Преждевременные роды (ПР) являются одной из значимых проблем современного акушерства во всем мире, осложняющих примерно до 10% всех беременностей [1]. С ними связано примерно 75% гибели детей и 50% неврологических нарушений в последующей жизни [2]. Работами многих авторов показано, что такие заболевания, как ожирение, сахарный диабет 2-го типа, артериальная гипертензия и многие другие связаны с нарушенным вектором развития эмбриона/плода и ПР [3, 4]. ПР являются полиэтиологичной патологией, значительную часть (примерно 11%) в которую вносит преэклампсия [5].
Выяснение механизмов и факторов, способствующих индукции ПР, является важнейшей научно-практической задачей. Сравнение таких состояний, как угроза ПР со срочными родами, где объединяющим началом служит сокращение матки, а также с преэклампсией, где объединяющий момент – воспаление, позволяет вычленить общие механизмы и факторы, способствующие индукции ПР.
Иммунологические механизмы в индукции ПР являются во многом ведущими [6]. Выбор интерлейкина (ИЛ)-6 был не случаен, а обусловлен следующими моментами. Во-первых, это классический цитокин, обладающий как провоспалительной, так и антивоспалительной активностью. Во-вторых, он является важным миокином, высоко экспрессируемым скелетными мышцами при их сокращении. Повышение экспрессии вызывает увеличение его секреции в кровь, где он необходим для изменения метаболизма и обеспечения энергетики сокращения мышц. Показано также, что он экспрессируется клетками миометрия матки [7] при их сокращении. Другими словами, исследуя ИЛ-6, можно проследить связь между сокращением миометрия при срочных родах и при угрозе ПР. Сами срочные роды мы рассматриваем как сложный многоуровневый процесс, одним из компонентов которого является локальная воспалительная реакция в шейке и нижнем сегменте матки [8]. При ПР также часто регистрируются элементы воспаления и, соответственно, активации иммунной системы.
Растворимый рецептор 1 сосудисто-эндотелиального фактора роста (р-СЭФРр1) – это классический антиангиогенный фактор, продуцируемый клетками трофобласта, блокирующий действие СЭФР и фактора роста плаценты [9]. СЭФР обладает провоспалительной активностью [10]. С этих позиций его растворимый рецептор контролирует как рост сосудов, так и провоспалительную активность.
Цель исследования: изучить содержание и возможную роль ИЛ-6, его растворимого рецептора (рИЛ-6р), а также р-СЭФРр1 при срочных родах, угрозе ПР и преэклампсии.
Материал и методы исследования
В соответствии с целью исследования обследованы 85 беременных, которые были разделены на 4 группы. Первая группа (35 человек) – физиологические роды, первый период, среднее время сокращения миометрия составляло 5,29±0,6 часа. Вторая группа (11 человек) – с угрозой ПР, обязательным компонентом которых был сокращающийся миометрий. Третья группа (23 человека) – с преэклампсией. Четвертая группа (контроль) – 16 соматически здоровых беременных с неотягощенным акушерско-гинекологическим анамнезом и физиологическим течением беременности.
Диагноз преэклампсия ставили при сочетании повышенного артериального давления – 140/90 мм рт. ст. и выше и протеинурии 300 мг/сутки и выше в суточной моче, не обусловленной заболеванием почек. В основу диагностики преэклампсии положена американская классификация [11] и МКБ-10. Всем беременным проведено традиционное комплексное клинико-лабораторное обследование и ультразвуковое исследование. Параллельно с этим в сыворотке крови методом твердофазного иммуноферментного анализа исследован ИЛ-6 с использованием коммерческих наборов (ЗАО «Вектор Бест», Новосибирск), рИЛ-6р и рСЭФРр1 с использованием высокочувствительных наборов (human sIL-6R Platinum ELISA, human sVEGF-R1 platinum ELISA, eBioscience, Австрия) в соответствии с рекомендациями фирмы-производителя. Чувствительность наборов – 0,5 пг/мл, 0,01 нг/мл и 0,03 нг/мл соответственно.
Математическую обработку полученных данных проводили с использованием непараметрического U-критерия Манна–Уитни, программа Statistica, версия v 10.0. Достоверными считались результаты при р<0,05.
Результаты исследования и обсуждение
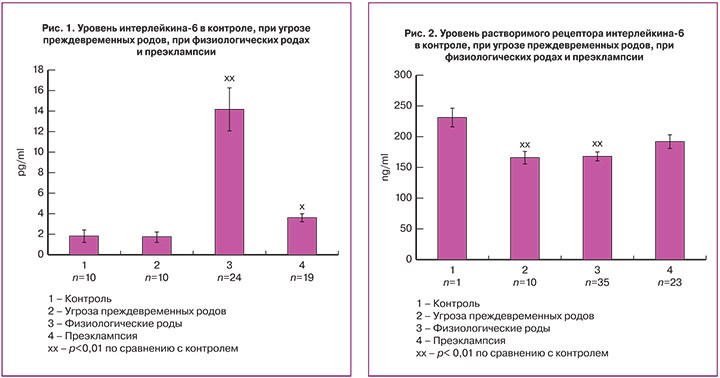
У беременных контрольной группы уровень ИЛ-6 в сыворотке был около 2 пг/мл (рис. 1). При угрозе ПР его уровень был примерно таким же. Однако при физиологических родах, при интенсивно сокращающемся миометрии этот показатель был в 7 раз выше, чем в контроле (рис. 1). Другими словами, сокращение миометрия невысокой интенсивности при угрозе ПР не ведет к повышению в сыворотке ИЛ-6, в то время как интенсивное сокращение миометрия во время физиологических родов повышает уровень ИЛ-6 в сыворотке крови. Индивидуальные показатели содержания ИЛ-6 в первом периоде родов были гетерогенными, при цифрах, максимально превышающих контроль почти в 20 раз. Эти данные показывают, что, возможно, интенсивно сокращающийся миометрий синтезирует и секретирует ИЛ-6. Параллельно отметим, что при длительном и интенсивном сокращении скелетных мышц уровень ИЛ-6 начинает повышаться уже через 30 минут, и его уровень может достигать 100-кратного увеличения. Такого повышения ИЛ-6 достигает только при одном состоянии – сепсисе [12, 13]. При преэклампсии также отмечается повышение ИЛ-6, но не такое значительное, как при физиологических родах (рис. 1). Наиболее вероятной причиной повышения ИЛ-6 при ПЭ является активация иммунной системы, а при родах повышение обусловлено как активацией иммунной системы, так и сокращающимся миометрием. Сравнение этих групп показывает, какой возможный уровень ИЛ-6 дает сокращающийся миометрий. Способность миометрия в состоянии растяжения экспрессировать и секретировать ИЛ-6 показана в работе S.B. Rajagopal и соавт. [14].
Анализ индивидуальных показателей ИЛ-6 при физиологических родах показывает, что примерно в 25% случаев его уровень превышал контроль в 10 раз. Такое повышение важно как для иммунной, так и для эндокринной системы, так как оно не только стимулирует иммунную систему, но и имеет эндокринологическую направленность, меняя метаболизм в первую очередь в печени, включая гликогенолиз и направляя поток глюкозы в интенсивно сокращающийся миометрий по аналогии с сокращающимися скелетными мышцами [15]. Последствия такого повышения ИЛ-6 для организма в условиях беременности не известны. Однако можно предполагать, что этот момент имеет отношение к таким послеродовым осложнениям, как эндометриты и маститы, из-за того что повышение ИЛ-6 не локальное, а системное.
 При рассмотрении полученных данных под углом миокинов – цитокинов, нарабатываемых и секретируемых сокращающимися мышечными клетками, обращает на себя внимание специфика сокращения миометрия во время родов, где периоды сокращения чередуются с периодами покоя. Вероятно, данная тактика сокращения мышечных клеток тормозит гиперпродукцию ИЛ-6. Известно, что уровень ИЛ-6 повышается при ПР [16]. Полученные нами индивидуальные данные показывают, что только примерно в 10% случаев при угрозе ПР уровень ИЛ-6 превышал контрольные цифры. Учитывая приведенные данные, можно предполагать, что это повышение может перевести угрозу в реальные ПР. Умеренное повышение ИЛ-6 при ПЭ также говорит в пользу того, что только нарастающий тренд может быть предиктором перехода угрозы в ПР.
При рассмотрении полученных данных под углом миокинов – цитокинов, нарабатываемых и секретируемых сокращающимися мышечными клетками, обращает на себя внимание специфика сокращения миометрия во время родов, где периоды сокращения чередуются с периодами покоя. Вероятно, данная тактика сокращения мышечных клеток тормозит гиперпродукцию ИЛ-6. Известно, что уровень ИЛ-6 повышается при ПР [16]. Полученные нами индивидуальные данные показывают, что только примерно в 10% случаев при угрозе ПР уровень ИЛ-6 превышал контрольные цифры. Учитывая приведенные данные, можно предполагать, что это повышение может перевести угрозу в реальные ПР. Умеренное повышение ИЛ-6 при ПЭ также говорит в пользу того, что только нарастающий тренд может быть предиктором перехода угрозы в ПР.
Уровень рИЛ-6р имел во многом обратное соотношение к ИЛ-6 (рис. 2). Он имел четко выраженное снижение, наиболее значимое в ситуации с физиологическими родами, где выраженность воспалительного процесса максимальна. Ответ на ИЛ-6 обусловливается его взаимодействием с растворимой формой рецептора. Показано также, что ИЛ-6 через свой растворимый рецептор является инструментом, контролирующим рекрутирование лейкоцитов при воспалении [17], и, что самое главное, регулирует переход от нейтрофильной к моноцитарной инфильтрации, что знаменует переход от острого воспаления к регенерации и разрешению воспаления. С этих позиций снижение рИЛ-6р, найденное нами, отражает степень нарастания инфильтрации нейтрофилами при изученных процессах, наиболее выраженное при физиологических родах, где эта инфильтрация максимальна. Так, например, количество нейтрофилов в шейке матки и нижнем сегменте матки перед и во время родов увеличивается в 196 раз, а моноцитов/макрофагов – в 8 раз [8].
Возможно и то, что снижение рИЛ-6р может являться одним из механизмов, сдерживающих нарастание воспаления при угрозе ПР.
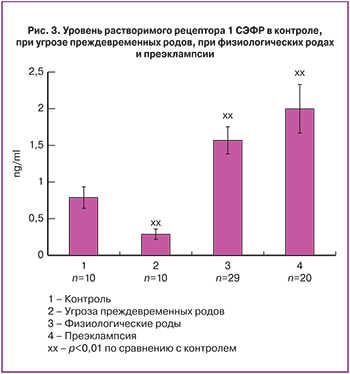
Уровень рСЭФРр1 имел несколько иные показатели при исследуемых процессах. Так, он снижался почти в 2 раза при угрозе ПР (рис. 3), повышался при физиологических родах и еще более значимо был повышен при преэклампсии. Его повышение при преэклампсии – известный факт [18]. С современных позиций его повышение как антиангиогенного фактора является одним из патогенетических механизмов развития преэклампсии. Какую роль несет повышение этого рецептора при физиологических родах, неизвестно. Можно высказать только предположение, что в ответ на гипоксию в комплексе с иммунологическими факторами во время родов может происходить повышение экспрессии рСЭФРр1 в трофобласте. Так, T. Cindrova-Davies и соавт. [19] показали, что во время родов экспрессия рСЭФРр1повышается в 2,5 раза в плаценте. Параллельно отметим, что миометриальные гладкомышечные клетки экспрессируют как СЭФР, так и рСЭФРр1 [14, 19]. Полученные нами данные в отношении повышения при физиологических родах антиангиогенного фактора рСЭФРр1 имеют очень важное практическое значение. Их можно трактовать двояко: 1) кратковременное повышение этого антиангиогенного фактора не имеет вредных последствий для организма в виде повышения артериального давления и протеинурии; 2) возможно, существуют пути, позволяющие избегать осложнений в условиях повышения рСЭФРр1. Последнее положение особенно привлекательно, так как манипуляции с блокадой СЭФР аналогично действию рСЭФРр1 в настоящее время являются одним из важнейших терапевтических направлений в лечении онкологических заболеваний, где сдерживающим моментом служат осложнения в виде подъема артериального давления и протеинурии [20].
Сложнее объяснить найденное нами снижение рСЭФРр1 при угрозе ПР. Возможно, это связано с тем, что повышение этого растворимого рецептора повышает риск ПР [21].
На основании полученных результатов мы предлагаем следующую схему, объясняющую степень сокращения миометрия при физиологических родах и при угрозе ПР. Миометриальные клетки матки в состоянии растяжения экспрессируют и секретируют СЭФР.
СЭФР, как хемоаттрактант, через свой рецептор рСЭФРр1 обеспечивает миграцию моноцитов [22], в данном случае в миометрий. Взаимодействие моноцитов с гладкомышечными клетками миометрия ведет к повышению продукции миоцитами ИЛ-6, 8, моноцитарного хемоаттрактантного протеина 1 и к усилению сокращения миоцитов. Повышенный уровень ИЛ-6 обеспечивает эндокринное и энергетическое сопровождение интенсивно сокращающегося миометрия. При преэклампсии повышенный уровень рСЭФРр1 обеспечивается трофобластом. С этих позиций моноциты при патологическом процессе мигрируют в плаценту по пути высокого градиента хемоаттрактанта. По сути, так и происходит в реальной ситуации. Биологическая сущность повышения рСЭФРр1, блокирующего действие СЭФР, и фактора роста плаценты во многом не понятна. Исходя из нашей схемы, можно только по-иному взглянуть на этот вопрос. Растворимый СЭФРр1 повышается для контроля воспаления, блокируя провоспалительный цитокин СЭФР.
Заключение
Сделана попытка сравнить угрозу ПР с физиологическими родами. Во-первых, объединяющим моментом обоих процессов является умеренно в первом и интенсивно во втором случае сокращающийся миометрий. Во-вторых, показано резкое нарастание такого миокина, как ИЛ-6, и снижение его растворимого рецептора при физиологических родах, при сокращении миометрия высокой интенсивности и отсутствие такового эффекта при угрозе ПР, то есть при сокращении миометрия низкой интенсивности. В-третьих, полученные данные позволяют говорить о возможной роли растворимого рецептора СЭФР как для срочных родов, так и для ПР. Использованный в работе подход к классическим цитокинам с позиций миокинов позволяет по-новому взглянуть на проблему как срочных родов, так и ПР. Как видно из полученных данных, выяснение механизмов индукции нормальных родов и сравнение их с угрозой ПР с позиций миокинов может помочь в разработке новых диагностических и лечебных стратегий для более успешной терапии ПР.



