Safety of regional anesthesia during delivery in pregnant women with placenta increta
Background. The incidence of abnormal placental invasion has increased fourfold in the past 40 years. There is no consensus of opinion on intraoperative analgesia in this pathology. Regional anesthesia techniques have been used more frequently to improve delivery outcomes.Korolev A.Yu., Pyregov A.V., Fedorova T.A., Shmakov R.G., Gerasimov Yu.A., Shpiluk M.A., Medvedeva A.A.
Description. This paper presents 3 clinical cases of delivery in patients with confirmed placenta increta.
Conclusion. Regional anesthesia can currently serve as the technique of choice for cesarean section in patients with placenta increta. The decision to transfer to general anesthesia is made depending on the specific clinical situation.
Combined spinal and epidural anesthesia is safe and effective during elective surgical delivery in patients with placenta increta. The emergency nature of surgery and the uncontrolled bleeding-related need for hysterectomy are the risk factors for transition to general anesthesia.
Keywords
Over the last ten years, the incidence of the abnormally invasive placenta has increased by 30%, from 20.6 per 10 000 to 26.9 per 10 000 population [1]. Abnormally invasive placenta is associated with half of cesarean hysterectomies and the increased need for intensive care. There is a direct link between abnormal placentation and massive blood loss [2, 3]. The risk of recurrence of placenta abnormalities during subsequent pregnancies is 19.9% [4]. The stillbirth rate in patients with a history of abnormal placentation is 3.3% compared with 0.6% in its absence [1]. The term “abnormally invasive placenta” was introduced in 2013 and defined as a placenta that cannot be removed spontaneously or manually without causing severe bleeding [5]. The abnormal invasive placenta, which has been termed “placental ingrowth” in the domestic literature, occurs when the placenta penetrates too deep into the uterine wall. Depending on the depth of placental penetration, it is classified into three categories: placenta accreta, placenta increta and placenta percreta [6]. Important predisposing factors for the formation of abnormally invasive placenta include preceding cesarean section and placenta previa [7]. Another risk factor along with the post-cesarean section uterine scar is a history of abortions [8]. The maternal mortality rate associated with placenta increta and its complications reaches up to 6–7% in a series of cases [9, 10]. Over the past 40 years, the incidence of this pathology has increased from 1 per 2500 pregnancies in the 1980s to about 1 in 731 pregnancies at present, which is associated with increasing cesarean delivery rates [6, 11, 12]. Therefore, for the anesthesiologist, a chance for a clinical encounter with the abnormally invasive placenta becomes quite high. Currently, there is no consensus about the choice of anesthesia for patients with placenta increta. Anesthetic management strategy often depends on the qualification of the anesthesiologist, surgeon, availability of medical equipment and some other factors. Currently, regional anesthesia tends to be widely adopted in clinical practice.
Here we report a series of three cases of the abnormally invasive placenta, which were managed with abdominal delivery.
Case 1. Patient K. was a 39-year-old woman, who presented for an elective cesarean section at 34 weeks’ gestation for total placenta previa and placental penetration into the post-cesarean section uterine scar. Her past obstetric history included an emergency cesarean section; the current pregnancy was the second for her. Her comorbidities included chronic arterial hypertension and gestational diabetes mellitus, which was a correction with diet. On the evening before the operation, the patient was transferred to the anesthesiology and intensive care unit (ICU) for preoperative preparation. She underwent ultrasound-guided right internal jugular vein catheterization and received 500 ml of a balanced crystalloid solution. Control of the central catheter position was performed by chest x-ray after surgery. In the operating room, the patient received premedication including dexamethasone 4 mg, tranexamic acid 1000 mg, amoxicillin-clavulanate 1.2 g. After that, with the patient in the left lateral position, combined spinal-epidural anesthesia (CSEA) was performed at the L2-L3 level using the needle-through-needle technique: 2.4 ml of hyperbaric bupivacaine was injected intrathecally (height 170 cm), and an epidural catheter was threaded 4 cm cranially. After laying the patient on her back and tilting the table to the left, a continuous infusion of norepinephrine was started at the rate of 20 ng/kg/ min. The dosage of norepinephrine during surgery was from 20 to 120 ng/kg/min; it was discontinued at the time of suturing the anterior fascia of the rectus abdominis muscle. A skin incision was made 6 minutes after induction of anesthesia. The operation consisted of lower midline laparotomy with incision taken to the left of the umbilicus, low transverse cesarean section, uterine repair, and uterine balloon tamponade. The newborn was delivered at 3 minutes and had Apgar scores 7 and 8 at 1 and 5 minutes, respectively. The operative time was 78 minutes. Blood loss during surgery was 1600 ml. The patient underwent intraoperative cell salvage and autologous blood (500 ml, hematocrit 60%) transfusion using the Cell Saver. When the blood loss reached 1200 ml, she was given 1000 mg of tranexamic acid and 1020 ml (15 ml/kg) of fresh frozen plasma (FFP). The total infusion volume during surgery was 3020 ml including 1500 ml of balanced crystalloid solutions and 500 ml of 6% gelatin solution. No donor red blood cells were transfused. The infusion-transfusion therapy was aimed to preserve blood volume and maintain mean arterial pressure at least 60 mm Hg, prevent tachycardia and ensure a normal urine output. Controlled hypotension was also used to reduce blood loss. Blood pressure during the operation ranged from 91/46 to 118/70 mm Hg, heart rate (HR) from 61 to 96 bpm. No oxygen therapy was required. To ensure the patient’s comfort, she was sedated with a combination of ketamine and midazolam from the 15th minute of the operation and until the aponeurosis was sutured. Sixty minutes after the intrathecal injection of the anesthetic, a solution of ropivacaine 0.375% - 15 ml was infused via the epidural catheter. After completion of the operation, the patient was transported to the ICU in a stable condition, without any cardiovascular or respiratory complications and was fully awake. The changes in laboratory parameters are presented in Table 1. The intrauterine balloon was removed after 1 hour. Post-surgery blood loss during the first postoperative day was 300 ml. The early postoperative period was uneventful. The patient received antibiotic therapy, venous thromboembolism prevention, multimodal anesthesia with emphasis on the epidural component. The patient was ambulated 6 hours after surgery. After 24 hours, she was transferred to the postpartum ward in a satisfactory condition and discharged on the 6th day after delivery.

Case 2. Patient P. was a 47-year-old woman, who underwent an elective cesarean delivery at 34 weeks’ gestation for placenta previa with the placental penetration into the isthmus, cervix, and post-cesarean section uterine scar; she also had multiple uterine fibroids. Current pregnancy was the first for her and occurred after IVF with frozen embryo transfer. On the evening before surgery, the patient was transferred to the ICU for preparation and received ultrasound-guided central venous catheter placement. Before the operation, 500 ml of crystalloid solution was infused. Premedication was given in the operating room and included dexamethasone 4 mg, tranexamic acid 1000 mg. After that, with the patient in the left lateral position, CSEA was performed at the L1-L2 level using the needle-through-needle technique: 2.5 ml of hyperbaric bupivacaine was injected intrathecally (height 172 cm), and an epidural catheter was placed 3 cm cranially. Before performing anesthesia, vasopressor support was started (norepinephrine 20 ng/kg/min). The dosage of norepinephrine during the operation ranged from 20 to 200 ng/kg/min; it was discontinued at the end of the operation. A skin incision was made 8 minutes after the induction of anesthesia. The patient underwent a lower midline laparotomy, low transverse cesarean section, ligation of the uterine arteries followed by uterine compression stitches and uterine balloon tamponade. The newborn was delivered at 3 minutes and had Apgar scores 7 and 8 at 1 and 5 minutes, respectively. After clamping the umbilical cord, amoxicillin-clavulanate (1.2 g) was administered. The operative time was 69 minutes. The patient underwent intraoperative cell salvage (1950 ml) and autologous blood (650 ml, hematocrit 60%) transfusion. When the blood loss reached 1000 ml, she was given 2000 mg of tranexamic acid. Intraoperatively and in the early postoperative period, she received 1070 ml of FFP. The total infusion-transfusion volume was 4140 ml including FFP and 1000 ml of 6% gelatin solution. Controlled hypotension was used by controlling the rate of continuous norepinephrine infusion. The blood pressure during the operation ranged from 94/57 to 119/65, the heart rate from 65 to 82 bpm. Fentanyl was given as a once-only dose of 100 μg for anesthesia during the upper abdominal cavity manipulations. The epidural component was used only for postoperative analgesia. After completion of the operation, the patient was transported to the ICU in a stable condition. The intrauterine balloon was removed at the end of the operation. Post-surgery blood loss during the first postoperative day after was 500 ml. No donor red blood cells were used (the minimum hemoglobin level was 84 g/l), the dynamics of laboratory parameters are presented in Table 2. Early ambulation, antibiotic therapy, venous thromboembolism prevention, and multimodal anesthesia were administered. A human albumin 20% solution (200 ml) was transfused. The patient was ambulated 8 hours after surgery. After 24 hours, she was transferred to the postpartum ward in a satisfactory condition and discharged on the 5th day after delivery.
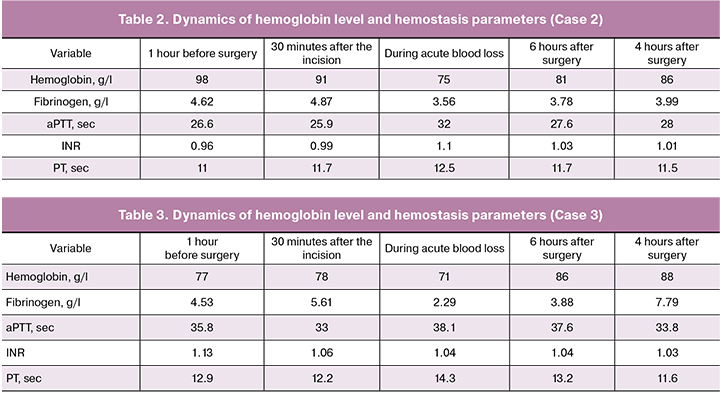
Case 3. Patient U. was a 26-year-old woman, who underwent an emergency cesarean delivery at 31 weeks’ gestation of the 4th pregnancy due to the onset of premature labor and the presence of placenta previa with placental penetration into the uterine scar after three previous cesarean sections. This pregnancy was the fifth for her. It should be noted that she might have a history of preeclampsia during the first pregnancy. On admission, the patient had severe iron deficiency anemia (hemoglobin 77 g/l) and was administered intravenous iron preparations. Twelve hours before delivery, the patient was transferred to the ICU for observation and preparation for surgery due to the threatened premature birth. Central venous catheter was placed in the right internal jugular vein. Due to the onset of the first stage of labor, the patient was taken to the operating room. The operation included a lower midline laparotomy, adhesions separation, cesarean section, hysterectomy without adnexectomy, resection of the bladder, omental resection, and drainage of the abdominal cavity. Before the operation, the patient received 270 ml blood transfusion to increase the blood oxygen capacity because of the initially low level of hemoglobin and due to the high risk of massive blood loss during the operation. The surgery was started under spinal-epidural anesthesia. Premedication included 2000 mg of tranexamic acid. CSEA was performed at the L1-L2 level using the needle-through-needle technique; 10 mg of hyperbaric bupivacaine was injected into the spinal space and an epidural catheter was placed 4 cm cranially. Surgery was started 5 minutes after the anesthesia was applied. The operative time was 113 minutes. On the 4th minute, a male infant was delivered, with Apgar scores 7 and 8 at 1 and 5 minutes, respectively. With the start of the operation, vasopressor support using 40 to 150 ng/kg/min norepinephrine was initiated; it was discontinued at the end of the operation. After clamping the umbilical cord, amoxicillin-clavulanate (1.2 g) was administered. At the 23rd minute of the operation, the patient required conversion to general anesthesia, as the surgery moved to the upper abdominal cavity, and she began to experience nausea, discomfort, and pain. A rapid sequential induction was performed with endotracheal intubation and ventilation in the VCV mode with 6 liter MV. Blood loss during surgery was 2220 ml, and the patient received 700 ml of autologous blood (hematocrit 60%) to replace the globular volume; also, 1400 ml of plasma was transfused in the operating room. The total infusion-transfusion volume was 3640 ml including FFP and 500 ml of 6% gelatin solution. The blood pressure tended to decrease and was supported by norepinephrine; during the operation, the blood pressure ranged from 88/53 to 118/72 mm Hg with the heart rate from 68 to 102 bpm. After the operation, the patient was extubated and transferred to the ICU in a stable condition. The lowest hemoglobin level before the completion of autologous blood transfusion was 71g/l. Blood loss during the day after surgery was 200 ml. No blood transfusion after surgery was required since the minimum hemoglobin level was 86 g/l (Table 3). Early ambulation, antibiotic therapy, venous thromboembolism prevention, multimodal anesthesia, intravenous iron preparations, and human albumin solution were administered. The patient was ambulated 12 hours after surgery. After 48 hours, she was transferred to the postpartum ward in a satisfactory condition and discharged on the 8th day after delivery.
Discussion
Many authors recommend epidural anesthesia or CSEA for elective delivery in patients with placenta accreta. At the same time, according to the literature, up to 45% of clinical situations require a conversion to general anesthesia because of massive hemorrhage [13,14,15]. Another strategy is to switch to general anesthesia as soon as possible, but after a newborn delivery. Due to expected bleeding, early access to the airways is considered the safest approach in these patients. Hemodynamic control and patient comfort with continued bleeding improve when the transition to general anesthesia occurs at an early stage. In this regard, a common approach is one in which combined anesthesia is applied. In the study comprising 43 patients with abnormally invasive placenta, 70% had combined anesthesia, 9% received only general anesthesia, and 21% of operations were performed under CSEA. It should be noted that among 34 cases of general anesthesia, there was one case with difficult airways, accompanied by repeated failed intubation attempts. Esteves-Pereira A.P. and the co-authors concluded that neuraxial anesthesia is the preferred method in obstetric practice, including for abnormal placentation [16]. In some clinics, the determining factor is directly the anatomical component. In a review by Radnia N. and co-authors, which included 96 cases of abnormally invasive placenta, there was a significant difference in anesthetic management, which was associated with the severity of placental penetration. In cases of placenta accreta, 91.8% of operations were performed under spinal anesthesia, and only in 2 out of 45 cases required a conversion to general anesthesia. In patients with placenta increta, 66.7% of operations were performed under spinal anesthesia and the conversion to general anesthesia was required in every fifth case. Among patients with placenta percreta, general anesthesia was required in 86% of cases, although in 71.4% of cases the surgery was started under spinal anesthesia. The authors of the article recommended spinal anesthesia as the primary choice for elective cesarean section complicated by the abnormally invasive placenta, and general anesthesia for placenta percreta [17]. In a survey conducted in 26 clinics in Israel, most respondents reported that they perform general anesthesia for patients with suspected placenta increta, including for elective surgery [18].
CSEA is safe in women undergoing cesarean delivery for placenta increta and has several advantages. First, CSEA eliminates the risk of airway-related complications, including aspiration of gastric contents. Secondly, there is practically no effect of anesthetics on the fetus, and the woman is present at the birth of her child. Thirdly, cesarean delivery under regional anesthesia is associated with lower blood loss than under general anesthesia [13, 19]. The epidural component of CSEA provides the ability to prolong the period of anesthesia and achieve optimal postoperative analgesia.
- Regional anesthesia, in particular, CSEA, is safe and effective in patients with placenta increta undergoing elective operative delivery.
- Necessary conditions for cesarean delivery under CSEA in women with placenta increta include the availability of equipment for intraoperative cell salvage and autologous blood transfusion, adequate reserve of FFP and donor red blood cells, hemostatic agents (fibrinolysis inhibitors, prothrombin complex concentrates, activated coagulation factor VII), and the feasibility of vasopressor support.
- Risk factors for conversion to general anesthesia are an emergency cesarean delivery and hysterectomy for uncontrolled bleeding. Further research is needed to identify the risk factors for conversion to general anesthesia.
References
- Baldwin HJ, Patterson JA, Nippita TA, Torvaldsen S, Ibiebele I, Simpson JM. Maternal and neonatal outcomes following abnormally invasive placenta: a population-based record linkage study. Acta Obstet Gynecol Scand. 2017; 96(11):1373-1381
- Brookfield K.F., L.T. Goodnough, D.J. Lyell, A.J. Butwick. Perioperative and transfusion outcomes in women undergoing cesarean hysterectomy for abnormal placentation. Transfusion. 2014; 54: 1530-1536.
- Mhyre JM, Shilkrut A, Kuklina EV, Callaghan WM, Creanga AA, Kaminsky S.Massive blood transfusion during hospitalization for delivery in New York State, 1998–2007. Obstet Gynecol. J. 2013; 122:1288-1292.
- Cunningham K.M., Anwar A, Lindow S. The recurrence risk of placenta accreta following uterine conserving management. J Neonatal Perinatal Med. 2015; 8(4): 293-296.
- Silver, Robert M. Abnormal Placentation: Placenta previa, vasa previa and placenta accreta. Obstet Gynecol. 2015; 126; 654-668.
- Eshkoli T, Weintraub AY, Sergienko R, Sheiner E. Placenta accreta: risk factors, perinatal outcomes, and consequences for subsequent births. Am J Obstet Gynecol. 2013; 208(3):1-7.
- Rosenberg T, Pariente G, Sergienko R, Wiznitzer A, Sheiner E. Critical analysis of risk factors and outcome of placenta previa. Arch Gynecol Obstet. 2011; 284(1):47-51
- Hung TH, Hsieh CC, Hsu JJ, Lo LM, Chiu TH, Hsieh TT. Risk factors for placental abruption in an Asian population. Reprod Sci. 2007; 14(1): 59-65.
- O’Brien B, Smoleneic J. Cervical varicosities and placenta praevia. Obstet Gynaecol. 2013;53(1): 453-454.
- Колчина В.В., Азарова Л.В. Взаимосвязь аномалий расположения плаценты с факторами инфекционного генеза. Фундаментальные исследования. 2014; 7(14):723–727. [Kolchina V.V., Azarova L.V. The relationship of anomalies of the location of the placenta with factors of infectious genesis. Basic research. 2014; 7 (14): 723–727.(in Russian)]
- Faiz AS, Ananth CV. Etiology and risk factors for placenta previa: an overview and meta-analysis of observational studies. J Matern Fetal Neonatal Med. 2003; 3(3):175–190.
- Chantraine F., J. Langhoff-Roos. Abnormally invasive placenta–AIP. Awareness and pro-active management is necessary. Acta Obstet Gynecol Scand. 2013; 4: 369-371.
- Belfort M.A. Placenta accreta. Am J Obstet Gynecol. 2010; 20(3); 430-435.
- Clark SL, Koonings PP, Phelan JP. Placenta previa/accreta and prior cesarean section. Obstet Gynecol. 1985; 66: 89-90.
- Silver RM, Barbour KD. Placenta accreta spectrum: accreta, increta, and percreta. Obstet Gynecol Clin North Am.2015; 42:381-383.
- Esteves-Pereira AP, Deneux-Tharaux C, Nakamura-Pereira M, Saucedo M. Caesarean Delivery and Postpartum Maternal Mortality: A Population-Based Case Control Study in Brazil. PLoS One. 2016;11(4): 18-26.
- Radnia N, Manouchehrian N, Shayan A, Shirmohamadi N, Eskandarloo T, Otogara M. Frequency and causes of emergency hysterectomy along with vaginal delivery and caesarean section in Hamadan, Iran. Electron Physician. 2017; 6: 4643-4647.
- Fitzpatrick KE, Sellers S, Spark P, Kurinczuk JJ, Brocklehurst P, Knight M. Incidence and risk factors for placenta accreta/increta/percreta in the UK: a national case-control study. PLoS ON. 2012; 7(12).
- Kramer MS, Berg C, Abenhaim H, Dahhou M, Rouleau J.Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol. 2013;209(5):449.
Received 11.04.2018
Accepted 20.04.2018
About the Authors
Korolev, Aleksey Yu., doctor of the Department of Anaesthesiology and Resuscitation, assistant of the Department of Anesthesiology and Resuscitation,National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia.
117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954387777. E-mail: a_korolev@oparina4.ru, nimbeks@mail.ru
Pyregov, Aleksey V., MD, associate professor, head of the Department of Anesthesiology and Resuscitation, National Medical Research Center for Obstetrics,
Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia.
117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954387777. E-mail: a_pyregov@oparina4.ru
Fyodorova, Tatyana A., MD, professor, head of Transfusiology and Extracorporal Hemocorrection Department, National Medical Research Center for Obstetrics,
Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia.
117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +7495438-77-77. E-mail: t_fyodorova@oparina4.ru
Shmakov, Roman G., MD, professor, chief medical officer, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician
V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954382489. E-mail: r_shmakov@oparina4.ru
Gerasimov, Yuri A., head of Clinical Work, Department of Anesthesiology and Resuscitation, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954387777. E-mail: u_gerasimov@oparina4.ru
Shpiluk, Margarita A., researcher of Laboratory of Clinical Immunology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +7495438-44-44. E-mail: m_shpiluk@oparina4.ru
Medvedeva A.A., 6th year student, Pirogov Russian National Research Medical University.
117997, Russia, Moscow, Ostrovityanova str. 1.Tel.: +79652895738. E-mail: medvedevavnya@mail.ru
For citations: Korolev A.Yu., Pyregov A.V., Fedorova T.A., Shmakov R.G., Gerasimov Yu.A., Shpiluk M.A., Medvedeva A.A. Safety of regional anesthesia during delivery in pregnant women with placenta increta. Akusherstvo i Ginekologiya/
Obstetrics and Gynecology. 2019; (1): 92-7. (in Russian)
http://dx.doi.org/10.18565/aig.2019.1.92-97



