Association between interleukin-6 and thrombomodulin levels and pathomorphological changes in early reproductive losses
Taizhanova D.Zh., Zubkov D.V., Kamyshansky E.K., Komlichenko E.V., Magalov I.Sh., Sorokina M.A.
Objective: The objective of the study was assessment of relationship between the prognostic values of laboratory serum markers and histopathological changes in early pregnancy loss.
Materials and methods: The study included 269 women of reproductive age seeking healthcare in hospital due to reproductive loss before 12 weeks of pregnancy. Histomorphological structural changes were evaluated followed by comparative analysis of these changes and laboratory parameters, such as fibrinogen, leukocytes, interleukin-6 (IL-6), platelets, thrombomodulin (TM), and plasminogen activator inhibitor 1 (PAI-1), which are most often used in predicting reproductive losses.
Results: Our study found statistically significant difference between the group of inflammatory changes versus the group of hemorrhagic/ischemic changes. The interquartile ranges for IL-6 were 5730–8840 ng/ml and 3540–6910 ng/ml, respectively, that can serve as a prerequisite for determination of reference values for prediction of inflammatory factors at the pre-gravid stage. Also, there was statistically significant difference in TM levels. The interquartile range of TM in the group of inflammatory changes was 5430–6510 ng/ml versus 7120–9030 ng/ml in the group of hemorrhagic/ischemic changes, that indicated a significant correlation between laboratory markers and the results of histological analysis of hemorrhagic changes. There were no statistically significant differences between the other laboratory parameters.
Conclusion: According to analysis of histopathological changes before 12 weeks of pregnancy and two or more adverse pregnancy outcomes in history in female population in Kazakhstan, the main causes of reproductive losses are inflammatory and hemorrhagic disorders. The study showed that IL-6 as a predisposing factor of the causes of reproductive losses and TM as the gold standard for identification of coagulation and hemorrhagic defects, that cause miscarriage, can be considered to be the most significant prognostic laboratory criteria.
Authors' contributions: Taizhanova D.Zh., Zubkov D.V., Komlichenko E.V. – the concept and design of the study;
Taizhanova D.Zh., Zubkov D.V., Kamyshansky E.K., Magalov I.Sh. – manuscript writing; Zubkov D.V., Sorokina M.A., Kamyshansky E.K. – material collection and processing; Taizhanova D.Zh, Zubkov D.V., Kamyshansky E.K. – contribution to the final manuscript.
Conflicts of interest: The authors confirm that they have no conflicts of interest to declare.
Funding: The study was carried out at the authors’ own expense.
Ethical Approval: The study was approved by the Ethics Committee at Medical School of Medical University of Karaganda,
No. 23.
Patient Consent for Publication: The patients have signed informed consent for participation in the study and publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Taizhanova D.Zh., Zubkov D.V., Kamyshansky E.K., Komlichenko E.V., Magalov I.Sh.,
Sorokina M.A. Association between interleukin-6 and thrombomodulin levels and
pathomorphological changes in early reproductive losses.
Аkusherstvo i Gynecologia/Obstetrics and Gynecology.2024; (10): 91-100 (in Russian)
https://dx.doi.org/10.18565/aig.2024.160
Keywords
The issue of predicting reproductive losses in population remains relevant for many years. Over the past decades, various prognostic criteria have been studied, including social, clinical, laboratory, genetic, molecular, that could serve as predictors of miscarriage. Currently, there is scant information on morphological changes in spontaneous abortion [1].
According to various authors, laboratory parameters, such as fibrinogen, leukocytes, interleukin-6 (IL-6), platelets, thrombomodulin (TM), and plasminogen activator inhibitor (PAI-1) are most frequently considered as prognostic laboratory markers.
Fibrinogen is a soluble plasma protein synthesized in the liver and directly involved in thrombus formation. The role of fibrinogen in the pathogenesis of adverse pregnancy outcomes is confirmed in many studies [2–6]. However, the prognostic significance of fibrinogen remains debatable. Some studies reported fibrinogen as the main predictive marker [2, 5, 7], although according to the other research findings, there were no statistically significant changes in this parameter [3, 6].
IL-6 belongs to the family of cytokines that have a pleiotropic effect and participate in a wide range of physiological processes (inflammation, immune reactions, etc.). According to literature data, it is a recognized as an inflammatory cytokine that mediates innate and adaptive immunity and numerous physiological processes, including those associated with pregnancy [8, 9].
The number of platelets per unit blood volume is the most important parameter in assessment of systemic inflammation [10, 11]. When pregnancy occurs, various immune effectors and molecules involved in the immune microenvironment create specific maternal–fetal tolerance [12].
TM is a membrane protein expressed on the endothelium. It plays an important role in maintaining vascular homeostasis. In hypercoagulable state due to endothelial injury, TM is released into the intravascular space [13]. Currently, TM is considered as one of the most sensitive and specific predictors of reproductive losses [14]. The prognostic value of TM in impaired intravascular homeostasis, even in the absence of such pathological factors as antiphospholipid syndrome and Factor V Leiden has been described, which have long been considered to be the main coagulopathic causes of miscarriage in women [6, 15].
PAI-1 is expressed in interstitial extravillous trophoblasts and vascular trophoblasts. During implantation and placentation, it inhibits extracellular matrix degradation, thereby inhibiting trophoblast invasion. Elevated plasma level of PAI-1 is detected in recurrent miscarriage [16].
In this respect, it is important to evaluate laboratory predictors in association with morphological changes in adverse pregnancy outcomes to stratify the risk group for adverse outcomes at the pregravid stage.
The objective of the study was assessment of relationship between the prognostic values of laboratory serum markers and histopathological changes in early miscarriages.
Material and methods
Patient selection criteria
The prospective study was conducted at the Regional clinical hospital in Karaganda, the Republic of Kazakhstan from January, 2022 to May, 2023.
The study included 269 women of reproductive age seeking healthcare in hospital due to reproductive loss before 12 weeks of pregnancy.
Inclusion criteria: confirmed US diagnosis of adverse pregnancy outcome or spontaneous abortion before 12 weeks; absence of external genital pathology in history, acute inflammatory diseases, surgical intervention during gestation period, absence of severe somatic symptom disorder and chronic diseases at the stage of decompensation in history.
Exclusion criteria: multiple pregnancy; ectopic pregnancy; pronounced autolytic changes or absence/insufficient quantity (less than 5 cross sections) of chorionic villi in the sample.
All tissues obtained after induced abortion, aspiration or uterine curettage were sent for histological examination in accordance with national legislative standards.
In each case after completion of routine histopathological specimen examination, pathology reports, slides and paraffin blocks were requested. The slides and paraffin blocks were retrieved from the archive of histology laboratory and compared with the data in the laboratory request forms that were received with the specimen and archived in the laboratory. Pathology reports were also retrieved and carefully analyzed. In cases, when there were doubts about the quality of the pathology report, the slides were reviewed.
According to histopathological changes, 5 study groups were formed.
Group 1 (n=106) – abnormal branching of chorionic villi and dysmorphic chorionic villi: highly irregular villous shapes/outlines; at least one trophoblast invasion and/or multiple intussusceptions [17, 18].
Group 2 (n=31) – inflammatory changes: acute and chronic villitis, acute intervillositis, massive perivillosial fibrin deposition, chronic histiocytic intervillositis, lymphoplasmacytic deciduitis, decidual vasculitis [19, 20].
Group 3 (n=25) – hemorrhagic/ischemic changes and/or impaired vasculogenesis: early vascular karyorrhexis, intervillous hemorrhage, hemorrhagic endometrium during pregnancy, parabasal strokes and hemorrhages in the basal plate with parabasal necrosis, severe chorionic villus hypoplasia (grade IIB) and avascular villi (grade III) according to classification system by Hakvoort R.A. [21].
Group 4 (n=39) – other impairments: reactive cellular infiltration in decidual tissue, chorionic villous edema with myxoid stromal changes, chorionic villous sclerosis/fibrosis, diffuse hydropic enlargement of chorionic villi with trophoblast hyperplasia, neoplasia.
Group 5 (n=68) – without pathology.
Laboratory tests of serum markers
The anamnestic data of patients, who were seeking care in hospital, were collected. The analysis of their digital health passports was performed to rule out external genital pathology and to form the study group. After the patients signed voluntary informed consent to participate in the study, venous blood samples were collected for further testing at the Medical University of Karaganda. The blood samples were collected from the participants on an empty stomach. Platelet indices and leucocyte count were analyzed using SYSMEX XS-500i Hematology Analyzer (Japan). Fibrinogen levels were determined with automatic blood coagulation analyzer ACL ELITE PRO for in vitro diagnostics (“Instrumentation laboratory Co., USA). Serum TM, IL-6 and PAI-1 levels were measured using chromogenic enzyme-linked immunosorbent assay (ELISA) with IVD kits and automated immunoassay analyzer Evolis (Bio-Rad Laboratories, Inc., USA) and Freedom EVO Clinical automation platform (Tecan, Switzerland).
Histological examination
Before histological examination, tissue samples were fixed in 10% formaldehyde solution at 4°C for 24 hours, washed with tap water and dehydrated using a series of increasing concentrations of alcohols (70%, 90%, 95%, 100%). Tissue samples were then immersed in xylene and embedded in paraffin blocks. Tissue sections of 3 µm thickness were cut using a microtome and placed on glass slides. The slides were then deparaffinized and stained.
Tissue sections were immersed in Mayer's hematoxylin for 15 minutes and then rinsed with water for 5 minutes. Sections were then stained with eosin for 1 minute.
Intrauterine pregnancy was confirmed, when in addition to other tissues, such as decidual tissue or secretory endometrium, fetal tissue or chorionic villi were identified. Histological identification of chorionic villi was done according to classification system by Vogel (1996).
Ethics statement
The study was carried out in accordance with the Declaration of Helsinki developed by the World Medical Association (2013). The study was approved by the Ethics Committee at the Medical School of Medical University of Karaganda, No.23. There were no experimental interferences and changes in the study protocols. Written informed consent was obtained from all patients before enrollment in the study.
Statistical analysis
Statistical software packages Statistica 12.6 and IBM SPSS Statistics (version 26, IBM, Armonk, NY, USA) were used for statistical analysis.
All quantitative variables were tested for normality using Shapiro–Wilk test. The data were represented by descriptive statistics: the quantitative data distribution different from normal was represented as The median, the upper quartile and lower quartile (Q1; Q3) and interquartile range. The categorical data were represented as absolute numbers and the ratio between a part and the whole group. The comparative analysis of qualitative variables in unpaired (independent) groups, where distribution differed from normal, was performed using the non-parametric Mann–Whitney U-test. All p-values were corrected for multiple pairwise comparisons with respect to the number of tests using the Bonferroni correction (p=0.005). Non-parametric Pearsons’ chi-squared test (χ2) was used to test categorical variables between the groups; with 1 degree of freedom, Yates's correction for continuity was used, and with a small number of observations (<5) Fisher’s exact test was used.
Results
Clinical characteristics of women
In the study groups, the following histopathological changes were found: branching abnormalities and dysmorphic chorionic villi in 39.4% of tissue specimens, inflammatory changes in 11.5%, hemorrhagic/ishemic changes and/or impaired vasculogenesis in 9.3%, other histopathological changes in 14.5%, and there were no histopathological changes detected in 25.3%.
Histopathological changes in early pregnancy loss and clinical characteristics of women are represented in Table 1.
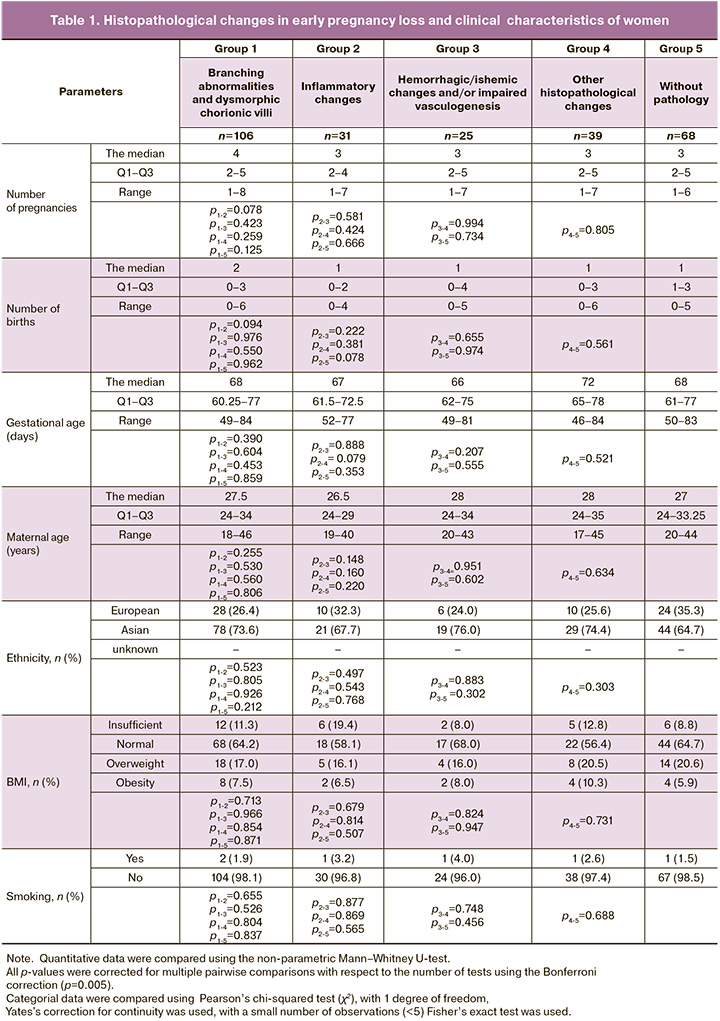
In Group 1 (Abnormal branching of chorionic villi and dysmorphic chorionic villi), the median number of pregnancies was 4 (Q1–Q3: 2–5, interquartile range 1–8), the median number of births was 2 (Q1–Q3: 0–3, interquartile range: 0–6), the median gestational age was 68 days, the median age of women was 27.5 years. Most patients – 78/106 (73.6%) were of Asian origin, 28/106 (26,4%) were of European origin. More than half of women – 68/106 (64.2%) had normal body mass index (BMI), 18/106 (17.0%) had overweight, 12/106 (11,3%) had lack of weight, 8/106 (7,5%) had obesity.
In group 2 (Inflammatory changes), the median number of pregnancies was 3 (Q1–Q3: 2–4, interquartile range: 1–7), the median number of births was 1 (Q1–Q3: 0–2, interquartile range: 0–4), the median gestational age was 67 days, the median age of women was 26.5 years. There were 21/31 patients (67.7%) of Asian origin and 10/31 patients (32.3%) of European origin in the group. Most women 18/31 (58.1%) had normal BMI, 6/31 (19.4%) – had lack of weight, 5/31 (16.1%) – had overweight, 2/31 (6.5%) – had obesity.
In group 3 (Hemorrhagic/ischemic changes and/or impaired vasculogenesis), the median number of pregnancies was 3 (Q1–Q3: 2–5, interquartile range: 1–7), the median number of births was 1 (Q1–Q3: 0–4, interquartile range: 0–5), the median gestational age was 66 days, the median age of women was 28 years. There were 19/25 patients (76.0%) of Asian origin and 6/39 (24,0%) of European origin in the group. More than half of women 17/25 (68.0%) had normal BMI, 4/25 had overweight (16.0%), 2/25 had lack of weight (8.0%), 2/25 (8.0%) had obesity.
In group 4 (Other impairments), the median number of pregnancies was 3 (Q1–Q3: 2–5, interquartile range: 1–7), the median number of births was 1 (Q1–Q3: 0–3, interquartile range: 0–6), the median gestational age was 72 days, the median age of women was 28 years. There were 29/39 women (74.4%) of Asian origin, 10/39 (25.6%) of European origin in the group. Normal BMI had 22/39 women (56.4%) 8/39 (20.5%) had overweight, 5/39 (12.8%) had lack of weight, 4/39 (10.3%) had obesity.
In group 5 (Without pathology), the median number of pregnancies was 3 (Q1–Q3: 2–5, interquartile range: 1–6), the median number of births was 1 (Q1–Q3: 1–3, interquartile range: 0–5), the median gestational age was 68 days, the median age of women was 27 years. More than half of patients – 44/68 (647%) were of Asian origin, 24/68 (35.3%) were of European origin. Normal BMI had 44/68 women (64.7%), 14/68 (20.6%) had overweight, 6/68 (8.8%) had lack of weight, 4/68 (5.9%) had obesity.
Serum laboratory parameters in women
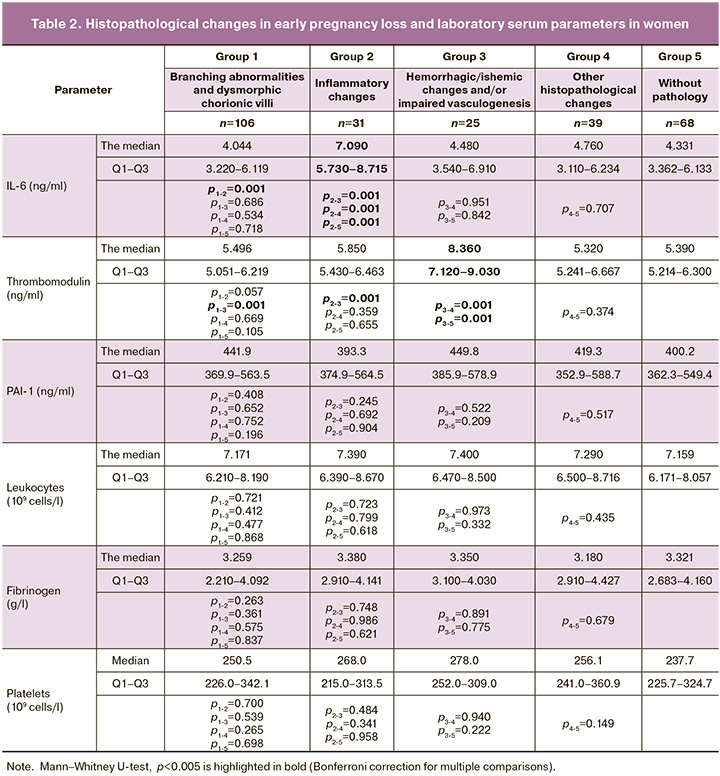
Serum levels of IL-6 were statistically significantly higher in group 2 (inflammatory changes) compared to other groups (p<0.005). The median value of IL-6 in group 2 was 7.090, interquartile range (Q1–Q3): 5.730–8.715. In 19 cases (61.3%) in group 2, massive acute inflammatory infiltrates were found both in chorionic villi and decidual tissue (Fig. 1 a, b). In 5 cases (16.1%), moderate and diffuse inflammatory infiltrates were detected, mainly characterized by macrophages, lymphocytes and neutrophils in the decidua. Additionally, there were areas of necrosis in 7 specimens (22.5%). Normally, immune cells are detected in dicidua in miscarriage. However, in these cases, tissue infiltration specific for deciduitis was found to be much more massive and extensive compared to group 4 (other impairments).
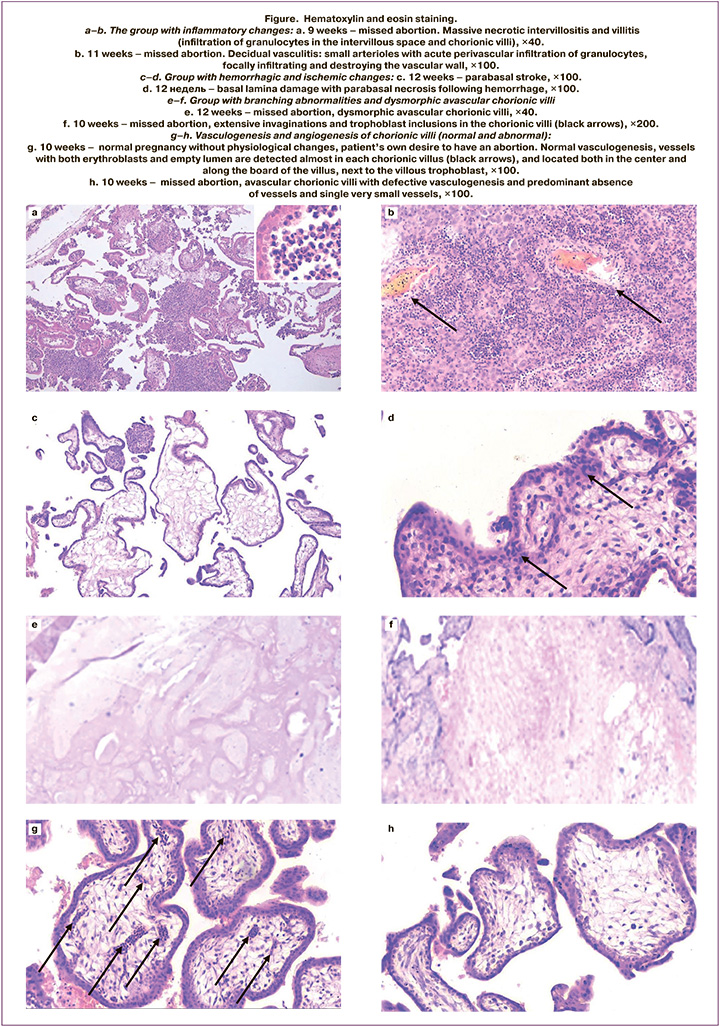
TM levels were significantly higher in group 3 (hemorrhagic/ischemic changes and /or abnormal vasculogenesis) compared to other groups (p<0.005). The median value of TM in group 3 was 8.360, interquartile range (Q1–Q3): 7.120–9.030. Early vascular karyorrhexis and intervillous hemorrhage was found in 4 samples (16%). Parabasal strokes and hemorrhages in the basal plate in 7 specimens (28%) (Fig. e, f).
Vascularization in 15 samples (60%) showed that severe dysplasia was in 10 samples 10 (66.7%) (predominantly avascular chorionic villi, and the vessels with one or more erythroblasts in one chorionic villus), and avascularity was in 5 samples (33.3%) (Fig. g, h).
The values of other parameters (leucocytes, platelets, PAI-1 and fibrinogen) were not statistically different in all study groups (p>0.005) (Table 2).
Discussion
The most important result of this study was detection of high levels of IL-6 and TM as the markers of inflammatory and hemorrhagic/ischemic histopathological changes in early pregnancy losses.
Statistically significant difference in the levels of IL-6 was found between the samples with histopatological changes versus other study groups (p<0.005). The obtained results are consistent with previously published data by Zhang M. et al. (2017), where a significant correlation between changes in interleukin levels and reproductive losses was demonstrated, and the authors recommended to consider the values of IL-6 as the important criterion for prediction recurrent miscarriage [9]. The study found that the median and interquartile ranges of IL-6 in the group of inflammatory changes was 7.090 (5.730–8.715), that can serve as prerequisite to establish reference values for prediction or diagnostics of inflammation, as well as expands and complements the diagnosing causes of early pregnancy failure.
In the group of hemorrhagic/ischemic changes and/or abnormal vasculogenesis the median value of TM was 8.360 (7.120–9.030), that was significantly higher compared to other study groups T (p<0.005).
TM is recommended to be the gold standard for prediction of early reproductive losses [14, 15]. Our study found association between high levels of TM and histological vascular malformations, that opens opportunities to study pathogenesis of these malformations. The results of our study highlights the importance of TM as the key marker, which can not only predict reproductive losses, but also helps in understanding the complex biological processes underlying vascular malformations.
The results of the study showed a tendency towards severe hypovascularization and avascularization of chorionic villi in the group with elevated TM levels. The literature describes that vascularization of chorionic villi may reduce after fetal death and postmortem autolytic changes [22, 23]. However, in our study hypoplasia of the vascular system with significantly fewer vessels and predominantly hypovascular/avascular chorionic villi was found predominantly in group with high levels of TM. Based on the obtained results, it can be concluded that the observed changes in the vessels in chorionic villi are the result of pathological development due to abnormal or defective vasculogenesis, but not due to postmortem changes. High levels of TM may contribute to defective vasculogenesis or be the marker of the process leading to impaired vasculogenesis irrespective of postmortem changes evaluated by autopsy. This indicates that TM plays a specific role in abnormal development of the vascular system of the chorionic villi. These highlights require further research.
The benefit of this study include the use of standardized histological nomenclature and large sample size.
The limitation of the study is that the sample size included case series at one healthcare facility, and description of pathologies was mainly based on pathology reports provided by pathologists in performing their routine work. Another limitation was lack of the group to be compared with the group, where pregnancy was terminated due to social or medical indications or by woman’s own desire.
Conclusion
Increased level of IL-6 should be considered to be the marker of hemorrhagic/ischemic changes and defective vasculogenesis. Monitoring of the level of these markers will enable to detect women at high risk group, that will make it possible to perform targeted medical examination and correction of potential factors influencing unfavorable course of pregnancy.
References
- Кушубекова А.К., Самигуллина А.Э., Бообекова А.А. Невынашивание беременности: гистологическое исследование соскобов из полости матки. Международный журнал прикладных и фундаментальных исследований. 2019; 5: 66-71. [Kushubekova A.K., Samigullina A.E., Boobekova А.А. Non-care of pregnancy: histological study of cerebrates from the cavity of the uterus. International Journal of Applied and Fundamental Research. 2019; (5): 66-71. (in Russian)].
- Beniuk V.O., Ginzburg V.H., Chebotarova A.S., Hychka N.M., Kovaliuk T.V., Beniuk S.V. et al. Pathogenetic background of occurrence of venous thromboembolic complications in women with antenatal fetal death. Pol. Merkur. Lekarski. 2021; 49(293): 341-5.
- Perés Wingeyer S., Aranda F., Udry S., Latino J., de Larrañaga G. Inherited thrombophilia and pregnancy loss. Study of an Argentinian cohort. Med. Clin. (Barc.). 2019; 152(7): 249-54. https://dx.doi.org/10.1016/j.medcli.2017.12.019.
- Sekiya A., Hayashi T., Kadohira Y., Shibayama M., Tsuda T., Jin X. et al. Thrombosis prediction based on reference ranges of coagulation-related markers in different stages of pregnancy. Clin. Appl. Thromb. Hemost. 2017; 23(7): 844-50. https://dx.doi.org/10.1177/1076029616673732.
- Wang W., Long K., Deng F., Ye W., Zhang P., Chen X. et al. Changes in levels of coagulation parameters in different trimesters among Chinese pregnant women. J. Clin. Lab. Anal. 2021; 35(4): e23724. https://dx.doi.org/10.1002/jcla.23724.
- Зубков Д.В., Тайжанова Д.Ж., Амирбекова Ж.Т., Турдунова Г.С., Беспалова Н.В. Информативность скринингового обследования параметров коагуляции для прогнозирования раннего выкидыша: обзор литературы. Репродуктивная медицина (Центральная Азия). 2022; 4: 55-62. [Zubkov D.V., Taizhanova D.Zh., Amirbekova Zh.T., Turdunova G.S., Bespalova N.V. The informative value of coagulation parameter screening examination to predict early miscarriage: a literature review. Reproductive Medicine (Central Asia). 2022; (4): 55-62. (in Russian)]. https://dx.doi.org/10.37800/RM.3.2022.55-62.
- Cui C., Yang S., Zhang J., Wang G., Huang S., Li A. et al. Trimester-specific coagulation and anticoagulation reference intervals for healthy pregnancy. Thromb. Res. 2017; 156: 82-6. https://dx.doi.org/10.1016/j.thromres.2017.05.021.
- Vilotić A., Nacka-Aleksić M., Pirković A., Bojić-Trbojević Ž., Dekanski D., Jovanović Krivokuća M. IL-6 and IL-8: an overview of their roles in healthy and pathological pregnancies. Int. J. Mol. Sci. 2022; 23(23): 14574. https://dx.doi.org/10.3390/ijms232314574.
- Zhang M., Xu J., Bao X., Niu W., Wang L., Du L. et al. Association between genetic polymorphisms in interleukin genes and recurrent pregnancy loss - a systematic review and meta-analysis. PLoS One. 2017; 12(1): e0169891. https://dx.doi.org/10.1371/journal.pone.0169891.
- Akin M.N., Kasap B., Yuvaci H.U., Turhan N. Association between platelet indices and first trimester miscarriage. Blood Coagul. Fibrinolysis. 2016; 27(5): 526-30. https://dx.doi.org/10.1097/MBC.0000000000000445.
- Wang W., Sung N., Gilman-Sachs A., Kwak-Kim J. T helper (Th) cell profiles in pregnancy and recurrent pregnancy losses: Th1/Th2/Th9/Th17/Th22/Tfh cells. Front. Immunol. 2020; 11: 2025. https://dx.doi.org/10.3389/fimmu.2020.02025.
- Pei C.Z., Kim Y.J., Baek K.H. Pathogenetic factors involved in recurrent pregnancy loss from multiple aspects. Obstet. Gynecol. Sci. 2019; 62(4): 212-23. https://dx.doi.org/10.5468/ogs.2019.62.4.212.
- Watanabe-Kusunoki K., Nakazawa D., Ishizu A., Atsumi T. Thrombomodulin as a physiological modulator of intravascular injury. Front. Immunol. 2020; 11: 575890. https://dx.doi.org/10.3389/fimmu.2020.575890.
- Yang Y., Hu Y., Wu M., Xiang Z. Changes of new coagulation markers in healthy pregnant women and establishment of reference intervals in Changsha. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022; 47(4): 469-78. https://dx.doi.org/10.11817/j.issn.1672-7347.2022.210536.
- Martínez-Zamora M.A., Tàssies D., Creus M., Reverter J.C., Puerto B., Monteagudo J. et al. Higher levels of procoagulant microparticles in women with recurrent miscarriage are not associated with antiphospholipid antibodies. Hum. Reprod. 2016; 31(1): 46-52. https://dx/doi.org/10.1093/humrep/dev278.
- Ye Y., Vattai A., Zhang X., Zhu J., Thaler C.J., Mahner S. et al. Role of plasminogen activator inhibitor type 1 in pathologies of female reproductive diseases. Int. J. Mol. Sci. 2017; 18(8): 1651. https://dx.doi.org/10.3390/ijms18081651.
- Kliman H.J., Firestein M.R., Hofmann K.M., Milano K.M., Holzer P.H., Brink L.T. et al. Trophoblast inclusions in the human placenta: identification, characterization, quantification, and interrelations of subtypes. Placenta. 2021; 103: 172-6. https://dx.doi.org/10.1016/j.placenta.2020.10.014.
- Firestein M.R., Kliman H.J., Sania A., Brink L.T., Holzer P.H., Hofmann K.M. et al. Trophoblast inclusions and adverse birth outcomes. PLoS One. 2022; 17(3): e0264733. https://dx.doi.org/10.1371/journal.pone.0264733.
- Pinar M.H., Gibbins K., He M., Kostadinov S., Silver R. Early pregnancy losses: review of nomenclature, histopathology, and possible etiologies. Fetal Pediatr. Pathol. 2018; 37(3): 191-209. https://dx/doi.org/10.1080/15513815.2018.14557755.
- Redline R.W. Early pregnancy loss with normal karyotype. Ch. 2. In: Redline R.W., Boyd T.K., Roberts D.J., eds. Placental and gestational pathology. Diagnostic pediatric pathology. Cambridge University Press; 2017: 9-15. https://dx.doi.org/10.1017/9781316848616.003.
- Hakvoort R.A., Lisman B.A., Boer K., Bleker O.P., van Groningen K., van Wely M. et al. Histological classification of chorionic villous vascularization in early pregnancy. Hum. Reprod. 2006; 21(5): 1291-4. https://dx.doi.org/10.1093/humrep/dei456.
- Lisman B.A., Boer K., Bleker O.P., van Wely M., van Groningen K., Exalto N. Abnormal development of the vasculosyncytial membrane in early pregnancy failure. Fertil. Steril. 2004; 82(3): 654-60. https://dx.doi.org/10.1016/j.fertnstert.2004.02.119.
- Чучалина Л.Ю. Роль искусственного прерывания беременности в первом триместре в формировании вторичного бесплодия. Акушерство и гинекология. 2016; 11: 113-6. [Chuchalina L.Yu. The role of induced abortion in the first trimester of pregnancy in the development of secondary infertility. Obstetrics and Gynecology. 2016; (11): 113-6. (in Russian)]. https://dx.doi.org/10.18565/aig.2016.11.113-116.
Received 08.07.2024
Accepted 30.09.2024
About the Authors
Dana Zh. Taizhanova, Dr. Med. Sci., Professor at the Department of Internal Diseases, Karaganda Medical University, Republic of Kazakhstan, Karaganda, Gogol str., 40, https://orcid.org/0000-0001-6971-8764Dmitry V. Zubkov, doctoral student PhD, teacher-researcher at the Department of Obstetrics, Gynecology and Perinatology, Karaganda Medical University,
Republic of Kazakhstan, Karaganda, Gogol str., 40, +7(707)302-11-31, Zubkov@qmu.kz, gipokrat999999@gmail.com, https://orcid.org/0000-0002-6298-7096
Evgeny K. Kamyshansky, PhD, Associate Professor, Head of the Pathological and Anatomical Unit, Clinic of the Karaganda Medical University, Republic of Kazakhstan, Karaganda, Ardak str., 1-3, https://orcid.org/0000-0002-8125-6643
Eduard V. Komlichenko, Dr. Med. Sci., Institute of Perinatology and Pediatrics, V.A. Almazov National Medical Research Center, St. Petersburg, Russian Federation,
https://orcid.org/0000-0003-2943-0883
Islam Sh. Magalov, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology, Baku branch of I.M. Sechenov First Moscow State Medical University, Baku, Azerbaijan, Scopus ID: 36485060700.
Marina A. Sorokina, PhD, Associate Professor, Head of the Department of Informatics and Biostatistics, Karaganda Medical University, Republic of Kazakhstan,
Karaganda, Gogol str., 40, https://orcid.org/0000-0001-5333-1362
Corresponding author: Dmitry V. Zubkov, Zubkov@qmu.kz, gipokrat999999@gmail.com



