Association of the polymorphic variant G-105a of the SEPS1 gene and the polymorphic variant C3872T of the CRP gene with preterm birth
Objective. To determine an association of the polymorphic variant G-105A of the SEPS1 gene and the polymorphic variant C3872T of the CRP gene with preterm birth in European women.Musalaeva I.O., Tarasenko E.V., Galina Т.V., Zheludova E.M., Azova M.M., Kirbasova N.P., Olenev A.S.
Subjects and methods. Sixty-five women with preterm birth at 23.5 to 37 weeks’ gestation and 65 women with full-term pregnancy were genotyped. The polymorphic variant G-105A of the SEPS1 gene was determined by the polymerase chain reaction (PCR), followed by restriction analysis, the polymorphic variant C3872T of the CRP gene was done by the allele-specific PCR followed by electrophoretic detection.
Results. There was an association of the polymorphic variant G-105A of the SEPS1 gene with the risk of preterm birth in Caucasian women. The frequency of the A allele of the SEPS1 gene was 20%, which was significantly higher than that in the control group (8%) (χ2 = 5.025, p = 0.025). The incidence of heterozygotes was also considerably higher in the preterm birth group (χ2 = 6.002; p = 0.014).There was no association of the T allele in the polymorphic variant C3872T of the CRP gene with preterm birth.
Conclusion. The A allele in the polymorphic variant G-105A of the gene SEPS1 can act as one of the predictors of preterm birth.
Keywords
According to World Health Organization (WHO), about 15 million preterm babies are born each year [1]. Preterm birth (PB) is considered to occur between 22 and 37 weeks of gestation. PB accounts for about 75% of perinatal deaths. The causes of spontaneous PB are not fully understood. Risk factors include obstetric and gynecological history (gynecological diseases, outcomes of previous pregnancies and births), complications of the current pregnancy (gestosis, multiple pregnancy, polyhydramnios, and placental presentation), social causes (age, bad habits, living conditions), concomitant diseases (heart disease, kidney disease, acute infectious diseases). PB frequency in different countries ranges from 5% to 18% [1]. About 30% of spontaneous PB is caused by the presence of infection, and 80% of children born before the 30th week of pregnancy are diagnosed with histologically verified chorioamnionitis [2, 3].
Inflammatory processes play a significant role in the development of many diseases. Currently, a sufficiently large number of genes have been identified, the products of which can be considered as markers of inflammation, or which are directly related to the implementation of infectious processes in the body [4-9]. These include more than 200 cytokines (interleukins (IL), tumor necrosis factors (TNF-α), interferons, growth factors), C-reactive protein, transforming growth factor (TGF-β) and many others.
The active study of gene polymorphisms of interleukins allowed the scientists to establish the interconnection between different allele variants of these genes with the development of PB, asthma, cancer, viral hepatitis, rheumatoid arthritis and a number of other diseases [6, 10-13]. Currently, a large amount of data on the role of inflammation in the development of PB has been accumulated. For example, the correlation of IL-1β and toll-like receptors (TLR) genes polymorphism with miscarriage was revealed [4, 10].
There is also a special class of proteins named selenoproteins which contain one or more residues of selenium-containing amino acid of selenocysteine. A special non-standard protein in the selenoprotein family is S-selenoprotein. It is involved in immune and inflammatory signaling pathways [7]. S-selenoprotein can be classified as a new membrane protein preventing stress response to the activation of the inflammatory cascade. Several polymorphisms have been found in the SEPS1 genes which have not been studied so far. As for SEPS1 G-105A polymorphism (rs28665122), a correlation with the risk of PB in women in the Chinese population [5], the risk of gastric cancer in the Japanese and Chinese [14, 15], and the risk of lung cancer in the Norwegians [12] was shown. At the same time, there is evidence that genetic manifestations may vary depending on ethnicity and the different effects of environmental factors.
The CRP-gene encodes a C-reactive protein which is a marker of systemic inflammatory response [16]. C-reactive protein is a factor of innate immune response. The production of this protein is nonspecific and is characterized by high correlation between its concentration in the blood, the cause, severity and stage of the disease. A large number of CRP-gene polymorphisms are known [17]. Polymorphism C3872T of the CRP-gene in Russia was studied in patients with cardiovascular diseases, liver diseases and primary erysipelas [13, 18, 19]. Currently, there are controversial data on the association of C-reactive protein and intraamniotic infection [8, 9]. In clinical practice the experience in determining the level of C-reactive protein in patients with PB as a predictor has already been gained, but CRP-gene polymorphisms have not been studied.
Therefore, the purpose of this research is to study the association of polymorphisms G-105A (rs28665122) of the SEPS1 gene and С3872Т of the CRP-gene with the PB in Caucasian women.
Materials and Methods
This research included 65 women with PB (the study group) at 23.5 to 37 weeks gestation. The control group consisted of 65 women with full-term pregnancy. Blood for the research was obtained from patients of maternity hospitals №29 and №8 in Moscow. Blood was collected in EDTA tubes and stored at -20 ° C.
DNA was isolated from blood leukocytes with sets of „DNA-express-blood” produced by LLC „Syntol”. The following primers were used to amplify the site containing the polymorphic variant G105A of the SEPS1 gene [5]:
5’-TCCTTGGCTTCAGTGTCCAAT-3’
5’-CGCGGACAGAGACTCCTCTT-3’
Polymerase chain reaction (PCR) was carried out in a 25 µl reaction mixture containing 20 ng of DNA, 0.2 µm of each primer, 0.2 µm of deoxynucleoside triphosphate and 0.5 units of Tag polymerase in a 1× buffer (LLC „Syntol”). The reaction conditions were 95° C – 2 min, then 35 cycles: 94° C – 30 sec, 57° C – 30 sec, 72° C – 30 sec, final elongation – 72° C – 7 min.
As a result of PCR, a 370 base-pair DNA fragment was formed. The DNAs were transplanted and incubated for 12 hours at -20° C by 3 volumes of ethyl alcohol after amplification. Then the amplicons were treated with endonuclease Mox 20 I (SibEnzyme LTD.), the work was carried out according to the technique described by Wang Y. et al. [5]. The electrophoresis in 3% agarose gel was performed to analyze the results.
The fragments were formed after the restriction with the sizes of 370, 233 and 137 base pairs (bp). The band of 370 bp corresponds to a homozygous GG, the bands of 233 and 137 bp correspond to a homozygous AA. All three fragments are found in heterozygotes.
Polymorphic variant C3872T of CRP-gene was determined by allele-specific PCR using a set of reagents of the Litech company. The results were detected by electrophoresis in 3% agarose gel.
R-language programs were used for statistical processing of the data [20]. Differences at the significance level p≤0.05 were considered statistically significant. The χ2 criterion was used to compare the frequencies of genotypes and alleles in the samples [21].
The work was carried out on the basis of Scientific Molecular Laboratory at Medical Institute of Peoples’ Friendship University of Russia, Moscow. DNA isolation was performed at Research and Educational Centre, Peoples’ Friendship University of Russia, Moscow.
Results and Discussion
The age of the examined women in the study group with PB ranged from 21 to 43 years, the average age was 32.2±5.8 years; in the control group (full-term pregnancy) the women’s age ranged from 19 to 41 years, the average age was 29.2±5.2 years (p=0.007). The body mass index was 26.9±6.4 and 25.5±4.4 kg/m2, respectively (p=0.187). The comparative characteristics of the groups revealed statistically significant differences in the number of pregnancies in the history: in the study group the average number was 2.6±1.6 and in the control group it was 1.7±1.1 (p=0.001). The comparative characteristics of social and family status showed no significant differences (p=0.312, p=0.931, respectively).
Since the causes may be different in the early and late stages of PB, the patients with PB (the study group) were divided into two subgroups: there were 21 patients with PB from 22 to 28 weeks gestation in the first subgroup, and 44 women with PB from 29 to 37 weeks gestation in the second subgroup.
The assessment of the vaginal microbial landscape in patients in the group with PB was performed. It revealed that 35 of 65 (53.8%) women had nonspecific vulvovaginitis with an increase in the number of leukocytes in smear to 40-80 per high power field, predominantly coccal and coccobacillary flora. The result of vaginal discharge microscopy corresponded to nonspecific vaginitis in 21 cases out of 65 (32.3%) in patients with full-term pregnancy. Comparing the data obtained by the criterion χ2, significant differences between these groups were obtained (χ2=6.149, p=0.0132). There was a tendency to an increase in nonspecific vulvovaginitis in pregnant women of the first subgroup, compared with the second subgroup (52.4 and 31.8%, respectively) (p=0.1177).
A marked increase in opportunistic microorganisms in 42 patients out of 65 (64.6%) was revealed in the analysis of the data on bacteriological examination of cervical smears in patients with PB. The following microorganisms prevailed: Candida albicans - 24 (57.%), Enterococcus faecalis - 18 (42.9%), Streptococcus agalactiae B - 15 (35.7%), Escherichia coli - 9 (21.4%). The growth of opportunistic flora was observed in 30 out of 65 patients (46.2%) in the group with full-term pregnancy. The predominance of opportunistic flora in the group with PB was found, compared with the control group (χ2=4.483; p=0.0342). The hazard risk of PB in the presence of conditionally pathogenic microorganisms was 1.4, 95% CI 1.02 – 1.92; and odds ratio was 2.13, 95% CI 1.05 – 4.31.
The majority of women were engaged in professional work: 44 women (67.7%) in the study group, and 50 women (76.9%) in the control group. There were no statistically significant differences between the groups (χ2 =1.383, p=0.2396).
Pregnancy in patients of both groups was somatically complicated (56% and 56%, p>0.05). Chronic hypertension was diagnosed with approximately the same frequency (3% and 4%, p>0.05). Anemia was diagnosed with approximately the same frequency in every second or third patient in both groups (44% and 40%, χ2 =0.284, p=0.5943).
Diseases of the genitourinary system in group with PB occurred in 12 patients (18.5%), while 8 patients (12.3%) of group with full-term pregnancy had such diseases. There were no significant differences between the groups (χ2=0.945, p=0.3309). Gastrointestinal diseases occurred in 12% of patients in both groups (p>0.05). Diseases of the respiratory system and thyroid disease were found among the patients with approximately the same frequency, in every twelfth or thirteenth patient (χ2= 0.050, p=0.8239).
A survey of respondents on sexual characteristics revealed that more than one in two (53.8%) from group with PB indicated that her first sexual intercourse was before the age of 17, and the same frequency was observed in the group with full-term pregnancy (46.2%) (χ2=0.769, p=0.3805). Every third patient in both groups had the first sexual intercourse at the age of 18 and later (37% and 39%, χ2 =0.130, p=0.7184) .
 The frequency of alleles of the SEPS1 gene in different populations has not been studied enough. According to Wang Y. et al., the average population frequency of allele A of the SEPS1 gene in the Chinese population is 6%, with the occurrence of heterozygotes 11.1%, homozygous AA 0.2%. This paper shows the correlation of the polymorphic variant G-105A (rs28665122) of the SEPS1 gene with the risk of PB [5].
The frequency of alleles of the SEPS1 gene in different populations has not been studied enough. According to Wang Y. et al., the average population frequency of allele A of the SEPS1 gene in the Chinese population is 6%, with the occurrence of heterozygotes 11.1%, homozygous AA 0.2%. This paper shows the correlation of the polymorphic variant G-105A (rs28665122) of the SEPS1 gene with the risk of PB [5].
The results obtained in the study of the distribution of genotypes and alleles in the polymorphic variant G105A of the SEPS1 gene in the studied samples are presented in Table 1.
In the group of women with PB the frequency of allele A in the polymorphic variant G-105A of the SEPS1 gene is 20%, which is significantly higher than in the control group (7.7%) (χ2=8.254; p=0.0041). The incidence of heterozygous AG is 40% and also significantly higher in the group of women with PB, compared with 15.4% in the group of women with full-term pregnancy (χ2=9.835, p=0.0017). AA homozygotes were not detected in any of the groups.
There were no significant differences in the incidence of polymorphic variant G105A of the SEPS1 gene between subgroups (10 out of 21, (47.6%) and 16 out of 44 (36.4%) (p=0.3932).
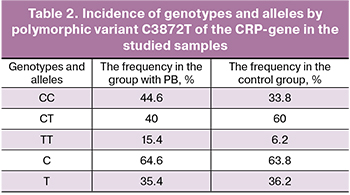 The results obtained in the study of the distribution of genotypes and alleles by polymorphic variant C3872T of CRP-gene in the studied samples are presented in Table 2.
The results obtained in the study of the distribution of genotypes and alleles by polymorphic variant C3872T of CRP-gene in the studied samples are presented in Table 2.
Association of the T-allele at the polymorphic variant С3872Т of the CRP-gene with PB was not detected (χ2=0.017, p=0.8971). However, there is a tendency to increase in the frequency of TT homozygotes in the group of women with PB (χ2=2.882, p=0.088). It is possible that the presence of the homozygous genotype of T-allele predisposes women to PB. This hypothesis requires further research in larger samples.
A comparative analysis between the groups on the growth of opportunistic flora and the incidence of polymorphic variant G-105A (rs28665122) of the SEPS1 gene revealed no correlation (χ2=0.833, p=0.361).
Conclusion
The results of the research revealed significant differences in the frequency of heterozygotes and A-allele of SEPS1 gene (G-105A) in Caucasian women with PB and full-term pregnancy. Thus, the A-allele of the polymorphic variant G105A of the SEPS1 gene can act as one of the PB predictors.
There were no statistically significant differences in the analysis of alleles and genotypes incidence by polymorphic variant C3872T of CRP-gene in Caucasian women with PB and full-term pregnancy.
References
- Всемирная организация здравоохранения. Информационные бюллетени. Available at: http://www.who.int/mediacentre/factsheets/fs363/ru/
- Lu G.C., Goldenberg R.L., Cliver S.P., Kreaden U.S., Andrews W.W. Vaginal fetal fibronectin levels and spontaneous preterm birth in symptomatic women. Obstet. Gynecol. 2001; 97(2): 225-8.
- Noguchi T., Sado T., Naruse K., Shigetomi H., Onogi A., Haruta S. et al. Evidence for activation of Toll-like receptor and receptor for advanced glycation end products in preterm birth. Mediators Inflamm. 2010; 2010: 490406. https://dx.doi.org/10.1155/2010/490406.
- Allen-Daniels M.J., Serrano M.G., Pflugner L.P., Fettweis J.M., Prestosa M.A., Koparde V.N. et al. Identification of a gene in Mycoplasma hominis associated with preterm birth and microbial burden in intraamniotic infection. Am. J. Obstet. Gynecol. 2015; 212(6): 779. e1-779. e13. https://dx.doi.org/10.1016/j.ajog.2015.01.032.
- Wang Y., Yang X., Zheng Y., Wu Z.H., Zhang X.A., Li Q.P. et al. The SEPS1G-105A polymorphism is associated with risk of Sspontaneous preterm birth in a Chinese population. PLoS One. 2013; 8(6): e65657. https://dx.doi.org/10.1371/journal.pone.0065657.
- Langmia I.M., Apalasamy Y.D., Omar S.Z., Mohamed Z. Impact of IL1B gene polymorphisms and interleukin 1B levels on susceptibility to spontaneous preterm birth. Pharmacogenet. Genomics. 2016; 26(11): 505-9. https://dx.doi.org/10.1097/FPC.0000000000000243.
- Sun H.Y., Liu T.B., Wang Q.C., Wu W.Q., He Y.J. Single nucleotide polymorphism in the SEPS1 gene may contribute to the risk of various human diseases: a meta-analysis. Ann. Hum. Biol. 2016; 43(5): 469-79. https://dx.doi.org/10.3109/03014460.2015.1070903.
- Shahshahan Z., Iravani H. Comparison of CRP and ALK-P serum levels in prediction of preterm delivery. Adv. Biomed. Res. 2016; 5: 17. https://dx.doi.org/10.4103/2277-9175.175903.
- Stepan M., Cobo T., Musilova I., Hornychova H., Jacobsson B., Kacerovsky M. Maternal serum C-reactive protein in women with preterm prelabor rupture of membranes. PLoS One. 2016; 11(3): e0150217. https://dx.doi.org/10.1371/journal.pone.0150217.
- Yang X., Peng W., Zhu L N, Zhang X.A., Wang Y. Association between interleukin-1β C+3953T and genetic susceptibility to spontaneous preterm birth: a case-control study. Zhongguo Dang Dai Er Ke Za Zhi. 2016;18(11): 1123-9.
- Berenguer A.G., Fernandes A.T., Oliveira S., Rodrigues M., Ornelas P., Romeira D. et al. Genetic polymorphisms and asthma: findings from a case-control study in the Madeira island population. Biol. Res. 2014; 47: 40. https://dx.doi.org/10.1186/0717-6287-47-40.
- Hart K., Landvik N.E., Lind H., Skaug V., Haugen A., Zienolddiny S. A combination of functional polymorphisms in the CASP8, MMP1, IL10 and SEPS1 genes affects risk of non-small cell lung cancer. Lung Cancer. 2011; 71(2): 123-9. https://dx.doi.org/10.1016/j.lungcan.2010.04.016.
- Емельянова А.Н., Витковский Ю.А. Генетический полиморфизм IL-10 и CRP у больных циррозом печени вирусной этиологии. Сибирский медицинский журнал (Иркутск). 2013; 119(4): 39-41. [Emelyanova A.N., Vitkovsky Yu.A. Genetic polymorphism of IL-10 and CRP in patients with liver cirrhosis of viral etiology. Siberian Medical Journal (Irkutsk). 2013; 119 (4): 39-41. (in Russian)].
- Mao H., Cui R., Wang X. Association analysis of selenoprotein S polymorphisms in Chinese Han with susceptibility to gastric cancer. Int. J. Clin. Exp. Med. 2015; 8(7): 10993-9.
- Shibata T., Arisawa T., Tahara T., Ohkubo M., Yoshioka D., Maruyama N. et al. Selenoprotein S (SEPS1) gene -105G>A promoter polymorphism influences the susceptibility to gastric cancer in the Japanese population. BMC Gastroenterol. 2009; 9: 2. 1 https://dx.doi.org/10.1186/1471-230X-9-2.
- Gabay C., Kushner I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999; 340(6): 448-54.
- dbSNP https://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?geneId=1401
- Царенок С.Ю., Горбунов В.В., Дутова А.А. Частота аллелей и генотипов полиморфизма гена C-реактивного белка у женщин с ишемической болезнью сердца и постменопаузальным остеопорозом. Тихоокеанский медицинский журнал. 2017; 4: 65-8. [Tsarenok S.Yu., Gorbunov V.V., Dutova A.A. The frequency of alleles and genotypes of the polymorphism of the gene C-reactive protein in women with coronary heart disease and postmenopausal osteoporosis. Pacific Medical Journal. 2017; 4: 65-8. (in Russian)].
- Емельянов А.С., Витковский Ю.А., Емельянова А.Н. Частота аллелей и генотипов полиморфизма гена CRP (C3872T) при первичной роже. Забайкальский медицинский вестник. 2015; 4: 135-7. [Emelyanov A.S., Vitkovsky, Yu.A., Emelyanova A.N. Frequency of alleles and genotypes of the polymorphism of the CRP gene (C3872T) in primary erysipelas. Transbaikal Medical Gazette. 2015; 4: 135-7. (in Russian)].
- R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at:http://www.R-project.org
- Wang J., Shete S. Testing departure from Hardy-Weinberg proportions. Methods Mol. Biol. 2012; 850: 77-102. https://dx.doi.org/10.1007/978-1-61779-555-8_6.
Received 22.05.2018
Accepted 22.06.2018
About the Authors
Musalaeva, Indira O., Post-graduate Student, Department of Obstetrics and Gynecology, Institute of Medicine, Peoples’ Friendship University of Russia (RUDN University).117198, Russia, Moscow, Miklukho-Maklaya str. 6. Tel.: +79259301540. E-mail: i700@mail.ru.
Tarasenko, Ekaterina V., PhD (Biol.), associate professor, Department of Biology and General Genetics, Institute of Medicine, Peoples’ Friendship University of Russia
(RUDN University). 117198, Russia, Moscow, Miklukho-Maklaya str. 6. Tel.: +74954345288. E-mail: tarasenko_ev@inbox.ru.
Galina Tatyana V., MD, professor, Department of Obstetrics and Gynecology, Institute of Medicine, Peoples’ Friendship University of Russia (RUDN University).
117198, Russia, Moscow, Miklukho-Maklaya str. 6. Tel.: +74953604669. E-mail: tatiana.galina1@mail.ru.
Zheludova, Elena M., PhD (Biol.), associate professor, Department of Biology and General Genetics, Institute of Medicine, Peoples’ Friendship University of Russia
(RUDN University). 117198, Russia, Moscow, Miklukho-Maklaya str. 6. Tel.: +74954335131. E-mail: zheludova_em@pfur.ru.
Azova, Madina M., D.Sc. (Biol.), associate professor, head of the Department of Biology and General Genetics, Institute of Medicine, Peoples’ Friendship University
of Russia (RUDN University). 117198, Russia, Moscow, Miklukho-Maklaya str. 6. Tel.: +74954335131. E-mail: azovam@mail.ru.
Kirbasova, Nina P., MD, professor, I.M. Sechenov First Moscow State Medical University. 119991, Moscow, Trubetskaya str. 8, p. 2.
Olenev Anton S., PhD, head of the City Clinical Hospital No. 24 DZM, Perinatal Center.
127287, Russia, Moscow, 4th Vyatsky pereulok, 39. Tel.: +74956134509. E-mail: felidis@mail.ru.
For citation: Musalaeva I.O., Tarasenko E.V., Galina Т.V., Zheludova E.M., Azova M.M., Kirbasova N.P., Olenev A.S. Association of the polymorphic variant G-105a of the SEPS1 gene and the polymorphic variant C3872T of the CRP gene with preterm birth.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology.2019; (4): 34-8. (in Russian)
https://dx.doi.org/10.18565/aig.2019.4.34-38



