Carbohydrate and lipid metabolism and oxidative stress parameters in patients with menstrual irregularities and obesity
Aim. To investigate the association between oxidative stress and metabolic parameters in patients with obesity and menstrual irregularities.Glazkova O.L., Shmeleva S.V.
Materials and methods. The study included 24 obese women aged 20–35 years with menstrual irregularities (study group) and 15 obese women with regular menstrual cycles (control group).
Results. Body mass index ranged from 30.8 to 41.2 and from 29.7 to 40.9 kg/m2 in the study and control group patients, respectively. Study group patients had statistically significantly higher serum VLDL-C concentration. There were small but statistically significant differences between the study and control groups in the concentration of malondialdehyde [2.8 nmol/ml (0.56; 3.7) vs 1.5 nmol/ml (0.43; 2.1)]. Study group patients with PCOS had statistically significantly higher concentrations of malondialdehyde [3.5 (2.4; 3.9) nmol/ml],
and 8-OH-deoxyguanosine [0.36 (0.25; 0.44) ng/ml] than control subjects. Fasting and post-load insulin concentrations, serum concentrations of VLDL-C, LDL-C, SHBG, and free testosterone were statistically significantly higher in patients with PCOS than in control subjects.
Conclusion. Patients with menstrual irregularities and obesity had higher levels of metabolic and antioxidant defense abnormalities than women with obesity and a regular menstrual cycle. These differences were even more pronounced in women with PCOS.
Keywords
The relationship between obesity and female reproductive functions has been the focus of considerable attention among researchers for many decades. Obesity has become a significant and growing public health problem [1–3]. The negative effect of insulin resistance and the associated compensatory hyperinsulinemia characteristic of obesity on androgen metabolism, the metabolism of insulin-like growth factors, and apoptosis processes are no longer in doubt [4–9]. Adipokines, especially leptin, can affect the synthesis of gonadoliberin and hypothalamic activity [10, 11], and steroidogenesis. They can also affect the endometrium's status, especially decidualization and receptivity, and possibly other associated processes. Impaired feedback mechanisms regulating the menstrual cycle are also important, primarily due to changes in transport proteins production [4]. The accumulation of excess fatty tissue affects the adrenal glands' activity, can lead to a decrease in cortisol-binding globulin production and the intensification of cortisol conversion into inactive cortisone (with a subsequent compensatory increase in cortisol synthesis). All this, due to glucocorticoids' counter insulin action, aggravates insulin resistance and leads to increased androgen synthesis in the ovaries. It is presently considered that obesity may negatively affect meiosis and embryo implantation [12; 13].
Clinical manifestations of reproductive disorders associated with excess adipose tissue accumulation vary considerably. Obesity, being the most critical risk factor, does not always lead to gynecologic pathology development. The potential protective role of the genetically determined increased clearance of sex steroids and the deposition of their excess in adipose tissue is under debate. The activity of adipose tissue enzymes involved in the extra-glandular transformation of sex steroids can also affect the reproductive system's state, which is also genetically determined [14–17].
It can be assumed that there are other mechanisms by which obesity affects the reproductive system, contributing to the diversity of clinical manifestations. Currently, the metabolomic approach is increasingly used in research, and one should bear in mind a large number of roles of any molecule in the human body. Oxidative stress, which refers to the accumulation of excessive amounts of reactive oxygen species in the body, is of great interest. Under normal physiological conditions, reactive oxygen species are involved in anti-inflammatory and antitumor protection. But under oxidative stress conditions, excessive reactive oxygen species can damage cellular proteins, lipids, and DNA. Obesity is one of the causes of chronic oxidative stress in the human body [17–24].
The present study aimed to investigate the association between oxidative stress and metabolic parameters in patients with obesity and menstrual irregularities.
Materials and methods
The study included 24 obese women aged 20–35 years with menstrual irregularities (study group) [the mean age 29.1 (26.3; 33.4) years]. The control group consisted of 15 women of the same age with obesity and regular menstrual cycle [mean age 28.7 (25.3; 32.9) years]. Exclusion criteria were hyperprolactinemia, hypothyroidism, adrenal hyperandrogenism, and diabetes mellitus.
Height and body weight were measured to calculate the bodymassindex. Fastingserumlipidprofileswereanalyzed by the colorimetric photometric method. Fasting and 2-hour post glucose load insulin and glucose levels were measured using the chemiluminescent immunoassay and photometric method, respectively. Serum levels of total testosterone and sex hormone-binding globulin (SHBG) were determined by chemiluminescence immunoassay. Free testosterone concentration was calculated. To assess the level of oxidative stress by high-performance liquid chromatography, serum concentrations of malondialdehyde, 8-OH-deoxyguanosine, coenzyme Q-10, and glutathione (reduced) were determined [23, 24].
Statistical analysis
Statistical analysis was performed using the STATISTICA 10 software. The critical level of significance when the testing statistical hypothesis was considered at p <0.05. Quantitative variables were expressed as the median (Me) and interquartile range, where Q1 was the 25th quartile, Q3 was the 75th quartile. It was found that numerical variables were not normally distributed. The statistical significance of between-group differences for continuous variables was tested with the Mann–Whitney test.
Results and discussion
In the study group, 14 patients (58.33%) had opsomenorrhea, 10 (41.66%) had hyperpolymenorrhea or abnormal uterine bleeding, 6 (25%) had a single intramural fibroid ≤5 cm in diameter. Nine (37.5%) women had a history of childbirth, three of which became pregnant after ovulation stimulation (12.5%), two (8.33%) had a miscarriage, and 4 (16.66%) had infertility. The diagnosis of polycystic ovary syndrome (PCOS) according to the Rotterdam criteria was established in 16 women (66.66%) in the study group, of whom 7 (29.16%) had phenotype A.
The body mass index ranged from 30.8 to 41.2 kg/m2 in the study group patients and from 29.7 to 40.9 kg/m2 in the control group; that is, the groups were comparable in this respect.
The study and control groups did not have statistically significant differences in lipid profile such as the concentration of total cholesterol, triglycerides, low-density lipoproteins (LDL-C), high-density lipoproteins (HDL-C) (Table 1). In both groups, there were patients with lipid disorders. Only serum levels of very-low-density lipoproteins (VLDL-C) were insignificantly but statistically significantly higher in the study group patients, which is of interest since it most likely suggests the presence of endothelial dysfunction [18]. We also did not find statistically significant differences between the groups in carbohydrate metabolism parameters, including fasting and post-load hyperinsulinemia. Only the post-load serum glucose level was slightly higher in the study group patients. Differences in the concentration of testosterone, including free testosterone, as well as SHBG also had a trend character. Perhaps a slightly different result would have been obtained using the direct determination of free testosterone. The calculation of free testosterone concentration that we used does not consider a small amount of albumin-bound hormone.
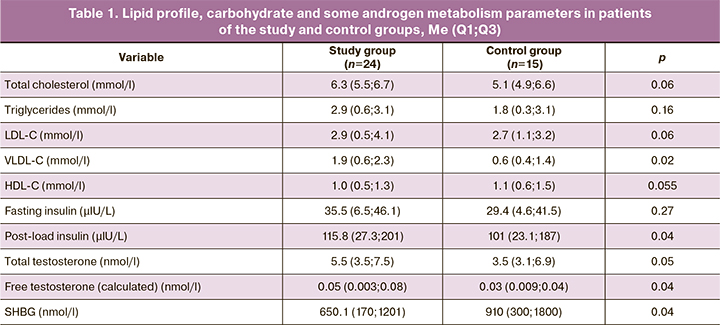
In the study group, the concentration of malonic dialdehyde, a substance formed from the arachidonic and other polyunsaturated fatty acids metabolism, was slightly but statistically significantly higher than in the control group (Table 2). Malondialdehyde interacts with phospholipids, amino acids, and nucleic acids. Determination of this substance's concentration is currently used for predicting and monitoring therapy in coronary heart disease and some kidney, infectious, and oncological diseases, and in anti-aging medicine. Current literature reports evidence about the increased concentration of this substance in patients with PCOS [22, 25]. The concentration of this oxidative stress marker is thought to be associated with lipid abnormalities. We found that its concentration increases in parallel with an increase in VLDL-C concentration in the study group, thus confirming this observation [19].
The concentration of the primary antioxidants – reduced glutathione and coenzyme Q10 – did not differ between patients groups. The same can be said about the concentration of 8-OH-deoxyguanosine, a modified nucleoside formed in a DNA molecule resulting from exposure to reactive oxygen species.
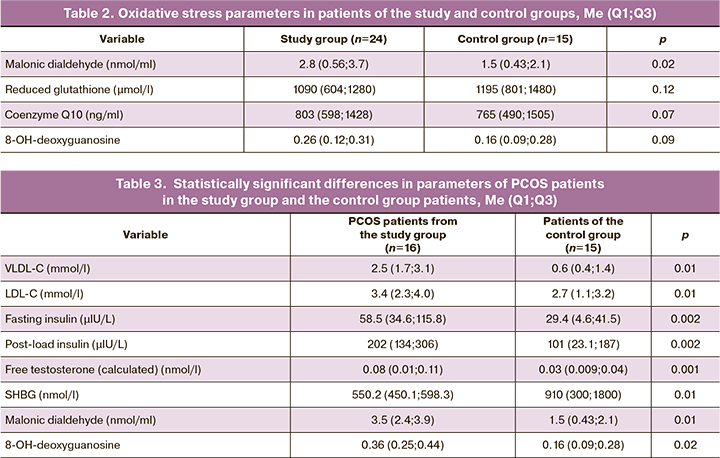
At the next study stage, we selected women with PCOS from the study group (n=16) and compared them with the control group. Table 3 shows only those variables for which the differences were statistically significant.
Patients with PCOS were characterized by the most pronounced lipid and carbohydrate metabolism abnormalities (insulin resistance), which is consistent with the literature. The decrease in SHBG production in these women naturally led to a higher calculated concentration of free testosterone.
Differences in malondialdehyde concentrations between the patients with PCOS and the control subjects were more significant than between the study and control group. Patients with PCOS also had some excess in the serum levels of 8-OH-deoxyguanosine, a modified nucleoside formed in a DNA molecule resulting from exposure to reactive oxygen species. It has mutagenic properties and is regarded as a marker of oxidative stress and risk of carcinogenesis.
Conclusion
The present study's findings suggest that oxidative stress can play a specific role in forming menstrual cycle disorders, affecting various levels of reproductive system regulation. Elucidation of the mechanisms of this negative influence can help in the development of personalized therapy. We have studied only some of the available parameters. At the same time, it is known that the antioxidant system includes many components – enzymes and low molecular weight substances, including vitamins. It seems promising to investigate these factors' whole spectrum in patients with menstrual irregularities to determine their diagnostic and prognostic value.
The most significant changes were found in patients with PCOS. Strictly speaking, they most significantly contributed to the differences between the groups. Whether patients with PCOS are more sensitive to oxidative stress or the disease itself contributes to its formation remains an open question. Insulin resistance in PCOS is tissue-specific, i.e., it is mainly related to peripheral tissues (for example, muscle), and ovarian tissues retain normal and even increased insulin sensitivity. This can be either a genetically determined feature or a consequence, for example, of active oxygen or inflammatory mediator action on tissues. The adverse effects of oxidative stress on the reproductive system may likely occur at an earlier age. Perhaps, analysis of oxidative stress parameters may be considered an additional diagnostic method in patients with PCOS in whom standard diagnostic procedures have been exhausted.
References
- Wang Y.C., McPherson K., Marsh T., Gortmaker S.L., Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011; 378(9793): 815-25. https://dx.doi.org/10.1016/S0140-6736(11)60814-3.
- Hales C.M., Carroll M.D., Fryar C.D., Ogden C.L. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS Data Brief. 2017; (288): 1-8.
- Michelle G., Zaher M., Erkan B. Role of Hormonal and Inflammatory Alterations in Obesity-Related Reproductive Dysfunction at the Level of the Hypothalamic-Pituitary-Ovarian Axis. Reprod. Biol. Endocrinol. 2018; 16(1): 45. https://dx.doi.org/10.1186/s12958-018-0366-6.
- Wei S., Schmidt M.D., Dwyer T., Norman R.J., Venn A.J. Obesity and menstrual irregularity: associations with SHBG, testosterone, and insulin. Obesity (Silver Spring). 2009; 17(5): 1070-6.
- Klenov V.E., Jungheim E.S. Obesity and reproductive function: a review of the evidence. Curr. Opin. Obstet. Gynecol. 2014; 26(6): 455-60. https://dx.doi.org/10.1097/GCO.0000000000000113.
- Wise M.R., Jordan V., Lagas A., Showell M., Wong N., Lensen S., Farquhar C.M. Obesity and endometrial hyperplasia and cancer in premenopausal women: a systematic review. Am. J. Obstet. Gynecol. 2016; 214(6): 689. e1-689. e17. https://dx.doi.org/10.1016/j.ajog.2016.01.175.
- Broughton D.E., Moley K.H. Obesity and female infertility: potential mediators of obesity's impact. Fertil. Steril. 2017; 107(4): 840-7. https://dx.doi.org/10.1016/j.fertnstert.2017.01.017.
- Arcidiacono B., Iiritano S., Nocera A., Possidente K., Nevolo M.T., Ventura V. et al. Insulin resistance and cancer risk: an overview of the pathogenetic mechanisms. Exp. Diabetes Res. 2012; 2012: 789174. https://dx.doi.org/10.1155/2012/789174.
- Kelly C.J., Stenton S.R., Lashen H. Insulin-like growth factor binding protein-1 in PCOS: a systematic review and meta-analysis. Hum. Reprod. Update. 2011; 17(1): 4-16. https://dx.doi.org/10.1093/humupd/dmq027.
- Livingstone C., Borai A. Insulin-like growth factor-II: its role in metabolic and endocrine disease. Clin. Endocrinol. (Oxf). 2014; 80(6): 773-81. https://dx.doi.org/10.1111/cen.12446.
- Lee H., Oh J.Y., Sung Y.A. Adipokines, insulin-like growth factor binding protein-3 levels, and insulin sensitivity in women with polycystic ovary syndrome. Korean J. Intern. Med. 2013; 28(4): 456-63. https://dx.doi.org/10.3904/kjim.2013.28.4.456.
- Brewer C.J., Balen A.H. The adverse effects of obesity on conception and implantation. Reproduction. 2010; 140(3): 347-64. https://dx.doi.org/10.1530/REP-09-0568.
- Tchernof A., Després J.P. Pathophysiology of human visceral obesity: an update. Physiol. Rev. 2013; 93(1): 359-404. https://dx.doi.org/10.1152/physrev.00033.2011.
- Kirschner M.A., Samojlik E., Silber D. A comparison of androgen production and clearance in hirsute and obese women. J. Steroid Biochem. 1983; 19(1B): 607-14. https://dx.doi.org/10.1016/0022-4731(83)90225-x.
- Samojlik E., Kirschner M.A., Silber D., Schneider G., Ertel N.H. Elevated production and metabolic clearance rates of androgens in morbidly obese women. J. Clin. Endocrinol. Metab. 1984; 59(5): 949-54. https://dx.doi.org/10.1210/jcem-59-5-949.
- Яковлев П.П., Ткаченко Н.Н., Коган И.Ю., Комарова Е.М., Лесик Е.А., Мекина И.Д., Крихели И.О., Гзгзян А.М. Возрастные изменения активности овариальной ароматазы у женщин с неэндокринными факторами бесплодия и синдромом поликистозных яичников. Проблемы репродукции. 2020; 26(1): 59-66. [Yakovlev P.P., Tkachenko N.N., Kogan I.Yu., Komarova E.M., Lesik E.A., Mekina I.D., Krikheli I.O., Gzgzyan A.M. Age-related changes in the activity of ovarian aromatase in women with non-endocrine factors of infertility and polycystic ovary syndrome. Problems of reproduction. 2020; 26(1): 59-66. (in Russian)].
- Gambineri A., Fanelli F., Tomassoni F., Munarini A., Pagotto U., Andrew R. et al. Tissue-specific dysregulation of 11β-hydroxysteroid dehydrogenase type 1 in overweight/obese women with polycystic ovary syndrome compared with weight-matched controls. Eur. J. Endocrinol. 2014; 171(1): 47-57. https://dx.doi.org/10.1530/EJE-13-1030.
- Клычникова Е.В., Матвеев С.Б., Рябинин В.А., Годков М.А., Голиков А.П., Ахметов В.В., Михайлов И.П. Окислительный стресс, липидный обмен и их взаимосвязь у больных с тяжелым течением гипертонической болезни в сочетании со стенозом сонных артерий. Клиническая лабораторная диагностика. 2012; 5: 20-2. [Klychnikova E.V., Matveev S.B., Riabinin V.A., Godkov M.A., Golikov A.P., Akhmetov V.V., Mikhailov I.P. The oxidation stress, lipid metabolism and their relationship in patients with severe course of hypertension disease in aggregate with carotid stenosis. Klin. Lab. Diagn. 2012; 5: 20-2. (in Russian)].
- Engin A. Endothelial dysfunction in obesity. Adv. Exp. Med. Biol. 2017; 960: 345-79. https://dx.doi.org/10.1007/978-3-319-48382-5_15.
- Ciaraldi T.P., Aroda V., Mudaliar S., Chang R.J., Henry R.R. Polycystic ovary syndrome is associated with tissue-specific differences in insulin resistance. J. Clin. Endocrinol. Metab. 2009; 94(1): 157-63. https://dx.doi.org/10.1210/jc.2008-1492.
- Agha K., Madmani Y., Shahrour Y., Essali A., Kadro W. Coenzyme Q10 for heart failure. Cochrane Database Syst. Rev. 2014; (6): CD008684. https://dx.doi.org/10.1002/14651858.CD008684.pub2.
- Захаров И.С., Букреева Е.Л. Оксидативный стресс при синдроме поликистозных яичников: прогностическое значение, возможности коррекции. Гинекология. 2018: 20(1): 35-8. [Zakharov I.S., Bukreeva E.L. Oxidative stress in polycystic ovary syndrome: prognostic value, correction possibilities. Gynecology. 2018: 20 (1): 35-8. (in Russian)]. https://dx.doi.org/10.26442/ 2079-5696 20.1.35-38.
- Тайц А.Н., Воробцова И.Н., Курдынко Л.В., Смирнов В.В., Шаповалова А.Б., Церцвадзе Г.К. Патофизиологические аспекты формирования инсулинорезистентности у женщин с синдромом поликистозных яичников. Медицина: Теория и практика. 2018; 3(2): 19-25. [Taits A.N., Vorobtsova I.N., Kurdykova L.V., Smironov V.V. Pathophysiological aspects of the formation of insulin resistance to women with polycystic ovary syndrome. Medicine: Theory and Practice. 2018; 3(2): 19-25. (in Russian)].
- Тоноян Н.М., Козаченко И.Ф., Франкевич В.Е., Чаговец В.В., Токарева А.О., Стародубцева Н.Л., Адамян Л.В. Анализ липидного состава плазмы крови методом масс-спектрометрии у пациенток с миомой матки. Проблемы репродукции. 2019; 25(6): 33-7. [Tonoyan N.M., Kozachenko I.F., Frankevich V.E., Chagovets V.V., Tokareva A.O., Starodubtseva N.L., Adamyan L.V.Analysis of lipid composition of blood plasma by mass spectrometry in patients with uterine myoma. Problems of reproduction. 2019; 25(6): 33-7. (in Russian)].
- Володина М.А., Хащенко Е.П., Уварова Е.В., Высоких М.Ю., Пятаева С.В., Суханова Ю.А., Тарасова Н.В., Сухих Г.Т. Патологические проявления системного оксидативного стресса на фоне митохондриальной дисфункции у подростков с синдромом поликистозных яичников. Репродуктивное здоровье детей и подростков. 2016; 2: 61-5. [Volodina M.A., Khashchenko E.P., Uvarova E.V., Vysokikh M.Yu., Pyataeva S.V., Sukhanova Yu.A., Tarasova N.V., Sukhikh G.T. Pathological manifestations of systemic oxidative stress against the background of mitochondrial dysfunction in adolescents with polycystic ovary syndrome. Reproductive health of children and adolescents. 2016; 2: 61-5. (in Russian)].
Received 10.06.2020
Accepted 28.09.2020
About the Authors
Olga L. Glazkova, Ph.D., Associate Professor at the Department of Obstetrics and Gynecology, Russian Medical Academy of Continuing Postgraduate Education,Ministry of Health of Russia. E-mail: glazkova-ol-le@yandex.ru. ORCID: 0000-0001-9324-2866. 125993, Russia, Moscow, Barrikadnaya str., 2/1-1.
Svetlana V. Shmeleva, Dr. Med. Sci., Professor at the K.G. Razumovsky Moscow State University of Technology and Management (PKU). E-mail: 89151479832@mail.ru. ORCID: 0000-0003-0390-194X. 109004, Russia, Moscow, Zemlyanoy Val str., 73.
For citation: Glazkova O.L., Shmeleva S.V. Carbohydrate and lipid metabolism and oxidative stress parameters in patients with menstrual irregularities and obesity.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 1: 104-109 (in Russian)
https://dx.doi.org/10.18565/aig.2021.1.104-109



