The association between rs2414098 polymorphism of the CYP19A1 gene and the risk of developing endometrial hyperplasia
Objective. To assess the prevalence of CYP19A1 rs2414098 polymorphism and to identify its association with the risk of endometrial hyperplasia in women.Fatkullin I.F., Gabidullina R.I., Smirnova G.A., Nukhbala F.R., Valeeva E.V., Orlova Yu.I., Shakirov A.A.
Subjects and methods. The investigation enrolled 180 female residents of the Republic of Tatarstan. A study group consisted of 79 patients with endometrial hyperplasia without atypia; a control group included 101 women without endometrial pathology. DNA samples were genotyped for CYP19A1 rs2414098 polymorphism with a real-time polymerase chain reaction assay. A chi-square test was carried out; an odds ratio was also estimated.
Results. The C allele and the C/C genotype of CYP19A1 rs2414098 polymorphism are the factors that increase the risk of endometrial hyperplasia without atypia in women living in the Republic of Tatarstan. The prevalence of the alleles and genotypes of CYP19A1 rs2414098 proved to be comparable to the European sample data. Overweight (BMI >25 kg/m2) was also found to be a risk factor for endometrial hyperplasia, which was independent of the CYP19A gene predictor.
Conclusion. The investigation has revealed that there are associations of the CYP19A1 rs2414098 polymorphism with the risk of endometrial hyperplasia without atypia.
Keywords
Endometrial hyperplasia (EH) is a spectrum of irregular morphological alterations, whereby abnormal proliferation of the endometrial glands results in an increase in gland-to-stroma ratio [1]. 2014 WHO classification divides EH into two groups: hyperplasia without atypia and atypical hyperplasia or endometrial intraepithelial neoplasia (EIN) [2]. The pathophysiology of EH involves excessive and continuous estrogenic stimulation of the endometrium [3]. The leading risk factors for the development of EH include chronic anovulation, polycystic ovary syndrome (PCOS), obesity, tamoxifen therapy, and estrogen monotherapy [3–5]. The incidence of EH is 133–208 per 100,000 women-years; the incidence rates for EH without atypia are 121 per 100,000 women-years and 16.8 per 100,000 women-years for atypical EH [6]. The clinical importance of a diagnosis of EH is associated with the occurrence of abnormal uterine bleeding and the long term risk of progression to endometrioid endometrial cancer (EC) [7].
The presence and severity of cytological atypia and morphology are key factors defining the risk for progression to carcinoma: in patients without atypia, malignancy occurs in 5% of patients within 20 years [7], while in 40% of patients with EIN endometrial cancer develops within a year after the EIN diagnosis [8].
There are data on the molecular genetic determinants of EH among women living in the Central-Chernozemniy Region of Russia. Ponomarenko I.V. et al. reported the association of ESR2 rs4986938 polymorphism with the development of endometrial hyperplasia [9]. The peripheral conversion of androgens, especially in the adipose tissue, is known to contribute to the circulating level of estrogens in women. The main enzyme that catalyzes the conversion of androstenedione and testosterone to estrone and estradiol, respectively, is CYP19A1 (aromatase) [10, 11]. CYP19A1 gene polymorphism is probably associated with the development of EH, especially in obese women.
The study aimed to investigate the prevalence of rs2414098 variants of CYP19A1 gene polymorphism and the association between the identified genotypes of this polymorphism and the risk of developing EH.
Material and methods
The study comprised 180 female residents of the Republic of Tatarstan. The study group consisted of 79 patients with EH without atypia (N85.0). The control group was made up of 101 women without endometrial pathology who were admitted for surgical treatment to the Department of Gynecology of Kazan State Clinical Hospital No. 7 with a diagnosis of female genital prolapse (N81). The mean age of patients with EH without atypia and of the control group was 41.8 (7.9) and 70.5 (5.4) years, respectively.
Inclusion criteria for study patients were living in the Republic of Tatarstan, white race, EH diagnosis at the time of the study, or absence of endometrial pathology. The criteria for exclusion of patients from the study were endometrial polyps, EH with atypia, and EC. Women were recruited into groups based on the results of a histological examination of endometrial biopsy specimens and surgical specimens of the removed uterus. Women’s participation was voluntary and confidential. The study protocol was approved by the Ethics Committee of Kazan State Medical University. Minutes No. 6, dated 06.25.2019. The study was conducted as per the principles of the Helsinki Declaration.
Genetic analysis of CYP19A1 rs2414098 polymorphism was performed using the real-time polymerase chain reaction (PCR) with a commercial set of reagents from TestGene (Ulyanovsk, Russia). DNA was isolated from the peripheral white blood cells using the Rapid-Genetics Assay reagent kits of DNA-Technology LLC (Moscow, Russia). Amplification and detection of the region of the target gene were performed on a CFX96 amplifier (BioRad, USA).
Statistical analysis was carried out using Microsoft Excel 2013 for Windows and GraphPad Prism 8.2.0 software. The distribution of continuous variables was tested for normality using the Kolmogorov-Smirnov test. Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD) and presented as M (SD); otherwise, the median (Me) and the quartiles Q1 and Q3 in the Me (Q1; Q3) format were reported.
Differences in mean values of variables following normal distribution were assessed using the Student t-test. Variables not showing normal distribution were compared by the Mann-Whitney test. Statistical significance was assumed for p < 0.05. The deviation from Hardy-Weinberg equilibrium was assessed with the χ2 test; odds ratios (OR) were calculated.
Results
The mean age at menarche did not significantly differ between the groups (table). All women in the control group were in menopause. Women with EH without atypia had a menstrual function. Noteworthy is the difference in the number of pregnancies and childbirth in women of different generations (p < 0.001). There was no difference between the groups in the rates of miscarriages; however, older women statistically significantly differed in the rates of induced abortions and term births (p < 0.001).
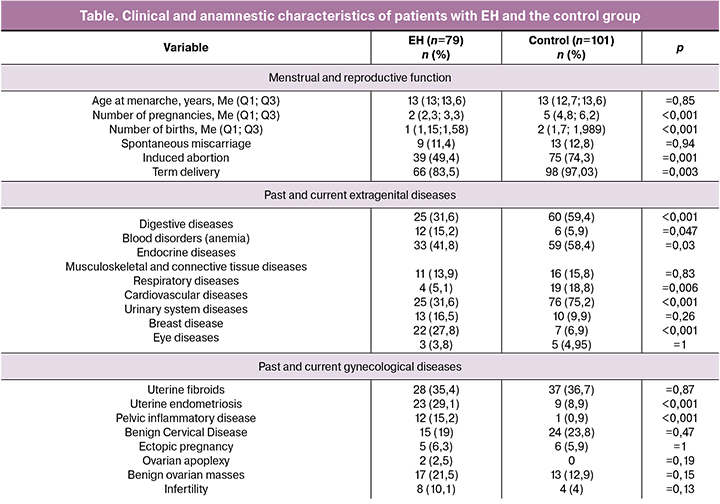
Women in the control group had statistically significant differences in the incidence of extragenital diseases. Women with EH without atypia were more likely to have uterine endometriosis and pelvic inflammatory diseases (p < 0.001).
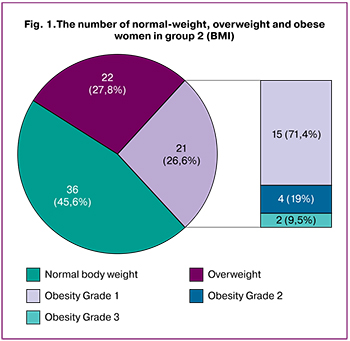 The groups differed in body mass index (BMI) with a BMI of 26.6 (5.7) and 28.9 (4.6) kg/m2 in the study group and control group, respectively (p <0.05). Among patients with EH without atypia, 36 (45.6%) patients had normal body weight, 22 (27.8%) were overweight, and 21 (26.6%) were obese. Among obese women, 15 (71.4%), 4 (19%), and 2 (9.5%) had grade 1, 2, and 3-grade obesity, respectively. More than half (n=43; 54.4%) of women with EH were overweight (BMI> 25 kg/m2) (Fig. 1).
The groups differed in body mass index (BMI) with a BMI of 26.6 (5.7) and 28.9 (4.6) kg/m2 in the study group and control group, respectively (p <0.05). Among patients with EH without atypia, 36 (45.6%) patients had normal body weight, 22 (27.8%) were overweight, and 21 (26.6%) were obese. Among obese women, 15 (71.4%), 4 (19%), and 2 (9.5%) had grade 1, 2, and 3-grade obesity, respectively. More than half (n=43; 54.4%) of women with EH were overweight (BMI> 25 kg/m2) (Fig. 1).
In the control group, 18 (17.82%) women had normal body weight, 46 (45.54%) were overweight, and 37 (36.63%) were obese, of which 24 (64%), and 13 (35.1%) had grade 1 and 2 obesity, respectively. Eighty-three (82.18%) women had increased body weight. The odds of developing EH without atypia increased significantly with increasing body weight (BMI ≥25 kg/m2) (OR = 3.86, 95% CI 1.97–7.58, p <0.001).
The distribution of genotype frequencies at the rs2414098 locus of the СYP19А1 gene in the study and control groups were close to those expected under Hardy Weinberg equilibrium (χ2 = 0.29, p = 0.59; χ2 = 0.04, p = 0.85, respectively). An analysis of CYP19A1 rs2414098 polymorphism revealed that in the group of patients with EH without atypia, the ratio of homozygotes for the C allele (CC), heterozygotes (CA) and homozygotes for the A allele (AA) was 40 (50.63%), 31 (39.24 %), 8 (10.13%), and in the control group it was 34 (33.7%), 50 (49.5%), 17 (16.8%), respectively. The prevalence of alleles and genotypes of the СYP19А1 rs2414098 gene in women of the control group was comparable with the European population (genotypes: SS - 35.8%, CA - 47.7%, AA - 16.5%; alleles: C - 59.6%, A - 40.4%) [11]. Allele C rate (70.3% versus 58.4%; χ2= 5.37, p = 0.02; OR = 1.68, 95% CI 1.08–2.61) and SS genotype (50.6% versus 33.7%; χ2 = 5.22, p = 0.02; OR = 2.02, 95% CI 1.10–3.70) were significantly higher in women with EH without atypia compared with the control group (Fig. 2 a, b).
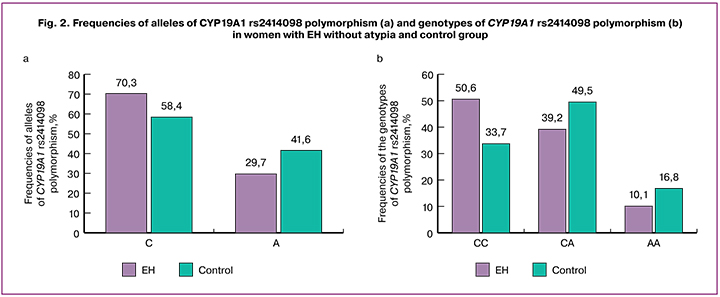
Therefore, the С allele of СYP19А1 rs2414098 and С/С genotype are significantly associated with increased odds for developing EH without atypia. The presence of the C allele and the C/C genotype of rs2414098 polymorphism of the CYP19A1 gene are risk factors for developing EH without atypia in women living in the Republic of Tatarstan.
The study analyzed the association between the CYP19A1 rs2414098 polymorphism in women with EH and their body weight. There was no statistically significant difference in the frequencies of the C allele and C/C genotypes in obese and normal-weight women compared with the control group (p > 0.05). Thus, increased body weight with a BMI> 25 kg/m2 is a risk factor for EH independent of the CYP19A1 gene as a genetic predictor.
Discussion
There are significant age-related differences in the synthesis of estrogen in women. In women with normal menstrual function, estrogens produced primarily by the ovaries, but in postmenopausal women, the ovaries cease to produce endogenous estrogens, at which point adipose tissue becomes the major source of estrogen. The final step of this transformation is catalyzed by aromatase [12]. This evidence suggests that the role of aromatase as the key enzyme catalyzing estrogen biosynthesis increases with increasing age.
In developed countries, about 200,000 new cases of EH are reported annually [13]. The highest (386 per 100,000 women) and lowest (6 per 100,000 women) incidence rates are observed in women aged 50–54 and below 30, respectively [7]. Considering that the mean age of EH and EC occurrence is 50–54 and 59– 62, respectively [14], the control group consisted of elderly and older women; 100% of the women in the control group were over 65 when the probability of endometrial pathology is decreased.
Based on the premise that a genetic predisposition to EH can affect the effectiveness of treatment and malignant transformation and taking into account the women’ age, we analyzed the association of CYP19A1 gene polymorphism with a risk of developing EH.
In the human genome, there is one copy of the aromatase gene (CYP19A1) located on the long arm of chromosome 15. The CYP19A1 gene has 10 exons, including 9 coding exons [15]. The aromatase gene CYP19A1 is highly polymorphic. Moreover, there are pronounced interpopulation differences in the frequencies of polymorphic variants of this gene. Of note, it has been suggested that some polymorphisms were significantly associated with hormone levels [16]. CYP19A1 intron single nucleotide polymorphisms may alter mRNA splicing, resulting in altered CYP19A1 activity, and can be considered a potential risk factor underlying the pathogenesis of EH [17].
The study findings suggest that the presence of the C allele and the C/C genotype of the rs2414098 polymorphism of the CYP19A1 gene are risk factors for developing EH without atypia in women living in the Republic of Tatarstan. Increased body weight was found to be an independent risk factor for developing EH without atypia. These results suggest the need for further studies investigating the impact of hereditary predisposition on the effectiveness of therapy for endometrial hyperplasia.
Conclusion
The study of 180 female residents of the Republic of Tatarstan revealed the association between rs2414098 polymorphism of the CYP19A1 gene and the risk of developing EH without atypia. The prevalence of alleles and genotypes of rs2414098 polymorphism of СYP19А1 gene was found to be comparable with that in the European region.
References
- Sanderson P.A., Critchley H.O., Williams A.R., Arends M.J., Saunders P.T. New concepts for an old problem: the diagnosis of endometrial hyperplasia. Hum Reprod Update. 2017; 23(2): 232–54.doi: 10.1093/humupd/dmw042.
- Kurman R., Carcangiu M., Herrington C., Young R. World Health Organisation classification of tumors of female reproductive organs, 4th edn. International Agency for Research on Cancer (IARC), 2014.
- Sobczuk K., Sobczuk A. New classification system of endometrial hyperplasia WHO 2014 and its clinical implications. Prz Menopauzalny. 2017; 16(3):107–11.doi: 10.5114/pm.2017.70589.
- Carlson M.J., Thiel K.W., Yang S., Leslie K.K. Catch it before it kills: progesterone, obesity, and the prevention of endometrial cancer. Discovery Medicine. 2012; 14: 215–22.
- Armstrong A.J., Hurd W.W., Elguero S., Barker N.M., Zanotti K.M. Diagnosis and management of endometrial hyperplasia. J Minim Invasive Gynecol. 2012; 19: 562–71. doi: 10.1016/j.jmig.2012.05.009.
- Royal College of Obstetrician and ginecologists. Management of Endometrial Hyperplasia. Green-Top Guideline No. 67: RCOG/BSGE, 2016.
- Lacey J.V., Chia V.M., Rush B.B., Carreon D.J., Richesson D.A., Ioffe O.B., Ronnett B.M., Chatterjee N., Langholz B., Sherman M.E., Glass A.G. Incidence rates of endometrial hyperplasia, endometrial cancer and hysterectomy from 1980 to 2003 within a large prepaid health plan. Int J Cancer.2012;131:1921-9. doi: 10.1002/ijc.27457.
- Коренная В.В., Масс Е.Е., Колода Ю.А., Полетова Т.Н. Гиперпластические процессы эндометрия: новый взгляд на проблему. Эффективная фармакотерапия. 2018;(26): 34–9.[ Korennaya V.V., Mass Ye.Ye, Koloda Yu.A., Poletova T.N. Hyperplastic Endometrial Processes: the New Approach to the Problem. Effektivnaya farmakoterapiya/Effective pharmacotherapy. 2018; (26): 34-39. (in Russian)].
- Пономаренко И.В., Полоников А.В., Чурносов М.И. Ассоциация полиморфизма rs4986938 гена ESR2 с развитием гиперплазии эндометрия. Акушерство и гинекология. 2019; 4: 66–72. [Ponomarenko I.V., Polonikov A.V., Churnosov M.I. Association of ESR2 rs4986938 polymorphism with the development of endometrial hyperplasia. Akusherstvo I Ginecologiya/Obstetrics and Gynecology. 2019; (4): 66–72. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.4.66-72.
- Cornel K.M.C., Bongers M.Y., Kruitwagen R.P.F.M., Romano A. Local estrogen metabolism (intracrinology) in endometrial cancer: A systematic review. Mol Cell Endocrinol. 2019; 489: 45–65. doi: 10.1016/j.mce.2018.10.004.
- 1000 Genomes Project Phase 3. Ensembl. 2019. Available at: http://www.ensembl.org
- Bulun S.E., Sebastian S., Takayama K., Suzuki T, Sasano H, Shozu M. The human CYP19 (aromataseP450) gene: update on physiologic roles and genomic organization of promoters. J Steroid Biochem Mol Biol. 2003; 86(3–5): 219–24. doi: 10.1016/s0960-0760(03)00359-5.
- Ozdegirmenci O., Kayikcioglu F., Akgul M.A., et al. Comparison of levonorgestrel intrauterine system versus hysterectomy on efficacy and quality of life in patients with adenomyosis. Fertil Steril. 2011; 95(2): 497–502. doi: 10.1016/j.fertnstert.2010.10.009.
- Сухих Г.Т., Ашрафян Л.А., Кузнецов И.Н. Ранняя диагностика основных локализаций рака органов репродуктивной системы у женщин: проблемы и перспективы. Доктор.Ру. 2018; 2(146): 6–9. [Sukhikh G.T., Ashrafyan L.A., Kuznetsov I.N. Early Diagnosis of the Most Common Female Reproductive Cancers: Challenges and Prospects. Doctor.Ru. 2018; 2(146): 6–9. (in Russian)]. eLIBRARY ID: 32474041
- Zhao H., Zhou L., Shangguan A.J., Bulun S.E. Aromatase expression and regulation in breast and endometrial cancer. J Mol Endocrinol. 2016. 57(1): 19–33. doi: 10.1530/JME-15-0310.
- Kidokoro K., Ino K., Hirose K., Kajiyama H., Hosono S., Suzuki T., Kawase T., Hiraki A., Hamajima N., Tanaka H., Tajima K., Kikkawa F., Matsuo K. Association between CYP19A1 polymorphisms and sex hormones in postmenopausal Japanese women. J Hum Genet. 2009; 54(2): 78–85.
- Starlard-Davenport A., Orloff M.S., Dhakal I., Penney R.B., Kadlubar S.A. Genotypic and Allelic Variability in CYP19A1 among Populations of African and European Ancestry. PLoS One. 2015; 10(2): e0117347. doi: 10.1371/journal.pone.0117347.
Received 01.10.2019
Accepted 04.10.2019
About the Authors
Ildar F. Fatkullin, MD, Professor, Head of the Department of Obstetrics and Gynecology named after V.S. Gruzdev KSMU. Tel.: +7(960)04-80-104,e-mail: fatkullin@yandex.ru
49 Butlerov Str., Kazan, Russian Federation, 420012.
Rushanija I. Gabidullina., MD, Professor of the Department of Obstetrics and Gynecology named after V.S. Gruzdev KSMU. Tel.: +7(917)28-99-310,
e-mail: ru.gabidullina@yandex.ru, orcid.org/0000-0002-7567-6043
49 Butlerov Str., Kazan, Russian Federation, 420012.
Gulnaz A. Smirnova, gynecologist of the City Clinical Hospital No. 7. Tel.: +7(917)250-54-25. E-mail: s89274281088@yandex.ru, orcid.org/0000-0002-5915-558X
54 Chuikov Str., Kazan, Russian Federation, 420132.
Fikret R. Nuhbala, postgraduate student of the Department of Obstetrics and Gynecology named after V.S. Gruzdev, KSMU. Tel.: +7(950)313-37-34,
e-mail: nuhbala_fikret@mail.ru, orcid.org/0000-0002-1244-7577
49 Butlerov Str., Kazan, Russian Federation, 420012
Elena V. Valeeva, Junior Researcher of the Laboratory of Molecular Genetics, Central Research Laboratory, KSMU. Tel.: +7(917)917-29-44,
e-mail: vevaleeva@ya.ru, orcid.org/0000-0001-7080-3878
49 Butlerov Str., Kazan, Russian Federation, 420012
Yuliya I. Orlova, 6th year student of the pediatric faculty, KSMU. Tel.: +7(917)878-84-85. E-mail: juliaorlova18@gmail.com, orcid.org/0000-0002-1992-9763
49 Butlerov Str., Kazan, Russian Federation, 420012.
Arseny A. Shakirov, 5th year student of the medical faculty, KSMU. Tel.: +7(927)414-12-54. E-mail: arseniy.shakirov@kazangmu.ru, orcid.org/0000-0001-9883-6989
49 Butlerov Str., Kazan, Russian Federation, 420012
For citation: Fatkullin I.F., Gabidullina R.I., Smirnova G.A., Nukhbala F.R.,
Valeeva E.V., Orlova Yu.I., Shakirov A.A. The association between rs2414098 polymorphism of the CYP19A1 gene and the risk of developing endometrial hyperplasia.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 2: 125-30(In Russian).
https://dx.doi.org/10.18565/aig.2020.2.125-130



