Cerebral-placental-uterine ratio as a novel combined parameter of obstetric Doppler ultrasonography
Aim. To identify the percentiles of the cerebral-placental-uterine ratio (CPUR) and investigate the likelihood of giving birth to low birth weight infants in patients with reduced CPUR. Materials and methods. The study retrospectively analyzed pregnancy outcomes and findings of 1780 Doppler studies performed in 1215 patients at 24–40 weeks of gestation. The Doppler study included measurement of pulsatility index in the fetal middle cerebral artery, umbilical cord arteries, and uterine arteries. CPUR was calculated as sequential divisions of the above parameters. Results. The study group included 79 (6.5%) newborns with birth weight <10th percentile; 1136 (93.5%) newborns with birth weight ≥10th percentile were assigned to the control group. The results of 1639 Doppler studies of the control group formed the basis of the CPUR percentile intervals. A decrease in CPUR <5th percentile statistically significantly increased the odds of giving birth to low birth weight infants [OR 5.0 (95%CI; 3.1–8.1) (p <0.0001)]. Conclusion. CPUR is a combined measure that is statistically significantly associated with increased odds of delivering low birth weight babies. These pilot values can be used for further prospective studies.Yarygina T.A., Bataeva R.S., Gus A.I.
Keywords
At the end of the last century, Barker D.J. (1990) established the hypothesis known as "fetal programming" [1], suggesting that epigenetic, environmental factors acting during embryonic and fetal life determine the risk of developing adult diseases, in particular non-infectious diseases. Mechanism of fetal programming involves maternal body composition, maternal dietary intake, uteroplacental blood flow, and placental function that produce permanent structural, physiological, and metabolic changes, thereby predisposing an individual to cardiovascular, metabolic, and endocrine disease in adult life.
Over the past decades, this concept has been further developed in connection with the potential to prevent complications by medical intervention in the preconception and intrauterine periods in high-risk patients, thus improving the quality and duration of a person's life [2, 3].
The normal placental function is a factor of paramount importance in the antenatal period, which has a long-term effect on a person's susceptibility to chronic diseases in adulthood [3, 4]. In particular, low birth weight has been associated with a higher risk of developing type 2 diabetes, obesity, neurological and cardiac pathology in later life [2, 4–6].
For the Russian Federation, low birth weight and prematurity constitute a significant public health problem, since stunted growth and malnutrition are diagnosed annually in more than 98 thousand newborns, accounting for 5.7% of all full-term babies and 14.5% of preterm infants. [7]. According to official statistics, among children aged under 14 years, 4.2% have endocrine diseases, nutritional and metabolic disorders, 9.1% have diseases of the nervous system, and 1.9% have circulatory diseases.
At the age of 15–17, these conditions' prevalence increases to 10.2%, 12.4%, and 5.2%, respectively [7]. Among the adult population, these diseases are among the leading causes of sickness, absence, disability, and mortality [8]
Given the potential for reducing these indicators by identifying high-risk pregnant women, it is imperative to develop and implement additional diagnostic tools for predicting and monitoring fetal status and possible prevention and treatment options for growth restriction [9, 10].
Fetal growth restriction is associated with redistribution of blood flow at an early stage in fetal adaptation to hypoxemia (so-called brain sparing) [11]. However, having an initially protective function, long-term fetal circulatory redistribution is associated with more severe neurodevelopmental impairments than those born with fetal growth restriction alone [12]. Neonatal studies demonstrate that persistently increased fetal brain perfusion may lead to hyperoxia and further damage to the brain tissue [13].
In recent years, studies addressing this issue have been increasingly focused on the cerebroplacental ratio (CPR), reflecting redistribution of cardiac output to the cerebral circulation and multidirectional changes in the pulsatility index (PI) of the fetal middle cerebral artery and umbilical cord artery. CPR was established by the International Consensus (2016) [14] and the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) (2019) [15] as an ultrasound parameter for the diagnosis of late fetal growth restriction.
A study by Monteith et al. (2019) [16] demonstrated that growth-restricted pregnancies with a CPR<1 have significantly increased risk of delayed neurodevelopment at three years of age when compared to pregnancies with abnormal Doppler measurements alone. The authors concluded that their results substantiate the benefit of routine assessment of CPR in pregnancies with fetal growth restriction and counseling parents regarding the long-term outcome of affected infants. Russian researchers, for example, O.V. Trokhanova et al. (2018) [16], support the definition of CPR as a mandatory indicator in the diagnosis of fetal status. They found a 17-fold increase in the incidence of fetal growth restriction among pregnancies with decreased CPR. Much research [17, 18] attributes the CPR reduction to markers of the fetus not reaching its growth potential, postulating the advisability of determining this ratio in ultrasound examination of the fetus, regardless of their expected birth weight. Hernandez-Andrade et al. (2018) [19] reported that a low CPR at 20–24 weeks of gestation could predict reduced fetal size later in pregnancy or at birth.
However, some other studies showed the opposite results. For example, Akolekar et al. (2019) [20] and Leavitt et al. (2019) [21] did not confirm the effectiveness of routine assessment of CPR at 35–37 weeks' gestation in for the prediction of neonatal small for gestational age and short-term adverse outcomes.
Another aspect and additional information were published by Zohav et al. (2019) [22]. In a large retrospective analysis, they demonstrated significant advantages of using local CPR reference ranges developed in their cohort of pregnant women, rather than external reference CPR values.
Uterine artery blood flow indicators were not included in the criteria for the diagnosis of fetal growth restriction after 32 weeks of pregnancy [14, 15] and are not used as criteria to determine the optimal time for delivery [23]. However, an increase in the uterine artery PI is significantly associated with fetal growth restriction [24], including in initially low-risk primigravida women [25], and with the development of perinatal hypoxic complications, regardless of fetal weight [26].
To include this vital indicator in a comprehensive Doppler blood flow assessment, MacDonald et al. [27] in 2019 proposed a novel Doppler variable combination, cerebral-placental-uterine ratio (CPUR), which includes a single-stage estimation of umbilical artery PI, middle cerebral artery PI, and CPR. In their prospective cohort study, conducted at 36 weeks of gestation in 347 primary pregnant women, the authors demonstrated that CPUR detected more fetal growth restriction cases than CPR alone.
In our country, no studies investigating CPUR have yet been published. Therefore it seems interesting to study both combined Doppler indicators, CPR and CPUR, with the determination of their percentile values for the Russian population, which was the aim of this study.
Materials and methods
We conducted a retrospective population study of pregnancy outcomes and analyzed findings of 1780 Doppler studies performed in 1215 patients at 11–13 and 24–40 weeks of gestation who were managed at the Fetal Medicine Centre in 2016–2018.
The inclusion criteria were singleton pregnancy that resulted in a live birth at 24 or more weeks, a live fetus in the uterine cavity at the time of the study, no data on the genetic and structural pathology of the fetus and child, recorded Doppler study results, informed consent of the patient to participate in the study with the provision of data on pregnancy outcomes.
The exclusion criteria were multiple pregnancies, fetal or newborn congenital malformations and chromosomal abnormalities, absence of Doppler flow measurements, patient refusal to participate in the study.
Fetal gestational age was estimated using the INTERGROWTH-21st standards, including the fetus's parietal-coccygeal length at 11–13 weeks of gestation [28].
Ultrasound examination after 24 weeks of pregnancy was performed at the discretion of attending physicians using the Voluson E8 Expert ultrasound system (GE Healthcare, USA) with a 4D multifrequency 2–8 MHz probes to measure the pulsatility index in the fetal middle cerebral artery, umbilical cord arteries, and uterine arteries following the recommendations of ISUOG [29]. CPR was calculated as the PI ratio in the middle cerebral artery to PI in the umbilical arteries. Fetal Medicine Foundation reference ranges from a large study by the Fetal Medicine Foundation (CPR-FMF) published by Ciobanu et al. (2019) were used to classify the percentiles of absolute CPR values [30]. CPUR was calculated as the ratio of CPR to PI in the uterine arteries.
Pregnancy outcome data were obtained from outpatient medical records and telephone interviews with patients.
The primary outcome was neonatal weight<10th percentile for a given gestational age [28]. Secondary outcomes were preterm birth (<37 weeks of gestation), operative delivery, hospitalization of the newborn in the intensive care unit, perinatal death, including antenatal fetal death after 22 weeks of pregnancy and death of the newborn before 28 days of life.
Statistical analysis
Statistical analysis and plotting were performed using MedCalc Statistical Software version 16.4.3 (MedCalc Software bv, Ostend, Belgium) and GraphPad Prism 6 (GraphPad Software, USA). Continuous variables showing normal distribution were compared with Student’s t-test for independent samples; otherwise, the Mann–Whitney U-test was used. The normality of the distribution was tested by the Shapiro-Wilk test. Categorical variables were compared using Fisher’s exact test. The odds ratio (OR) was calculated to assess the likelihood of having a low birth weight baby in cases with decreased CPR and CPUR<5th percentile compared with cases with normal CPR and CPUR ≥5th percentile. Differences between the groups were considered statistically significant at p<0.05.
Results
The final analysis included 1215 patients who underwent combined screening in the first trimester to determine the exact gestational age and at least one Doppler study at 24–40 weeks of gestation.
Mean birth weights were consistent with the 50th percentile of the Intergrowth-21st reference standards [28]. The study group (group 1) included 79 (6.5%) newborns with birth weight <10th percentile; 1136 (93.5%) newborns with birth weight ≥10th percentile were assigned to the control group (group 2).
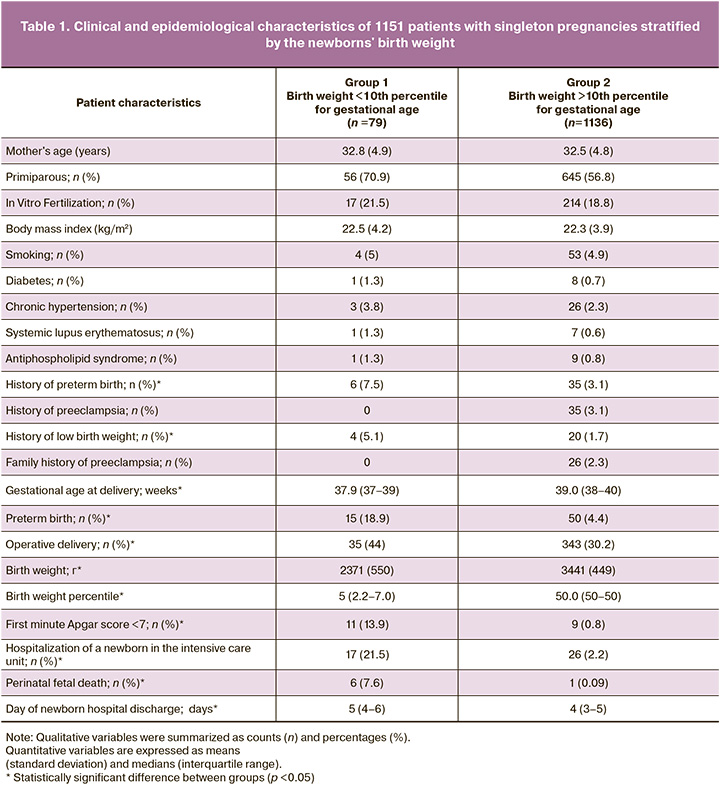
A total of 1780 Doppler studies were performed at various gestational ages, including 454, 905, 371, and 50 examinations at 24–29, 30–33, 34–37, and 38–40 weeks of pregnancy. Of the total number of 141 Doppler studies in group 1, examinations in the respective gestational ages accounted for 11%, 6.5%, 8.1%, and 4%. Table 1 summarizes the study cohort's clinical and epidemiological characteristics stratified by the newborns' birth weight. In comparison with group 2, mothers of low birth weight infants were more likely to have a history of preterm birth and small for gestational age birth (p <0.05). In current pregnancy, the incidence of perinatal death, preterm and operative birth, low Apgar scores, hospitalizations of the neonate in the intensive care unit, and hospital stay length were significantly higher in the study group than in the control group (p<0.001). CPR and CPUR percentile ranges, shown in Table 2, were created using the results of 1639 Doppler studies of patients in the control group were newborns’ birth weights were ≥10th percentile. Comparison of our CPR percentile ranges with the CPR-FMF reference ranges [30] revealed significant agreement: the differences between the 5th percentiles in the corresponding gestational ages ranged from 0.03 to 0.14.
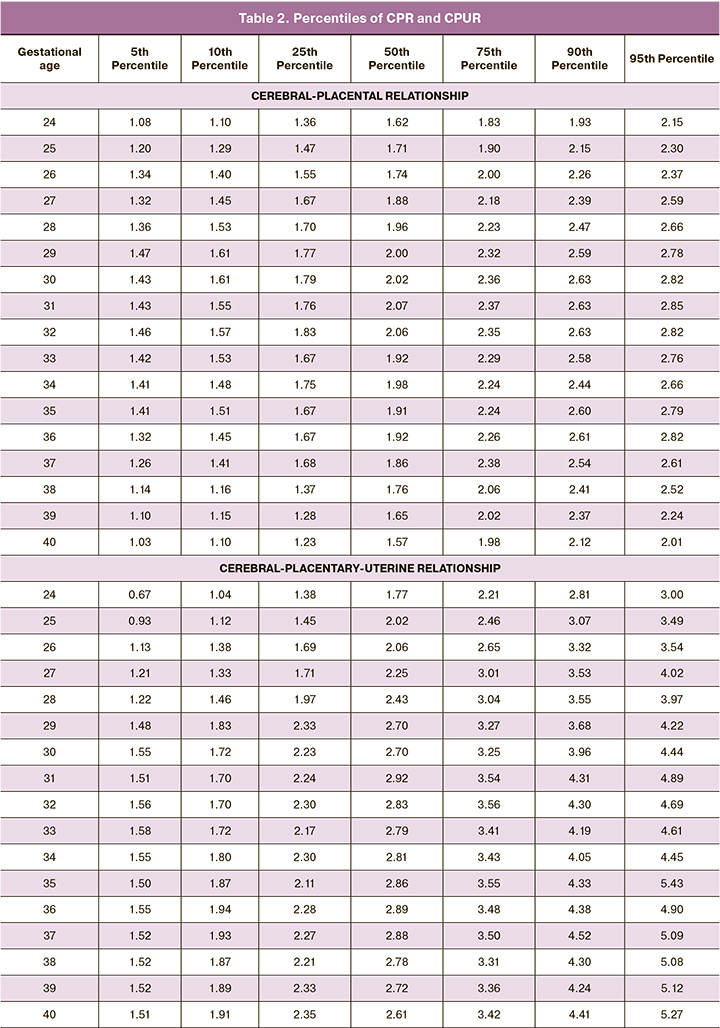
CPUR values (Table 2) changed depending on the gestational age: at 24–31 weeks, the 50th percentile CPUR increased from 1.77 to 2.92, and then slightly decreases to 2.61 by 40 weeks. It is noteworthy that the 5th percentile, which is essential for clinical use, had insignificant fluctuations in at 30–40 weeks (1.51– 1.58), which may facilitate the use of this cutoff value.
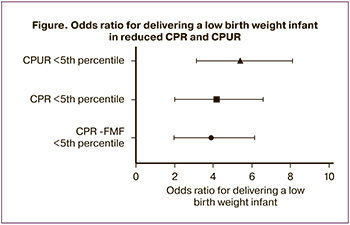 The odds ratio (OR), calculated for cases of CPR <5th percentile according to the reference CPR-FMF values [30], our study CPR and CPUR <5th percentile, revealed a statistically significant increase in the odds of having a low birth weight baby in comparison with cases having these parameters≥5th percentile (p <0.0001 for each of the indicators). In the analysis of CPR-FMF, OR was 3.56 (95% CI 1.95–6.61), for CPR OR was 3.9 (95% CI 2.0–6.6); for CPUR, OR was 5.0 (95% CI 3.1–8.1). A decrease in these parameters was found in 15, 22, and 27 out of 141 Doppler studies in group 1, 53, 74, and 74 out of 1639 Doppler studies in group 2, respectively (Figure).
The odds ratio (OR), calculated for cases of CPR <5th percentile according to the reference CPR-FMF values [30], our study CPR and CPUR <5th percentile, revealed a statistically significant increase in the odds of having a low birth weight baby in comparison with cases having these parameters≥5th percentile (p <0.0001 for each of the indicators). In the analysis of CPR-FMF, OR was 3.56 (95% CI 1.95–6.61), for CPR OR was 3.9 (95% CI 2.0–6.6); for CPUR, OR was 5.0 (95% CI 3.1–8.1). A decrease in these parameters was found in 15, 22, and 27 out of 141 Doppler studies in group 1, 53, 74, and 74 out of 1639 Doppler studies in group 2, respectively (Figure).
Discussion
An analysis of pregnancy outcomes in this study revealed a significant increase in complication rates, the need for intensive, and length of hospitalization in low birth weight infants compared with birth weight neonates ≥ 10 percentile. It is important to remember that the negative impact of the unfavorable course of the perinatal period on the child's health does not end with discharge from the maternity hospital. Research evidence demonstrates a strong association of intrauterine growth restriction and postponed perinatal hypoxia with impaired physical and psychomotor development in children in the first years of life [12, 13, 16]. In our country, annual rates of intrauterine hypoxia and asphyxia in childbirth are 3.8% for full-term and 21.8% for preterm infants, amounts to more than 77 thousand children [7]. Therefore, the problem of prenatal diagnosis and, more importantly, the prediction of these conditions is indisputably relevant. Numerous publications on CPR's effectiveness in assessing the risk of fetal growth restriction and hypoxia in low-birth-weight and normal-weight fetuses provided inconclusive results [16–21]. This fact indicates the need to continue research. The development of new combined indicators [27] seems to be interesting, since a unified criterion that includes the simultaneous assessment of all interrelated blood flow indicators in the mother-placenta-fetus unit, including uterine arteries, umbilical arteries, and fetal cerebral blood flow, potentially allows quantitative identification of the presence, degree of the severity and dynamics of impaired functioning of this system as a whole.
It is important to note that findings of this population study, conducted with strict adherence to the methodology for determining the gestational age and Doppler analysis, the mean weight of newborns corresponded to the 50th percentile of the reference intervals of the international project INTERGROWTH-21st [28], the total number of cases of fetal growth restriction (6.5 %) did not significantly differ from the all-Russian level of this indicator (6.2%) [7], and the calculated of CPR percentiles were comparable to that of Ciobanu et al. [30], one of the most extensive studies of the Fetal Medicine Foundation. This is the strengths of our study, confirming the validity of the data obtained on the increase in the odds of having a low birth weight child with a decrease in CPUR and allowing us to present the obtained pilot percentile CPUR values as the baseline for further prospective studies aimed at finding effective criteria for a high risk of low birth weight associated with perinatal and long-term complications.
Conclusion
CPUR is a combined measure that is statistically significantly associated with increased odds of delivering low birth weight babies. Prospective studies are needed to determine the effectiveness of this indicator.
References
1. Barker D.J. The fetal and infant origins of adult disease. BMJ. 1990; 301(6761): 1111. https://dx.doi.org/10.1136/bmj.301.6761.1111.
2. Kwon E.J., Kim Y.J. What is fetal programming?: a lifetime health is under the control of in utero health. Obstet. Gynecol. Sci. 2017; 60(6): 506-19. https:// dx.doi.org/10.5468/ogs.2017.60.6.506.
3. Cleal J.K., Lewis R.M. Ch. 22. The placenta and developmental origins of health and disease. In: Rosenfeld C.S., ed. The epigenome and developmental origins of health and disease. Elsevier Inc.; 2016: 439-61. https://dx.doi.org/10.1016/ B978-0-12-801383-0.00022-0.
4. Alexander B.T., Dasinger J.H., Intapad S. Fetal programming and cardiovascular pathology. Compr. Physiol. 2015; 5(2): 997-1025. https://dx.doi.org/10.1002/ cphy.c140036.
5. Faa G., Manchia M., Pintus R., Gerosa C., Marcialis M.A., Fanos V. Fetal programming of neuropsychiatric disorders. Birth Defects Res. C. Embryo Today. 2016; 108(3): 207-23. https://dx.doi.org/10.1002/bdrc.21139.
6. Marciniak A., Patro-Małysza J., Kimber-Trojnar Ż., Marciniak B., Oleszczuk J., Leszczyńska-Gorzelak B. Fetal programming of the metabolic syndrome. Taiwan J. Obstet. Gynecol. 2017; 56(2): 133-8. https://dx.doi.org/10.1016/j. tjog.2017.01.001.
7. Основные показатели здоровья матери и ребенка, деятельность службы охраны детства и родовспоможения в Российской Федерации за 2018 г. М.; 2019. 170с. [The main indicators of maternal and child health, the activities of the children and maternity facilities in the Russian Federation for 2018. Moscow: Ministry of Health of the Russian Federation. 2019; 170 p. (in Russian)].
8. Оксенойт Г.К., Никитина С.Ю., Агеева Л.И., Александрова Г.А., Зайченко Н.М., Кириллова Г.Н., Леонов С.А, Огрызко Е.В., Титова И.А., Харькова Т.Л., Чумарина В.Ж., Шубочкина Е.М. Здравоохранение в России. 2017: Статистический сборник. М.: Росстат; 2017. 170с. [Oxenoite G.K., Nikitina S.Yu. Public health in Russia. 2017: Stat. Sat Rosstat. M.; 2017.170 p. (in Russian)].
9. Ярыгина Т.А., Батаева Р.С., Гус А.И. Совершенствование тактики ведения беременности у пациенток с ложноположительным риском хромосомных аномалий плода. Акушерство и гинекология. 2020; 1: 71-7. https:// dx.doi.org/10.18565/aig.2020.1.71-77. [Yarygina T.A., Bataeva R.S., Gus A.I. Improving pregnancy management tactics in patients with a false-positive risk of fetal chromosomal abnormalities. Obstetrics and gynecology. 2020; 1: 71-7. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.1.71-77.
10. Ганичкина М.Б., Мантрова Д.А., Кан Н.Е., Тютюнник В.Л., Хачатурян А.А., Зиганшина М.М. Ведение беременности при задержке роста плода. Акушерство и гинекология. 2017; 10: 5-11. https://dx.doi.org/10.18565/ aig.2017.10.5.5-11. [Ganichkina M.B., Mantrova D.A., Kan N.E., Tyutyunnik V.L., Khachaturian A.A., Ziganshina M.M. Management of pregnancy with fetal growth retardation. Obstetrics and gynecology. 2017; 10: 5-11. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.10.5.5-11.
11. Giussani D.A. The fetal brain sparing response to hypoxia: physiological mechanisms. J. Physiol. 2016; 594(5): 1215-30. https://dx.doi.org/10.1113/ JP271099.
12. Murray E., Fernandes M., Fazel M., Kennedy S.H., Villar J., Stein A. Differential effect of intrauterine growth restriction on childhood neurodevelopment: a systematic review. BJOG. 2015; 122(8): 1062-72. https://dx.doi. org/10.1111/1471-0528.13435.
13. Cohen E., Baerts W., van Bel F. Brain-sparing in intrauterine growth restriction: Considerations for the Neonatologist. Neonatology. 2015; 108(4): 269-76. https://dx.doi.org/10.1159/000438451.
14. Gordijn S.J., Beune I.M., Thilaganathan B., Papageorghiou A., Baschat A.A., Baker P.N. et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet. Gynecol. 2016; 48(3): 333-9. https:// dx.doi.org/10.1002/uog.15884.
15. Salomon L., Alfirevic Z., Da Silva Costa F., Deter R., Figueras F., Ghi T. et al. ISUOG Practice Guidelines: ultrasound assessment of fetal biometry and growth. Ultrasound Obstet. Gynecol. 2019; 53: 715-23. https://dx.doi. org/10.1002/uog.20272.
16. Троханова О.В., Гурьев Д.Л., Гурьева Д.Д., Ермолина Е.А., Матвеев И.М., Мартьянова М.В. Неонатальные и постнеонатальные исходы при различных нарушениях фетоплацентарного кровотока. Доктор.Ру. 2018; 10: 10-7. https://dx.doi.org/10.31550/1727-2378-2018-154-10-10-17. [Trokhanova O.V., Guryev D.L., Guryeva D.D., Ermolina E.A., Matveev I.M., Martyanova M.V. Neonatal and postneonatal outcomes for various disorders of fetoplacental blood flow. Doctor.Ru. 2018; 10 (154): 10-7 (in Russian)]. DOI: 10.31550/1727-2378-2018-154-10-10-17.
17. Khalil A., Thilaganathan B. Role of uteroplacental and fetal Doppler in identifying fetal growth restriction at term. Best Pract. Res. Clin. Obstet. Gynaecol. 2017; 38: 38-47. https://dx.doi.org/10.1016/j.bpobgyn.2016.09.003.
18. DeVore G.R. The importance of the cerebroplacental ratio in the evaluation of fetal well-being in SGA and AGA fetuses. Am. J. Obstet. Gynecol. 2015; 213(1): 5-15. https://dx.doi.org/10.1016/j.ajog.2015.05.024.
19. Hernandez-Andrade E., Maymon E., Erez O., Saker H., Luewan S., Garcia M. et al. A low cerebroplacental ratio at 20-24 weeks of gestation can predict reduced fetal size later in pregnancy or at birth. Fetal Diagn. Ther. 2018; 44(2): 112-23. https://dx.doi.org/10.1159/000479684.
20. Akolekar R., Ciobanu A., Zingler E., Syngelaki A.., Nicolaides K.H. Routine assessment of cerebroplacental ratio at 35-37 weeks’ gestation in the prediction of adverse perinatal outcome. Am. J. Obstet. Gynecol. 2019; 221(1): 65. e1-65. e18. https://dx.doi.org/10.1016/j.ajog.2019.03.002.
21. Leavitt K., Odibo L., Nwabuobi C., Tuuli M.G., Odibo A. The value of introducing cerebroplacental ratio (CPR) versus umbilical artery (UA) Doppler alone for the prediction of neonatal small for gestational age (SGA) and short-term adverse outcomes. J. Matern. Fetal Neonatal Med. 2019; 21: 1-5. https://dx.doi.org/10. 1080/14767058.2019.1640206.
22. Zohav E., Zohav E., Rabinovich M., Shenhav S., Ovadia Y.S., Anteby, E.Y. et al. Local cerebroplacental ratio reference ranges are better predictors for adverse delivery outcomes in normal weight fetuses during pregnancy. J. Matern. Fetal Neonatal Med. 2019; Nov. 25: 1-6. https://dx.doi.org/10.1080/ 14767058.2019.1685968.
23. Ganzevoort W., Mensing Van Charante N., Thilaganathan B., Prefumo F., Arabin B., Bilardo C.M. et al. How to monitor pregnancies complicated by fetal growth restriction and delivery before 32 weeks: post-hoc analysis of TRUFFLE study. Ultrasound Obstet. Gynecol. 2017; 49(6): 769-77. https:// dx.doi.org/10.1002/uog.17433.
24. Običan S.G., Odibo L., Tuuli M.G., Rodriguez A., Odibo A.O. Third trimester uterine artery Doppler indices as predictors of preeclampsia and neonatal small for gestational age. J. Matern. Fetal Neonatal Local cerebroplacental ratio reference ranges are better predictors for adverse delivery outcomes in normal weight fetuses during pregnancy. J. Matern. Fetal Neonatal Med. Local cerebroplacental ratio reference ranges are better predictors for adverse delivery outcomes in normal weight fetuses during pregnancy. J. Matern. Fetal Neonatal Med. Med. 2020; 33(20): 3484-9. https://dx.doi.org/10.1080/14767058.2019.1575804.
25. Arrue M., García M., Rodriguez-Bengoa M.T., Landa J.M., Urbieta L., Maiztegui M. et al. Do low-risk nulliparous women with abnormal uterine artery Doppler in the third trimester have poorer perinatal outcomes? A longitudinal prospective study on uterine artery Doppler in low-risk nulliparous women and correlation with pregnancy outcomes. J. Matern. Fetal Neonatal Med. 2017; 30(7): 877-80. https://dx.doi.org/10.1080/14767058.2016.1190822.
26. Monaghan C., Binder J., Thilaganathan B., Morales-Roselló J., Khalil A. Perinatal loss at term: role of uteroplacental and fetal Doppler assessment. Ultrasound Obstet. Gynecol. 2018; 52(1): 72-7. https://dx.doi.org/10.1002/uog.17500.
27. MacDonald T.M., Hui L., Robinson A.J., Dane K.M., Middleton A.L., Tong S. et al. Cerebral-placental-uterine ratio as novel predictor of late fetal growth restriction: prospective cohort study. Ultrasound Obstet. Gynecol. 2019; 54(3): 367-75. https://dx.doi.org/10.1002/uog.20150.
28. Papageorghiou, A.T., Kennedy S.H., Salomon L.J., Ohuma E.O., Cheikh Ismail L., Barros F.C., Lambert A., Carvalho M., Jaffer Y.A., Bertino E., Gravett M.G., Altman D.G., Purwar M., Noble J.A., Pang R., Victora C.G., Bhutta Z.A., Villar J., Gravett M.G. (2014). International standards for early fetal size and pregnancy dating based on ultrasound measurement of crown– rump length in the first trimester of pregnancy. Ultrasound Obstet Gynecol. 2014: 44(6): 641-8. https://dx.doi.org/10.1002/uog.13448.
29. Bhide A., Acharya G., Bilardo C.M., Brezinka C., Cafici D., Hernandez-Andrade E. et al. ISUOG practice guidelines: use of Doppler ultrasonography in obstetrics. Ultrasound Obstet. Gynecol. 2013; 41(2): 233-9. https://dx.doi. org/10.1002/uog.12371.
30. Ciobanu A., Wright A., Syngelaki A., Wright D., Akolekar R., Nicolaides K.H. Fetal Medicine Foundation reference ranges for umbilical artery and middle cerebral artery pulsatility index and cerebroplacental ratio. Ultrasound Obstet. Gynecol. 2019; 53(4): 465-72. https://dx.doi.org/10.1002/uog.20157.
Received 03.06.2020
Accepted 07.09.2020
About the Authors
Tamara A. Yarygina, M.D., Ultrasonographer at the Ultrasound and Functional Diagnostics Department, Radiology Division, V.I. Kulakov NMRC for OG&P,Ministry of Health of Russia. Tel.: +7(495)531-44-44. E-mail: t_yarygina@oparina4.ru. https://orcid.org/0000-0001-6140-1930. 117997, Russia, Moscow, Oparina str., 4.
Roza S. Bataeva, M.D., Ph.D., Associate Professor at the Division of Diagnostic Ultrasound, Russian Medical Academy of Postgraduate Education;
Medical Director and Consultant, Fetal Medicine Centre, Moscow. Tel.: +7(495)215-12-15. E-mail: drbataeva@gmail.com. 101000, Russia, Moscow, Myasnitskaya str., 32-1.
Alexandr I. Gus, Dr. Med. Sci., Professor, Head of Ultrasound and Functional Diagnostics Department, Radiology Division, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. E-mail: a_gus@oparina4.ru. ORCID: 0000-0003-1377-3128. 117997, Russia, Moscow, Oparina str., 4.
For citation: Yarygina T.A., Bataeva R.S., Gus A.I. Cerebral-placental-uterine ratio as a novel combined parameter of obstetric Doppler ultrasonography. Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 10: 55-62 (in Russian)
https://dx.doi.org/10.18565/aig.2020.10.55-62



