Autoantibodies as risk factors for threatened miscarriage in women with early pregnancy loss
The association between obstetric complications and antiphospholipid antibodies (aPLs) and autoantibodies directed against hormones and complement components has attracted considerable scientific and practical interest.Menzhinskaya I.V., Ionanidze T.B., Van’ko L.V., Tetruashvili N.K., Krechetova L.V.
Aim. To investigate the diagnostic value of autoantibodies and risk factors for threatened miscarriage in women with early pregnancy loss.
Materials and methods. Eighty women with early pregnancy losses, including pregnant women with (subgroup 1, n=29) and without (subgroup 2, n=20) threatened miscarriage were tested for antibodies to cardiolipin (CL), β2-glycoprotein-I (β2-GP-I), phosphatidylserine/prothrombin complex (PS/PT), phosphatidylethanolamine (PE), component C1q, human chorionic gonadotropin (hCG), and progesterone (Pg) using ELISA.
Results. Antibodies directed against hormones were found in 40-45% of patients, 23.8%-33.8% had antibodies to β2-GP-I, CL, PE, PS, and 11.3% to PS/PT. IgA-antibodies to β2-GP-I and CL were detected in 16.3% and 6.3% of patients, respectively. Levels of aPL, antibodies to hormones, and C1q were higher in subgroup 1 than in subgroup 2. High odds ratios (>10) were observed for antibodies to β2-GP-I, PE, and Pg.
Conclusion. Determination of antibodies to β2-GP-I, CL, hormones, and C1q has a high diagnostic value for identifying early threatened miscarriage, and antibodies to β2-GP-I, PE, and PG are possible risk factors.
Keywords
Spontaneous pregnancy loss before 22 week of gestation (miscarriage) is a common obstetric complication occurring in about 15–25% of clinically confirmed pregnancies [1]. About 80% of all cases of sporadic pregnancy loss happen in the first trimester. A common risk factor is a history of pregnancy loss. The recurrent spontaneous miscarriage is observed in 2–4% of married couples [1]. Currently, a relationship has been found between this pregnancy complication and positivity for some autoantibodies, primarily antiphospholipid antibodies (aPL) [2]. It has been shown that from 7 to 25% of unexplained recurrent spontaneous miscarriages are associated with the presence of aPL [3].
Antibodies against cardiolipin (CL) and β2-glycoprotein-I (β2-GP-I) classes M and G and lupus anticoagulant represent currently adopted laboratory classification criteria for antiphospholipid syndrome (APS). One of the clinical manifestations of APS is recurrent spontaneous miscarriage before 10 weeks of gestation [4]. Screening of these classic AFLs is carried out according to the established procedure during examining women with recurrent spontaneous miscarriage [2]. However, a significant proportion of women with clinical signs of obstetric APS are negative for classical aFL. In these cases of seronegative APS, testing for a wide range of aFLs, not considered the laboratory criteria for APS, are of great importance [5]. A high prevalence of noncriteria aPLs was demonstrated in patients with APS compared with the control group [6].
Most of the studies have been focused on the study of aPL classes M and G. Some studies investigating the clinical significance of IgA antibodies against CL and β2-GP-I showed conflicting results [7]. These antibodies were not included in the 2006 APS classification criteria due to a lack of evidence. However, later, the XIII International Congress working group on aPL recommended testing for IgA antibodies against β2-GP-I in seronegative patients with clinical signs of APS [5].
Non-criteria aPL are detected in 58.3% of patients with seronegative APS, including antibodies to phosphatidylethanolamine (aPE) in 30.5% of cases [8]. Recurrent spontaneous miscarriage was found to have a strong association with kininogen-dependent aPEs [9]. aPEs and antibodies to the phosphatidylserine/prothrombin complex (PS/PT) may be the only aPLs found in patients with clinical manifestations of APS [10].
Of particular scientific interest are antibodies to the complement component C1q, which is a triggering molecule of the classical pathway of the complement system, belonging to the collecins family, which is widely present in the placenta and plays a regulatory role in maintaining pregnancy [11]. A recent study showed the prevalence of anti-C1q antibodies in women with unexplained recurrent pregnancy loss [12].
Besides, antibodies to pregnancy hormones, namely human chorionic gonadotropin (hCG) and progesterone (PG), are considered as a possible risk factor for recurrent miscarriage [13].
However, at present, the tests described above for non-criteria aPL, antibodies to C1q, and PG are not used in routine laboratory practice. Research continues on the relationship between antibodies of different specificity and their diagnostic value to identify obstetric pathology.
Given the above, this study aimed to investigate the diagnostic value of autoantibodies and risk factors for threatened miscarriage in women with early pregnancy loss.
Materials and methods
The study included 80 women with early pregnancy losses managed at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. They underwent clinical and diagnostic investigations and laboratory testing and were treated for the first trimester threatened miscarriage. Of them, 31 (38.8%) were non-pregnant and 49 (61.3%) were pregnant. Pregnant women were divided into subgroup of patients with the first trimester threatened miscarriage [subgroup 1, n=29 (59.2%)] women without threatened [subgroup 2, n=20 (40.8%)]. The mean age of women was 31.5 (5.6) years, and they had a history of 2.3 (1.3) cases of pregnancy loss at 7.5 (1.4) weeks of gestation.
The exclusion criteria were genital organs malformations, pregnancy after assisted reproductive technologies, the carriage of chromosomal rearrangements, fetal genetic abnormalities, severe endocrine disorders, severe somatic and autoimmune pathology, and acute infections.
The study protocol was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P. All participants provided signed informed consent to participate in the study and for the use of biomaterial.
Serum antibodies of classes A, M, and G to CL (aCL) and β2-GP-I (aβ2-GP-I), antibodies of classes M and G to PS (aPS), aFE were determined using the enzyme-linked immunosorbent assay (ELISA). The PS/PT complex (aPS/PT) IgG antibodies to the complement component C1q (aC1q) were measured using ORGENTEC Diagnostics and IBL International (Germany) kits. The determination of these antibodies was carried out following the instructions supplied with the kits. IgM and IgG antibodies to hCG (aHCG) and PG (aPG) were determined using highly purified hCG and PG 3- (O-carboxymethyl) oxime-BSA conjugate (Sigma-Aldrich, USA), monoclonal antibodies against IgM, Human IgG labeled with horseradish peroxidase (XEMA, Russia) using the previously described ELISA modifications [14, 15]. An Alegria automatic analyzer (ORGENTEC, Germany) or a Multiskan EX photometer (Thermo Electron (Shanghai) Instrument Co, China) were used to analyze and measure optical density (OD).
Statistical analysis
Statistical analysis was performed using Microsoft Office Excel 2010, MedCalc v. 12, and IBM SPSS Statistics v. 26 software. The distribution of continuous variables was tested for normality using the Kolmogorov-Smirnov and Shapiro – Wilk tests. Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD) and presented as M (SD); otherwise, the median (Me) and the quartiles Q1 and Q3 in the Me (Q1; Q3) format were reported. Categorical variables were compared by the χ2 test. Comparing numerical data with non-normal distribution was performed with a nonparametric Mann–Whitney U-test. Differences were considered statistically significant at a P <0.05. Correlation between two quantitative variables was analyzed using Pearson correlation coefficient and the dependent binary variable by conducting a ROC analysis (Receiver Operating Characteristics). The relationship between the risk factor and the outcome was assessed by the odds ratio (OR).
Results and discussion
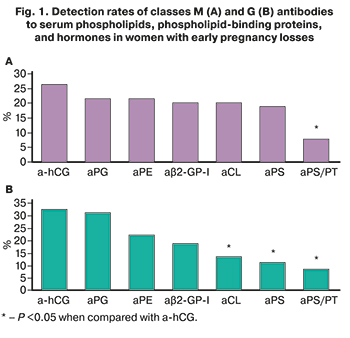 Among women with pregnancy losses class M and G aPLs and antibodies to hormones were detected in 37/80 (46.3%) and 50/80 (62.5%) women, respectively (P=0.04). aHCG and aPG were detected in 36/80 (45%) and 32/80 (40%) women, respectively. Among aPLs, the most frequently detected were aFE [in 27/80 (33.8%)], aβ2-HP-I [in 22/80 (27.5%)], aCL [in 22/80 (27.5%)] and aPS [in 20/80 (25%)]. aPS/PT were detected less frequently [in 9/80 (11.3%)] than antibodies to hormones and aPL of other specificity (P <0.05).
Among women with pregnancy losses class M and G aPLs and antibodies to hormones were detected in 37/80 (46.3%) and 50/80 (62.5%) women, respectively (P=0.04). aHCG and aPG were detected in 36/80 (45%) and 32/80 (40%) women, respectively. Among aPLs, the most frequently detected were aFE [in 27/80 (33.8%)], aβ2-HP-I [in 22/80 (27.5%)], aCL [in 22/80 (27.5%)] and aPS [in 20/80 (25%)]. aPS/PT were detected less frequently [in 9/80 (11.3%)] than antibodies to hormones and aPL of other specificity (P <0.05).
The most frequently detected IgM-a-hCG [in 21/80 (26.3%)], aPG [in 17/80 (21.3%)], aFE [in 16/80 (20%)], aCL [in 16/80 (20%)], aβ2-GP-I [in 16/80 (20%)] and aPS [15/80 (18.8%)] (Fig. 1A). At the same time, IgM-aPS/PT were found less frequently [in 6/80 (7.5%) women] of the above IgM-antibodies of different specificity (P <0.05).
IgG-a-hCG [in 26/80 (32.5%)] and aPG [in 25/80 (31.3%)] were detected more often than IgG-aCL [in 11/80 (13.8%)], aPS [in 9/80 (11.3%)], aPS/PT [in 7/80 (8.8%)] (Fig. 1B). A strong direct correlation was found between the levels of antibodies of classes M and G to CL, PS and β2-GP-I (r> 0.9; P <0.001), as well as between the levels of IgM-a-hCG and aPG (r=0.74; 95% CI (0.56–0.85); P <0.001).
Clinical and anamnestic characteristics of pregnant women with early pregnancy losses presented in table 1 showed that women of both subgroups were comparable in age, obstetric history, gynecological pathology, somatic and allergic diseases, and upper respiratory tract diseases. However, all pregnant women in subgroup 1 in the first trimester were diagnosed with early threatened miscarriage, in contrast to pregnant women in subgroup 2, whose pregnancy proceeded without threatened miscarriage.
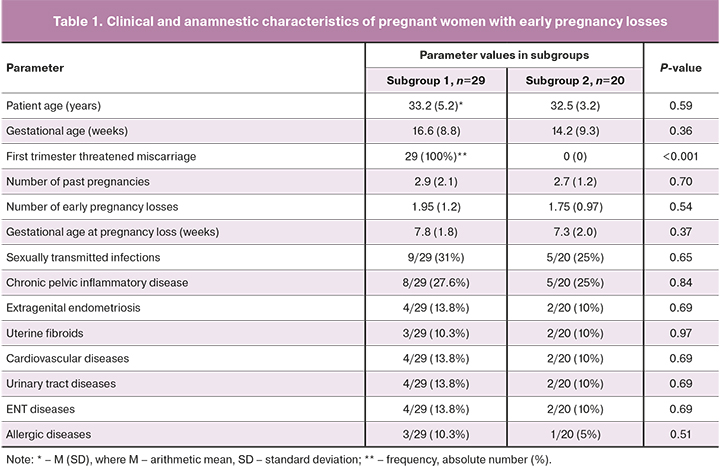
Pregnant women with threatened miscarriage (subgroup 1) had significantly higher median levels of IgG-aCL, aβ2-GP-I, aPS, aPS/PT, IgG-aPG, and aHCG and IgM-aPG than women without threatened miscarriage (subgroup 2) (Table 2).
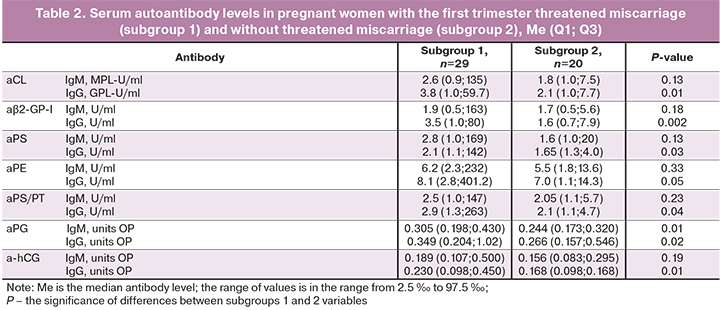
In subgroup 1, there was a higher detection rate for IgG-aCL [8/29 (27.6%)], aβ2-GP-I [10/29 (34.5%)] and aFE [11/29 (37.9%)] and IgM- and IgG-a PG [9/29 (31%) and 10/29 (34.5%)], compared with the frequency of detection of similar antibodies in subgroup 2 [1/20 (0.05%)]. The values of χ2 were, respectively, 3.9 (P=0.047), 5.8 (P=0.02), 6.8 (P=0.01), 4.8 (P=0.03) and 5.8 (P=0.02). High OR values were obtained for IgG-aβ2-GP-I (OR=10 (95% CI (1.2; 86.0); P=0.04), IgG-aPE (OR=11.6 (95% CI (1.36; 99.3); P=0.03), IgM-aPG (OR=19 (95% CI (1.04; 348.4); P=0.047) and IgG-aPG (OR=10 (95% CI (1.2; 86.0); P=0.04), which confirms a significant association of these antibodies with early threatened miscarriage in women with miscarriage.
Of particular interest was the prevalence of class A aPLs in women with miscarriage. IgA-aβ2-GP-I was found more often [in 13/80 (16.3%) women] than IgA-aCL [in 5/80 (6.3%) %) women] (P=0.046). ACL class A in all cases was detected in combination with IgA-aβ2-GP-I. IgA-aβ2-GP-I were found in 11 pregnant women [11/49 (22.4%)] and in 2 non-pregnant women [2/31 (6.5%)] (P=0.06), including 8/29 (27.6%) women with early threatened miscarriage. It should be noted that in 4/49 (8.2%) pregnant women, IgA-aβ2-GP-I was the only aPL detected. A moderate direct correlation was found between the levels of IgA- and IgG-a CL and aβ2-GP-I [r=0.49; 95% CI (0.27; 0.67); P=0.001]; r=0.34; 95% CI (0.09; 0.55); P=0.01); between the level of IgA antibodies of two specificities, there was a direct correlation of average strength [r=0.58; 95% CI (0.38; 0.73); P<0.001].
 These findings are consistent with the literature demonstrating more frequent detection of isolated IgA aβ2-GP-I in patients with APS than IgA aCL and a poor correlation of isolated positivity for IgA aCL with clinical manifestations of APS [16]. A higher prevalence of IgA-a β2-GP-I has been described in patients with APS compared to IgM-aβ2-GP-I and aCL, which are classified as APS criteria [16, 17].
These findings are consistent with the literature demonstrating more frequent detection of isolated IgA aβ2-GP-I in patients with APS than IgA aCL and a poor correlation of isolated positivity for IgA aCL with clinical manifestations of APS [16]. A higher prevalence of IgA-a β2-GP-I has been described in patients with APS compared to IgM-aβ2-GP-I and aCL, which are classified as APS criteria [16, 17].
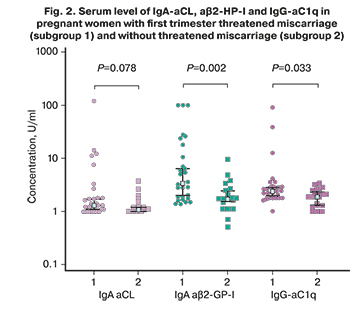
In our study, the median levels of IgA-aCL and aβ2-GP-I in pregnant women with early threatened miscarriage were 1.4 (1; 120) APL-U/ml and 3.4 (1.4; 100) U/ml, and in pregnant women without threatened miscarriage - 1.1 (1; 3.7) APL-U/ml and 1.7 (0.5; 9.6), respectively (Fig. 2). The median level of IgA-a β2-GP-I was significantly higher in subgroup 1 than in subgroup 2.
Noteworthy is that, along with aCL and aβ2-GP-I, AFE was often detected in women with miscarriage [in total, in 27/80 (33.8%) women]. It is important to note that 8/80 (10%) women had only aPE without aPL of different specificity. Women who were positive for AFE had a history of early pregnancy loss or first trimester threatened miscarriage. There was a tendency to an increase in the level of IgG-AFE in pregnant women with early threatened miscarriage, compared with pregnant women without threatened miscarriage (P=0.05).
According to some authors, AFEs are associated with the risk of early pregnancy loss. Recently, it was shown that in women with long-term persistent AFE, the frequency of miscarriages is significantly higher (40.7%) than in women with transient antibody elevation (20.0%; P=0.02) [18]. Long-term persistent AFEs are assumed to be pathogenic.
Although aPS/PT was detected in women with miscarriage significantly less frequently than antibodies to hormones and β2-GP-I, CL and PE, in 3/80 (3.8%) women, IgG-aPS / PT were the only aPL found. Their detection facilitated a more complete identification of patients with APS. Our results are consistent with the data of Zigon P. et al. (2015), who reported that aPS/PT was associated with recurrent early spontaneous miscarriage [19]. An international multicenter study has shown that IgG aPS/PT are found in both vascular and obstetric APS and can play the role of an additional marker of APS [10].
Mekinian A. et al. recently demonstrated the effectiveness of APS therapy to reduce the incidence of pregnancy loss in women who are seropositive for non-criteria aPE and aPS/PT [20]. The aPS/PT test is proposed for examining women with recurrent pregnancy loss [21]. Treatment of APS in patients with recurrent miscarriage and isolated positivity for non-criteria antibodies significantly increases live birth rates [22].
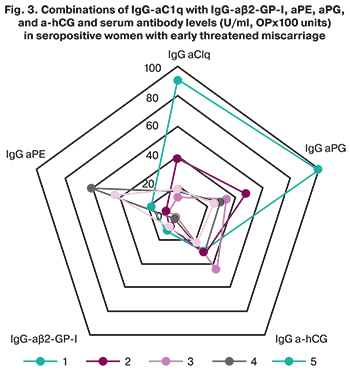 It has been established and experimentally proven that activation of complement through the classical pathway triggered by binding of the complement component C1q to immune complexes plays an essential role in developing obstetric complications of APS [23]. In APS, the formation of aC1q, capable of enhancing complement activation and disrupting the biological function of C1q associated with the removal of immune complexes and apoptotic cells, thereby promoting the development of autoimmunity [11, 23]. In women with early pregnancy losses and threatened miscarriage, of particular interest is investigating antibodies to phospholipids and hormones and poorly studied aC1q.
It has been established and experimentally proven that activation of complement through the classical pathway triggered by binding of the complement component C1q to immune complexes plays an essential role in developing obstetric complications of APS [23]. In APS, the formation of aC1q, capable of enhancing complement activation and disrupting the biological function of C1q associated with the removal of immune complexes and apoptotic cells, thereby promoting the development of autoimmunity [11, 23]. In women with early pregnancy losses and threatened miscarriage, of particular interest is investigating antibodies to phospholipids and hormones and poorly studied aC1q.
IgG-aC1q was detected in 8/80 (10%) patients: in 6/49 (12.2%) pregnant women, including 5/29 (17.2%) women with early threatened miscarriage and in 2/31 (6.5%) non-pregnant women. The median level of IgG-aC1q in women with threatened miscarriage [2.4 (1.0; 91.5) U/ml] was significantly higher than the median level of antibodies in pregnant women without threatened miscarriage (1.9 (1.0; 3.4 ) U/ml) (Fig. 2). aC1q were detected in various combinations with aPL and antibodies to hormones. At the same time, women who were positive for aC1q had an increased level of aβ2-GP-I, aFE, aPG, and a-hCG in three cases out of five (Fig. 3). It should be noted that there was a direct correlation of average strength between the levels of IgG-a C1q and aPG [r=0.57; 95% CI (0.37; 0.72); P <0.001].
These results are consistent with data on a higher prevalence of aC1q in primary APS [23] and patients with unexplained recurrent spontaneous miscarriage [12]. It is assumed that aC1q enhances complement activation and local tissue damage by interacting with C1q molecules associated with immune complexes, apoptotic cells, and directly with anionic phospholipids exposed on the cell surface, in particular trophoblast and endothelium [24]. High titers of aC1q can increase damage to placental tissue, leading to fetal loss or fetal growth restriction, especially in patients with recurrent pregnancy loss and obstetric APS [12].
According to the ROC-analysis, the determination of IgG and IgA-aβ2-GP-I, IgG-aCL, a-hCG and aC1q, IgM-aPG was characterized by high sensitivity, specificity, AUC, and positive predictive value in identifying early threatened miscarriage (table 3); test accuracy ranged from 60% to 67%. It should be noted that, according to several authors, the determination of IgA-αβ2-GP-I is characterized by a higher sensitivity for the diagnosis of APS than the determination of classical IgM-αβ2-GP-I and aCL [16, 17].
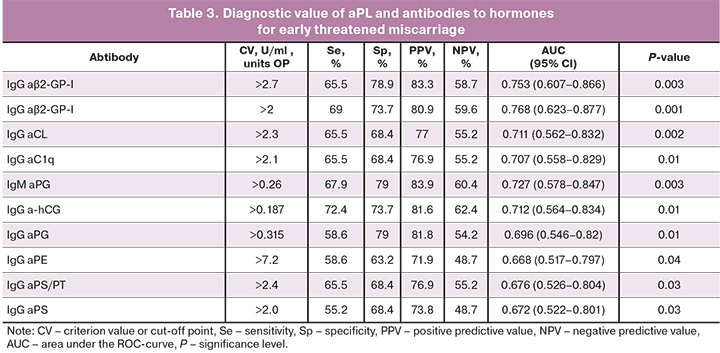
ROC curves allowed effective differentiation between patients with early threatened miscarriage and pregnant women without threatened miscarriage by the level of IgG- and IgA-aβ2-GP-I, IgG-aCL, a-hCG, and aC1q, IgM-aPG. The model's predictive ability was assessed by the values of AUC> 0.7 as good (Fig. 4).
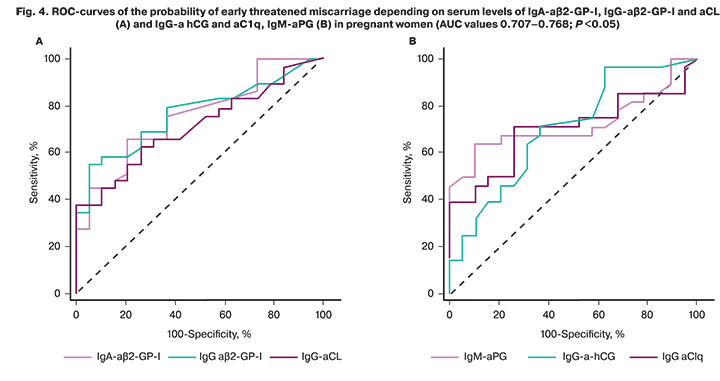
When determining IgG-aβ2-GP-I in combination with IgA-a β2-GP-I, the AUC increased to 0.788 (95% CI 0.646; 0.842); P=0.003; the accuracy of the tests reached 70.8%. The combination of tests for IgA- and IgG-aβ2-GP-I with a test for IgG-aHCG was the most effective for the diagnosis of early threatened miscarriage, compared with other tests. The AUC was 0.858 (95% CI 0.727; 0.942); P <0.001, the accuracy of the tests increased to 77.1%.
Conclusion
Women with early pregnancy loss are characterized by an increased formation of a wide range of autoantibodies, including antibodies to the hormones hCG and PG and aPL, both classical classes M and G to CL and β2-HP-I, referred to the criteria of APS, and non-criteria IgA-aCL and aβ2-GP-I, aFE, aPS/PT, aC1q. aC1q is often found in combination with aβ2-HP-I, and PE, aPG and a-hCG. IgA- and IgG-aβ2-GP-I, IgG-aCL, aC1q and aHCG, IgM-aPG have a high diagnostic value for early threatened miscarriage. The identification of non-criteria aPE, aPS/PT and IgA-aβ2-GP-I contributes to an increase in the accuracy of diagnosing obstetric APS. IgG-aβ2-GP-I, aFE, IgM- and IgG-aPG are possible risk factors for early threatened miscarriage.
References
- D'Ippolito S., Ticconi C., Tersigni C., Garofalo S., Martino C., Lanzone A. et al. The pathogenic role of autoantibodies in recurrent pregnancy loss. Am. J. Reprod. Immunol. 2020; 83(1): e13200. https://dx.doi.org/10.1111/aji.13200.
- Kwak-Kim J., Agcaoili M.S., Aleta L., Liao A., Ota K., Dambaeva S. et al. Management of women with recurrent pregnancy losses and antiphospholipid antibody syndrome. Am. J. Reprod. Immunol. 2013; 69(6): 596-607. https://dx.doi.org/10.1111/aji.12114.
- D'Ippolito S., Meroni P.L., Koike T., Veglia M., Scambia G., Di Simone N. Obstetric antiphospholipid syndrome: a recent classification for an old defined disorder. Autoimmun. Rev. 2014; 13(9): 901-8. https://dx.doi.org/10.1016/j.autrev.2014.05.004.
- Cervera R. Antiphospholipid syndrome. Thromb. Res. 2017; 151(Suppl. 1): S43-7. https://dx.doi.org/10.1016/S0049-3848(17)30066-X.
- Bertolaccini M.L., Amengual O., Atsumi T., Binder W.L., de Laat B., Forastiero R. et al. ‘Non-criteria’ aPL tests: report of a task force and preconference workshop at the 13th International congress on antiphospholipid antibodies, Galveston, TX, USA, April 2010. Lupus. 2011; 20(2): 191-205. https://dx.doi.org/10.1177/0961203310397082.
- Rodríguez-García V., Ioannou Y., Fernández-Nebro A., Isenberg D.A., Giles I.P. Examining the prevalence of non-criteria anti-phospholipid antibodies in patients with anti-phospholipid syndrome: a systematic review. Rheumatology 2015; 54(11): 2042-50. https://dx.doi.org/10.1093/rheumatology/kev226.
- Meijide H., Sciascia S., Sanna G., Khamashta M.A., Bertolaccini M.L. The clinical relevance of IgA anticardiolipin and IgA anti-β2 glycoprotein I antiphospholipid antibodies: a systematic review. Autoimmun. Rev. 2013; 12(3): 421-5. https://dx.doi.org/10.1016/j.autrev.2012.08.002.
- Conti F., Alessandri C., Sorice M., Capozzi A., Longo A., Garofalo T. et al. Thin-layer chromatography immunostaining in detecting anti-phospholipid antibodies in seronegative anti-phospholipid syndrome. Clin. Exp. Immunol. 2012; 167(3): 429-37. https://dx.doi.org/10.1111/j.1365-2249.2011.04532.x.
- Sugi T. Kininogen-dependent antiphosphatidylethanolamine antibodies and autoantibodies to factor XII in patients with recurrent pregnancy loss. J. Obstet. Gynaecol. Res. 2013; 39(7): 1223-9. https://dx.doi.org/10.1111/jog.12110.
- Amengual O., Forastiero R., Sugiura-Ogasawara M., Otomo K., Oku K., Favas C. et al. Evaluation of phosphatidylserine-dependent antiprothrombin antibody testing for the diagnosis of antiphospholipid syndrome: results of an international multicentre study. Lupus. 2017; 26(3): 266-76. https://dx.doi.org/10.1177/0961203316660203.
- Kouser L., Madhukaran S.P., Shastri A., Saraon A., Ferluga J., Al-Mozaini M., Kishore U. Emerging and novel functions of complement protein C1q. Front. Immunol. 2015; 6: Article 317. https://dx.doi.org/10.3389/fimmu.2015.00317.
- Ohmura K., Oku K., Kitaori T., Amengual O., Hisada R., Kanda M. et al. Pathogenic roles of anti-C1q antibodies in recurrent pregnancy loss. Clin. Immunol. 2019; 203: 37-44. https://dx.doi.org/10.1016/j.clim.2019.04.005.
- Менжинская И.В., Ванько Л.В. Ассоциация антител к гонадотропинам и женским половым гормонам с нарушениями репродуктивной функции. Акушерство и гинекология. 2017; 9: 20-7. https://dx.doi.org/10.18565/aig.2017.9.20-7. [Menzhinskaya I.V., Vanko L.V. Association of antibodies to gonadotropins and female sex hormones with reproductive disorders Obstetrics and Gynecology. 2017; 9: 20-27 (in Russian)]
- Менжинская И.В., Гладкова К.А., Сидельникова В.М., Сухих Г.Т. Антипрогестероновые антитела в клинике привычной потери беременности. Иммунология. 2008; 1: 34-7. [Menzhinskaya I.V., Gladkova K.A., Sidelnikova V.M., Sukhikh G.T. Anti-progesterone antibodies in the clinic of recurrent pregnancy loss. Immunology. 2008; 1: 34-37(in Russian)].
- Менжинская И.В., Кашенцева М.М., Ванько Л.В., Сухих Г.Т. Иммунохимические свойства аутоантител к хорионическому гонадотропину у женщин с невынашиванием беременности. Иммунология. 2015; 36(1): 30-5. [Menzhinskaya I.V., Kashentseva M.M., Vanko L.V., Sukhikh G.T. Immunochemical properties of autoantibodies to chorionic gonadotropin in women with miscarriage. Immunology. 2015; 36 (1): 30-35 (in Russian)]
- Pericleous C., Ferreira I., Borghi O., Pregnolato F., McDonnell T., Garza-Garcia A. et al. Measuring IgA anti-beta2-glycoprotein I and IgG/IgA anti-domain I antibodies adds value to current serological assays for the antiphospholipid syndrome. PLoS One. 2016; 11: e0156407. https://dx.doi.org/10.1371/journal.pone.0156407.
- Cabrera-Marante O., Rodríguez de Frías E., Serrano M., Lozano Morillo F., Naranjo L., Gil-Etayo F.J. et al. The Weight of IgA anti-β2glycoprotein I in the antiphospholipid syndrome pathogenesis: closing the gap of seronegative antiphospholipid syndrome. Int. J. Mol. Sci. 2020; 21(23): 8972. https://dx.doi.org/10.3390/ijms21238972.
- Yonezawa M., Kuwabara Y., Ono S., Ouchi N., Ichikawa T., Takeshita T. Significance of anti-phosphatidylethanolamine antibodies in the pathogenesis of recurrent pregnancy loss. Reprod. Sci. 2020; 27(10): 1888-93. https://dx.doi.org/10.1007/s43032-020-00208-4.
- Žigon P., Podovšovnik A., Ambrožič A., Tomšič M., Hočevar A., Gašperšič N. et al. Added value of non-criteria antiphospholipid antibodies for antiphospholipid syndrome: lessons learned from year-long routine measurements. Clin. Rheumatol. 2019; 38(2): 371-8. https://dx.doi.org/10.1007/s10067-018-4251-7.
- Mekinian A., Bourrienne M.C., Carbillon L., Benbara A., Noémie A., Chollet-Martin S. et al. Non-conventional antiphospholipid antibodies in patients with clinical obstetrical APS: Prevalence and treatment efficacy in pregnancies. Semin. Arthritis Rheum. 2016; 46(2): 232-7. https://dx.doi.org/10.1016/j.semarthrit.2016.05.006.
- Kitaori T., Sugiura-Ogasawara M., Oku K., Papisch W., Ebara T., Ozaki Y. et al. Determination of clinically significant tests for antiphospholipid antibodies and cutoff levels for obstetric antiphospholipid syndrome. Lupus. 2015; 24(14): 1505-19. https://dx.doi.org/10.1177/0961203315595128.
- Корнюшина Е.А., Чепанов С.В., Сельков С.А., Дадаева Д.Г., Шахалиев Р.А., Чудотворов К.Н., Немсцверидзе Н.Н. Профилактика потери беременности у женщин с циркуляцией аутоантител, не входящих в критерии антифосфолипидного синдрома. Журнал акушерства и женских болезней. 2018; 67(6): 24-30. [Kornyushina E.A., Chepanov S.V., Selkov S.A., Dadaeva D.G., Shakhaliev R.A., Chudotvorov K.N., Nemstsveridze N.N. Prevention of pregnancy loss in women with the circulation of autoantibodies that are not included in the criteria of antiphospholipid syndrome. Journal of Obstetrics and Women's Diseases. 2018; 67 (6): 24-30 (in Russian)]
- Oku K., Amengual O., Hisada R., Ohmura K., Nakagawa I., Watanabe T. et al. Autoantibodies against a complement component 1 q subcomponent contribute to complement activation and recurrent thrombosis/pregnancy morbidity in anti-phospholipid syndrome. Rheumatology (Oxford). 2016; 55(8): 1403-11. https://dx.doi.org/10.1093/rheumatology/kew196.
- Defendi F., Thielens N.M., Clavarino G., Cesbron J.Y., Dumestre-Pérard C. The immunopathology of complement proteins and innate immunity in autoimmune disease. Clin. Rev. Allergy Immunol. 2020; 58(2): 229-51. https://dx.doi.org/10.1007/s12016-019-08774-5.
Received 09.04.2021
Accepted 27.04.2021
About the Authors
Irina V. Menzhinskaya, Dr. Med. Sci., Leading Researcher at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.Tel.: +7(495)438-11-83. E-mail: i_menzinskaya@oparina4.ru. 4, Oparina str., Moscow, 117997, Russian Federation.
Teya B. Ionanidze, Junior Researcher at the 2nd Obstetric Department of Pathology of Pregnancy, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)438-14-88. E-mail: t_ionanidze@oparina4.ru. 4, Oparina str., Moscow, 117997, Russian Federation.
Ludmila V. Van'ko, Dr. Med. Sci., Professor, Leading Researcher at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)438-11-83. E-mail: LVanko@oparina4.ru. 4, Oparina str., Moscow, 117997, Russian Federation.
Nana K. Tetruashvili, Dr. Med. Sci., Head of the 2nd Obstetric Department of Pathology of Pregnancy, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)438-14-88. E-mail: n_tetruashvili@oparina4.ru. 4, Oparina str., Moscow, 117997, Russian Federation.
Lubov V. Krechetova, Dr. Med. Sci., Head of the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)438-11-83. E-mail: l_krechetova@oparina4.ru. 4, Oparina str., Moscow, 117997, Russian Federation.
For citation: Menzhinskaya I.V., Ionanidze T.B., Van’ko L.V., Tetruashvili N.K., Krechetova L.V. Autoantibodies as risk factors for threatened miscarriage in women with early pregnancy loss.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 8: 94-101 (in Russian)
https://dx.doi.org/10.18565/aig.2021.8.94-101



