Nowadays, the etiopathogenetic mechanisms of FGR development remain understudied. Oxidative stress is known to underlie great obstetric syndromes, including FGR [11–14]. Most studies indicate an increase in oxidative stress in FGR. However, at present, the data on differences in the functional state of the pro- and antioxidant systems in the early-onset and late-onset FGR are limited [15–17].
Therefore, the purpose of our study was to investigate the functional state of pro- and antioxidant systems of the blood plasma in pregnant women with in early- and late-onset FGR.
Materials and Methods
The study included 82 pregnant women. The main group consisted of 41 patients with FGR. Subgroup Ia consisted of 20 pregnant women with early-onset FGR (manifested before 34 weeks’ gestation), subgroup Ib included 21 patients with late-onset FGR (manifested after 34 weeks’ gestation). The control group included 41 women. The criteria for inclusion in the study were patients’ age 18–45 years, spontaneous singleton pregnancy, gestational age of 26–40 weeks, informed consent to participate in the study. Patients whose pregnancy was complicated by FGR were eligible for inclusion in the main group, and patients with singleton pregnancy without FGR were eligible for the control group. Exclusion criteria for both groups were severe extragenital pathologies, pregnancy following ART, preeclampsia, multiple pregnancy, fetal malformations, maternal genetic and acute infectious diseases.
All participants of the study were collected peripheral venous blood before delivery. Blood samples were taken in VACUETTE® tubes with EDTA, then centrifuged for 20 minutes at 300g ((t=4oC); the selected plasma was re-centrifuged for 10 minutes at 14 000g. The levels of markers of oxidative stress, lipid peroxidation products, MDA (malondialdehyde) and 4-hydroxynonenal were determined in the blood plasma samples of pregnant women using spectrophotometry; antioxidant activity, namely catalase was determined by polarographic method; glutathione peroxidase (GP), superoxide dismutase (SOD) and the contents of total, oxidized, and reduced glutathione were determined using spectrophotometric method; SOD1, SOD2, and catalase content was estimated by Western blot analysis.
Statistical analysis
For statistical processing of the results, R language scripts and the RStudio program were used, as well as Microsoft Office Excel 2016 and the «Statistica» v.13.0 software packages, StatSoft Inc. (USA). Compliance of the analyzed parameters with the law of normal distribution was evaluated using the Shapiro-Wilk test. To describe quantitative data with a normal distribution, the arithmetic mean (M) and standard deviation (SD) in the M (SD) format were used. Statistical analysis of quantitative data with normal distribution was performed using the Student’s t-test. Absolute values and percentages were used to describe qualitative indicators. The Pearson χ2 test was used to compare the groups. The odds ratio (OR) and its confidence interval (CI) were also determined. The threshold significance level p was assumed to be 0.05. Logistic regression models were developed to assess the possibility of classifying patients into groups of early-onset and late-onset FGR on the basis of the studied parameters. Parameters that were statistically significantly different between these groups were considered as independent variables in the model. The fact of the patient’s belonging to the group of early-onset or late-onset FGR was considered as an independentvariable.TheWaldcriterion,95%confidence interval (CI), odds ratio (OR), and its confidence interval were determined for each model. The quality of the designed model was determined by constructing the ROC curve, determining the area under the ROC curve (AUC), as well as calculating the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the developed model.
Results and Discussion
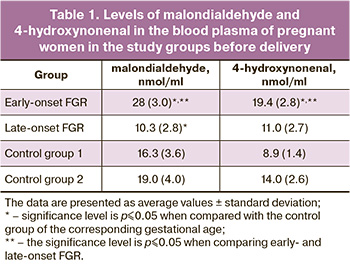 All patients included in the study were evaluated for age and weight-height parameters. It should be noted that there was a predominance of chronic arterial hypertension (CAH) (n=8; 40%) (OR=0.08; CI: 0.01– 0.7; p≤0.05), myopia (n=11; 55%) (OR=0.2; CI: 0.1–0.8; p≤0.05) and varicose veins (n=5; 25%; p=0.05) in the subgroup of early-onset FGR. The study of the course of pregnancy showed that arterial hypertension was more often observed in the group of patients with FGR: in the 1st trimester (n=3; 7.3%) (OR=0.3; CI: 0.03–3.2); p>0.05), in the 2nd (n=5; 12.1%) (OR=0.4; CI: 0.1–2.0; p>0.05) and in the 3rd trimesters (n=16; 39%) (OR=0.1; CI: 0.01–0.6; p≤0.05) with a predominance in the subgroup of early-onset FGR. Also, oligohydramnios was more frequently detected in the main group (n=26; 65%) (OR=5.1; CI: 2–13,1; p≤0.05), which should be considered as one of the manifestations of FGR. In order to have a complete understanding of the functional state of the pro- and antioxidant systems, as well as to determine the diagnostic role of markers of oxidative damage and antioxidant protection in early- and late-onset FGR, the oxidative status of the blood plasma in pregnant women of the study groups was evaluated. The comparative analysis revealed elevated levels of lipid peroxidation, MDA, and 4-hydroxynonenal in the blood plasma of pregnant women with early-onset FGR compared to the control group and late-onset FGR (p<0.001). A statistically significant decrease (p<0.001) in the level of MDA content in comparison with the control group was revealed in late-onset FGR. The data are presented in Table 1.
All patients included in the study were evaluated for age and weight-height parameters. It should be noted that there was a predominance of chronic arterial hypertension (CAH) (n=8; 40%) (OR=0.08; CI: 0.01– 0.7; p≤0.05), myopia (n=11; 55%) (OR=0.2; CI: 0.1–0.8; p≤0.05) and varicose veins (n=5; 25%; p=0.05) in the subgroup of early-onset FGR. The study of the course of pregnancy showed that arterial hypertension was more often observed in the group of patients with FGR: in the 1st trimester (n=3; 7.3%) (OR=0.3; CI: 0.03–3.2); p>0.05), in the 2nd (n=5; 12.1%) (OR=0.4; CI: 0.1–2.0; p>0.05) and in the 3rd trimesters (n=16; 39%) (OR=0.1; CI: 0.01–0.6; p≤0.05) with a predominance in the subgroup of early-onset FGR. Also, oligohydramnios was more frequently detected in the main group (n=26; 65%) (OR=5.1; CI: 2–13,1; p≤0.05), which should be considered as one of the manifestations of FGR. In order to have a complete understanding of the functional state of the pro- and antioxidant systems, as well as to determine the diagnostic role of markers of oxidative damage and antioxidant protection in early- and late-onset FGR, the oxidative status of the blood plasma in pregnant women of the study groups was evaluated. The comparative analysis revealed elevated levels of lipid peroxidation, MDA, and 4-hydroxynonenal in the blood plasma of pregnant women with early-onset FGR compared to the control group and late-onset FGR (p<0.001). A statistically significant decrease (p<0.001) in the level of MDA content in comparison with the control group was revealed in late-onset FGR. The data are presented in Table 1.
In the blood plasma of pregnant women with early-onset FGR, there was a reduced activity of the antioxidant enzymes SOD and GP in comparison with the control group and late-onset FGR; while the activity of catalase, on the contrary, was increased. SOD is known to neutralize superoxide anion to form peroxide, which is utilized by catalase. However, despite the increased activity of catalase, it is not enough for the utilization of peroxide, so adequate antioxidant protection cannot be provided; it is evidenced by the increased levels of lipid peroxidation products, MDA, and 4-hydroxynonenal in the blood plasma of pregnant women with early-onset FGR. It should be noted that despite the reduced activity of SOD and GP in early- onset FGR, the levels of SOD 1 and SOD 2 were also reduced in comparison with the control group. Low activity of the antioxidant enzymes SOD and GP was observed in late-onset FGR. At the same time, the levels of SOD1, SOD2, and catalase in late-onset FGR were statistically significantly lower in comparison with the control group (Table 2).
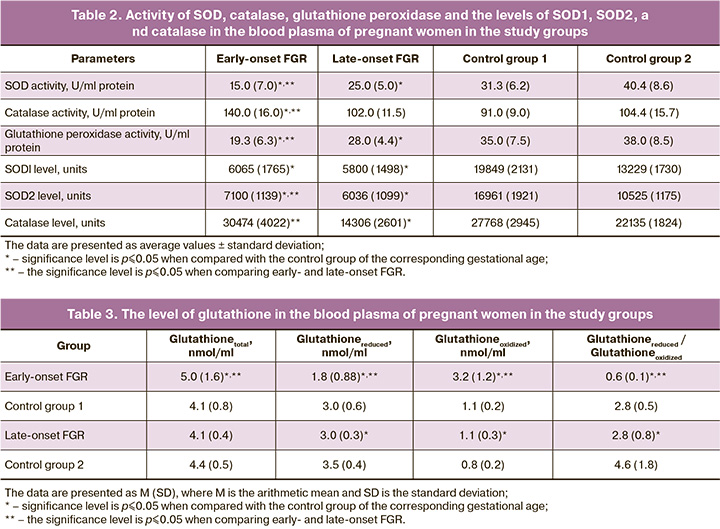
Summarizing the data on the state of the pro- and antioxidant systems in the mother, it can be concluded that the severity of oxidative stress is observed when there is a reduced antioxidant activity in the blood plasma of a pregnant woman with early-onset FGR. In late-onset FGR, despite low indicators of oxidative stress, the activity of antioxidant enzymes and their content were at a subthreshold level, which can be suggestive of a reduced overall protective antioxidant potential of the mother’s body. Oxidative stress is known to increase during normal pregnancy with gestational age and is accompanied by compensatory activity of antioxidant enzymes [15, 16]. Impaired remodeling of the spiral arteries and subsequent abnormal placentation trigger uncontrolled oxidative stress, which is observed in the case of early-onset FGR [16]. Low indicators of oxidative stress in late-onset FGR are probably associated with a low level of systemic inflammatory response of various origin, which makes it possible to prolong pregnancy taking into account the characteristics of the pregnant woman.
The data on the level of glutathione in the blood plasma of pregnant women are presented in Table 3. It should be noted that the ratio of glutathione reduced/glutathione oxidized was decreased in the mother’s blood plasma in both early- and late-onset FGR.
The results of the study indicate differences in the levels of oxidative stress in the early- and late-onset FGR. The data are presented in Figure.
Taking into account the obtained data, logistic regression models were developed that make it possible to differentiate early- and late-onset FGR. Each model was based on one of the parameters, the levels of the parameter were statistically significantly different between the groups of early- and late-onset FGR. The characteristics of the obtained models are shown in Table 4.
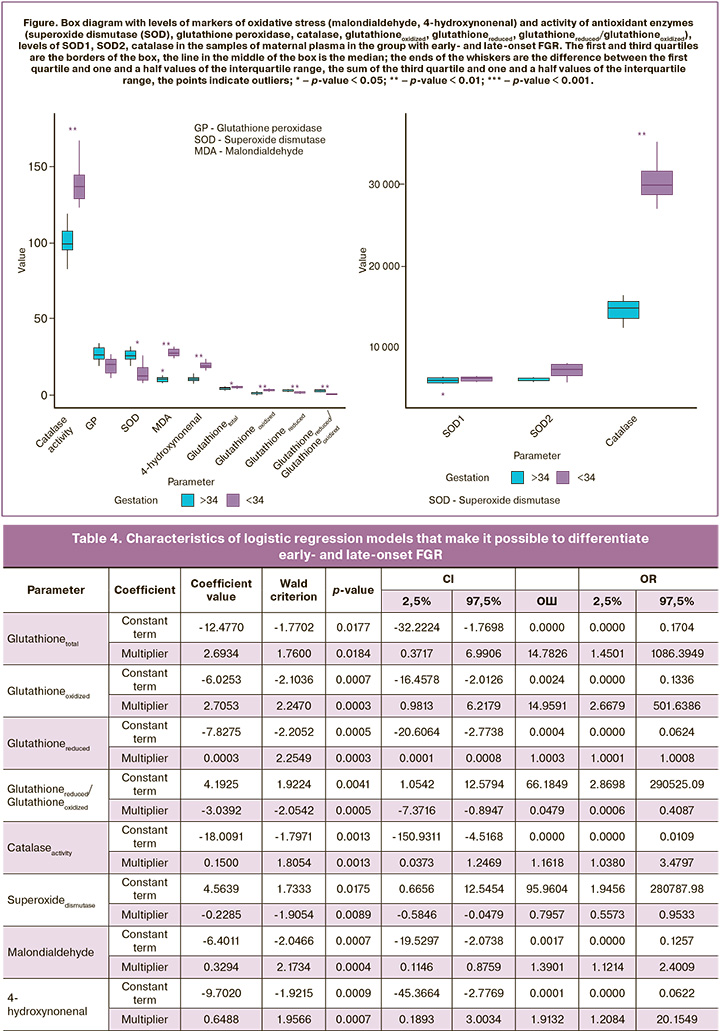
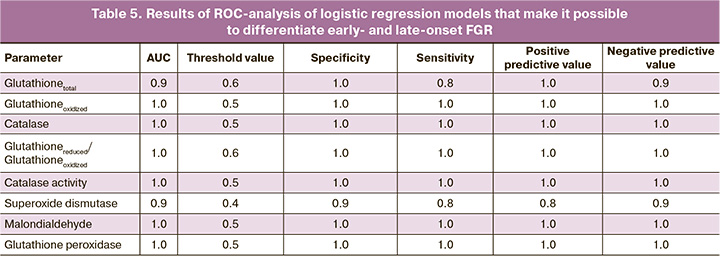
In order to evaluate the effectiveness of the models, ROC analysis was performed. The results of ROC analysis are presented in Table 5. ROC analysis demonstrated that early- and late-onset FGR can be differentiated with high sensitivity and specificity by identifying the above-mentioned specific markers of pro- and antioxidant systems in the blood plasma of pregnant women and using the proposed logistic regression models.
Conclusion
Thus, the obtained results, on the one hand, increased our understanding of the pathogenetic characteristics of early- and late-onset fetal growth retardation, and on the other hand, they allowed us to identify new non-invasive markers for differential diagnosis of pathogenetically different forms of this syndrome, which will enable clinicians to determine the optimal obstetric tactics for improving perinatal outcomes. There is a need for further research to identify the role of the detected changes in the pro- and antioxidant systems in the pathogenesis of fetal growth retardation.
References
- Стрижаков А.Н., Игнатко И.В., Тимохина Е.В., Белоцерковцева Л.Д. Синдром задержки роста плода. Патогенез. Диагностика. Лечение. Акушерская тактика. М.: ГЭОТАР-Медиа; 2014. 120 с. [Strizhakov A.N., Ignatko I.V., Timohina E.V., Belotserkovtseva L.D. Fetal growth retardation. Pathogenesis. Diagnostics. Treatment. Obstetric tactics. GEOTAR-Media; 2014; 120p. (in Russian)].
- Anderson N.H., Sadler L.C., Stewart A.W., McCowan L.M.E. Maternal and pathological pregnancy characteristics in customised birthweight centiles and identification of at-risk small-for-gestational-age infants: a retrospective cohort study. BJOG. 2012; 119(7): 848-56. https://dx.doi.org/10.1111/j.1471-0528.2012.03313.x.
- Gardosi J., Madurasinghe V., Williams M., Malik A., Francis A. Maternal and fetal risk factors for stillbirth: population based study. BMJ. 2013; 346: f108. https://dx.doi.org/10.1136/bmj.f108.
- Mayer C., Joseph K.S. Fetal growth: a review of terms, concepts and issues relevant to obstetrics. Ultrasound Obstet. Gynecol. 2013; 41(2): 136-45. https://dx.doi.org/10.1002/uog.11204.
- De Onis M., Blössner M., Villar J. Levels and patterns of intrauterine growth retardation in developing countries. Eur. J. Clin. Nutr. 1998; 52(Suppl. 1): S5-15.
- Макаров И.О., Юдина Е.В., Боровкова Е.И. Задержка роста плода. Врачебная тактика. Учебное пособие. 3-е изд. М.: МЕДпреcс-информ, 2016; 56 с. [Makarov I.O., Yudina E.V., Borovkova E.I. Fetal growth restriction. Management. Training manual. Medpress-inform. 2016; 56 p. (in Russian)].
- Crovetto F., Crispi F., Scazzocchio E., Mercade I., Meler E., Figueras F. et al. First-trimester screening for early and late small-for-gestational-age neonates using maternal serum biochemistry, blood pressure and uterine artery Doppler. Ultrasound Obstet. Gynecol. 2014; 43(1): 34-40. https://dx.doi.org/10.1002/uog.12537.
- Spinillo A., Gardella B., Bariselli S., Alfei A., Silini E., Dal Bello B. Placental histopathological correlates of umbilical artery Doppler velocimetry in pregnancies complicated by fetal growth restriction. Prenat. Diagn. 2012; 32(13): 1263-72. https://dx.doi.org/10.1002/pd.3988.
- Llurba E., Turan O., Kasdaglis T., Harman C.R., Baschat A.A. Emergence of late-onset placental dysfunction: relationship to the change in uterine artery blood flow resistance between the first and third trimesters. Am. J. Perinatol. 2013; 30(6): 505-12. https://dx.doi.org/10.1055/s-0032-1329181.
- Jacobsson B., Ahlin K., Francis A., Hagberg G., Hagberg H., Gardosi J. Cerebral palsy and restricted growth status at birth: population-based case-control study. BJOG. 2008; 115(10): 1250-5. https://dx.doi.org/10.1111/j.1471-0528.2008.01827.x.
- Biri A., Bozkurt N., Turp A., Kavutcu M., Himmetoglu O., Durak I. Role of oxidative stress in intrauterine growth restriction. Gynecol. Obstet. Invest. 2007; 64(4): 187-92. https://dx.doi.org/10.1159/000106488.
- Longini M., Perrone S., Kenanidis A., Vezzosi P., Marzocchi B., Petraglia F. et al. Isoprostanes in amniotic fluid: a predictive marker for fetal growth restriction in pregnancy. Free Radic. Biol. Med. 2005; 38(11): 1537-41. https://dx.doi.org/10.1016/j.freeradbiomed.2005.02.017.
- Kamath U., Rao G., Kamath S.U., Rai L. Maternal and fetal indicators of oxidative stress during intrauterine growth retardation (IUGR). Indian J. Clin. Biochem. 2006; 21(1): 111-5. https://dx.doi.org/ 10.1007/BF02913077.
- Mert I., Oruc A.S., Yuksel S., Cakar E.S., Buyukkagnici U., Karaer A. Role of oxidative stress in preeclampsia and intrauterine growth restriction. J. Obstet. Gynaecol. Res. 2012; 38(4): 658-64. https://dx.doi.org/10.1111/j.1447-0756.2011.01771.x.
- Toescu V., Nuttall S.L., Martin U., Kendall M.J., Dunne F. Oxidative stress and normal pregnancy. Clin. Endocrinol. (Oxf). 2002; 57(5): 609-13. https://dx.doi.org/10.1046/j.1365-2265.2002.01638.x.
- Duhig K., Chappell L.C., Shennan A.H. Oxidative stress in pregnancy and reproduction. Obstet. Med. 2016; 9(3): 113-6. https://dx.doi.org/10.1177/1753495X16648495.
- Хачатрян З.В., Кан Н.Е., Красный А.М., Садекова А.А., Куревлев С.В., Тютюнник В.Л. Метилирование генов в плаценте при задержке роста плода. Акушерство и гинекология. 2019; 12: 54-8. [Khachatryan Z.V., Kan N.E., Krasnyi A.M., Sadekova A.A., Kurevlev S.V., Tyutyunnik V.L. Methylation of genes in the placenta with fetal growth retardation. Akusherstvo i ginekologiya / Obstetrics and Gynecology. 2019; 12: 54-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.12.54-58.
Received 25.08.2020
Accepted 11.09.2020
About the Authors
Maria B. Ganichkina, graduate student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,Ministry of Health of Russia. Tel.: +7(915)239-77-43. E-mail: mariaganichkina@yandex.ru.
117997, Russia, Moscow, Ac. Oparina str., 4.
Mikhail Yu. Vysokikh, PhD, the Head of mitochondrial medicine research group, Academician V.I. Kulakov National Medical Research Center for Obstetrics,
Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(926)377-32-80. E-mail: m_vysokikh@oparina4.ru.
117997, Russia, Moscow, Ac. Oparina str., 4.
Victor L. Tyutyunnik, PhD, MD, deputy head doctor of the Perinatal Center of European Medical Center.
Tel.: +7(903)969-50-41. E-mail: tioutiounnik@mail.ru. ORCID: 0000-0002-5830-5099; Scopus ID: 56190621500; Researcher ID: B-2364-2015.
125040, Russia, Moscow, Pravda str. 15/1.
Vitaliy V. Chagovets, PhD, senior researcher of Laboratory of Proteomics and Metabolomics of Human Reproduction, Department of Systems Biology in Reproduction, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Tel.: +7(495)531-44-44. E-mail: v_chagovets@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Natalia E. Kan, PhD, MD, head doctor of the Perinatal Center of European Medical Center; Professor of the Department of Obstetrics and Gynecology,
Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Tel.: +7(926)220-86-55. E-mail: kan-med@mail.ru. ORCID: 0000-0001-5087-5946; Scopus ID: 57008835600; Researcher ID: B-2370-2015.
125040, Russia, Moscow, Pravda str. 15/1; 117997, Russia, Moscow, Ac. Oparina str., 4.
For citation: Ganichkina M.B., Vysokikh M.Yu., Tyutyunnik V.L., Chagovets V.V., Kan N.E. Early- and late-onset fetal growth restriction: differential diagnosis based on pro- and antioxidant system markers.
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2020; 9: 66-72 (in Russian)
https://dx.doi.org/10.18565/aig.2020.9.66-72



