Analysis of human embryo culture medium metabolites
Aim. To investigate metabolites of spent embryo culture media, including those containing granulocyte-macrophage colony-stimulating factor (GM-CSF), and identify molecular profiles that enable prediction of the human embryo implantation potential.Yarygina S.A., Smolnikova V.Yu., Kalinina E.A., Eldarov Ch.M., Gamisonia A.M., Makarova N.P., Bobrov M.Yu.
Materials and methods. We used samples of spent human embryo culture media with and without GM-CSF. The embryos were obtained from patients undergoing an IVF (ICSI). Metabolites were detected in the spent human embryo culture media using the HPLC-MS in the positive ion detection mode. After identifying chromatographic peaks, alignment of chromatograms, and better visualization of metabolite profiles in the compared samples, we conducted partial least squares discriminant analysis.
Results. Metabolic profiling enabled the detection of significant differences between the spent human embryo culture media of implanted and non-implanted embryos, regardless of the culture medium type. We identified molecular ions which levels were significantly changed in the culture media of the implanted embryos. A comparative analysis of potential metabolites in culture media showed that the presence of GM-CSF could potentially affect the metabolism of fatty acids in implanted embryos. The regulation of the metabolism of fatty acids involved in structural, nutritional, and signaling functions plays an essential role in early embryonic development. Therefore, the presence of GM-CSF in the culture medium can facilitate adequate embryonic development and exert a positive effect on implantation potential.
Conclusion. Metabolomic profiling of embryo culture media constituents offers a more accurate selection for elective single-embryo transfer to reduce the risks associated with multiple pregnancies.
Keywords
Soon after the first successful birth of a child after in vitro fertilization (IVF), various grading systems based on embryo cleavage rate and morphology were developed, leading to significant improvements in implantation and pregnancy rates. However, one of the major issues limiting the success of assisted reproductive technologies is the inability to estimate the reproductive potential of individual embryos accurately.
Women with multiple implantation failures account for over 30% of patients undergoing IVF. At the same time, couples undergoing infertility treatment are often subjected to lengthy, laborious treatment regimens, suffer complex psycho-emotional consequences, and experience financial problems [1].
In ART, multiple pregnancies are the major concern associated with preterm delivery and 3–4-fold higher perinatal morbidity and mortality than in singleton pregnancies, which increase in parallel with the number of fetuses [2, 3]. Given the medical complications associated with multiple pregnancies, some countries have introduced legal restrictions on the number of embryos transferred in IVF cycles.
Currently, the most significant goal of infertility treatment is to reduce the prevalence of multiple gestations while maintaining or improving overall pregnancy rates.
Traditionally, embryo selection for IVF is based on morphological criteria. Although this approach is the least technically challenging method to assess embryo quality, morphological assessment is not sufficient to accurately predict successful implantation [4].
Over the last decade, preimplantation genetic testing has emerged as one of the most effective approaches for improving IVF efficiency. However, this method has some limitations, including potential risks associated with embryo biopsy [5, 6]. These limitations have led to the search for non-invasive methods for assessing the quality and viability of embryos.
Metabolomics involves the comprehensive analysis of low-molecular-weight compounds in biological samples such as cell biological media, which are formed due to metabolic processes. In comparison with transcriptomic and proteomic approaches that require the object of study itself for analysis, metabolomic profiling can provide valuable information regarding the embryo viability by testing its culture environment [7, 8]. The embryo metabolism can be studied by analyzing the composition of the growth media used to culture the embryos until the transfer. The development of instrumental methods made it possible to investigate embryo culture media's composition to identify specific metabolites.
It is known that metabolic activity changes during in vitro embryo culture depending on the development stage reflecting the morphofunctional state of the embryo. Also, the metabolomic profiling data at a particular development stage may provide valuable characteristics of the embryo's normal or pathological phenotype [9] and help assess and predict its implantation potential [10]. The feasibility of using this approach to evaluate the quality of the embryo and its implantation potential remains unclear.
In recent years, to increase the effectiveness of embryo culturing, there have been continued efforts to develop optimal culture media, most suitable for the early development stages.
One of the approaches is embryo culture media supplementation with granulocyte-macrophage colony-stimulating factor (GM-CSF). As shown earlier, the use of GM-CSF results in acceleration of embryo development, an increase in the internal cell mass, and a decrease in proapoptotic processes activity [11–13]. The composition of commercial media used for human embryo culture varies significantly from relatively simple to multicomponent [14, 15]. Differences in composition, in turn, can affect the activity of the metabolic pathways of the embryo and lead to the formation of specific metabolites consistent with culture conditions. However, the evidence is lacking to demonstrate the effect of culture media composition on the embryonic metabolome.
To identify combinations of metabolites that can potentially serve as markers of successful implantation and facilitate the selection of the most promising embryo, we conducted a study aimed to identify differences in metabolite profiles of embryo culture media on days 3 and 5 of culture, depending on the type of culture media and implantation outcomes.
Materials and methods
This cross-sectional study with parallel groups analyzed samples of spent culture media used to culture human embryos of different morphological groups. We selected culture media samples of high-quality embryos based on Gardner’s criteria from 43 patients aged 24–37 undergoing the IVF (ICSI) program. The embryos were cultured in individual 30 μl drops of culture medium (Irvine CSC and Origio Embryogen). On days 3 and 5 of culture, a morphological assessment of the obtained embryos was conducted, followed by sampling in equal volumes of spent culture media, marked and frozen (-80°C).
Before performing high-performance liquid chromatography and mass spectrometry (HPLC-MS), the metabolites were extracted by adding three volumes of methanol to one volume of the incubation medium. After stirring, the precipitate underwent centrifugation at 14,000 g, and the supernatant was used for analysis.
For HPLC-MS analysis, 18 μL of the extract of each sample was taken, two μL of an internal standard with a final concentration of 25 μM was added, the separation of samples was performed on a Zorbax SB-C18 column (100 mm length×2.1 mm inner diameter×3.5μm particle size; Agilent Technologies) using an Ultimate 3000 Nano LC chromatography system (Thermo Scientific, USA).
Elution of the sample components was performed in an isocratic solution of 5% mobile phase B (0.1% formic acid in acetonitrile) for 15 minutes, then in a gradient of 5–95% mobile phase for 10 minutes at 40 μL/min flow rate. Then it was washed for 5 minutes (95% solution B), after which the initial concentration of phase B was returned to 5% within 1 minute, and the column was equilibrated for 3 minutes. The overall chromatography time for one sample was 34 minutes. Metabolites were detected on a Bruker MaXis Impact Hybrid quadrupole time-of-flight mass spectrometer (Bruker Daltoniks, Germany) in two measurements per sample. Mass spectra were collected at a resolution of 50,000 in the range of 100–1500 m/z in the mode of positively charged ions.
Peak detection, noise signal removal, and data processing and analysis were conducted using the Progenesis QI 2.0 software (Waters, Milford, USA). The mass spectra's processing and alignment were performed with the following parameters: instrument type – high-resolution mass spectrometer; minimum absolute peak intensity – 100 units; minimum peak width – 0.02 min. Differences between the compared groups were determined by partial least squares discriminant analysis (PLS-DA). The HMDB database (www.hmdb.ca) was used to search for potential metabolites with the corresponding molecular weights; the rate of changes in these metabolites between the samples' groups was determined using the Progenesis software package. To enrich the metabolic pathways involving potential metabolites, the KEGG database (www.kegg.jp) was used.
Results
In this study, two embryo culture protocols were used with the change of the culture medium on day three after fertilization. One group of embryos (group 1) was cultured before and after day 3 in Irvine CSC culture medium. Another group of embryos (group 2) was cultured for up to 3 days in Origio Embryogen medium supplemented with GM-CSF. Then culturing continued for five days in Irvine CSC medium. Media samples from both groups were divided into subgroups according to the cultivation period (days 3 and 5) and the outcomes of implantation (“+” and “-” positive and negative outcomes, respectively): group 1–3+, group 1–3-, group 1–5+, group 1–5- and group 2–3+, group 2–3-, group 2–5+, group 2–5-. We used 45 and 42 culture medium samples with embryo culture lengths of 3 and 5 days, respectively.
Metabolic profiling of culture media was performed using the HPLC-MS to obtain data sets for chromatographic separation and registration of molecular ions of the samples under study. After detecting chromatographic peaks and removing noise signals, the data were statistically analyzed by PLS-DA, which identified differences between the study groups. Figure 1 shows the analysis results for four comparisons made of 8 studied groups of samples. With both protocols, the analysis showed clustering of samples into distinct groups of implanted and non-implanted embryos. On day 3, according to the PLS-DA analysis, the differences between the groups were more pronounced.
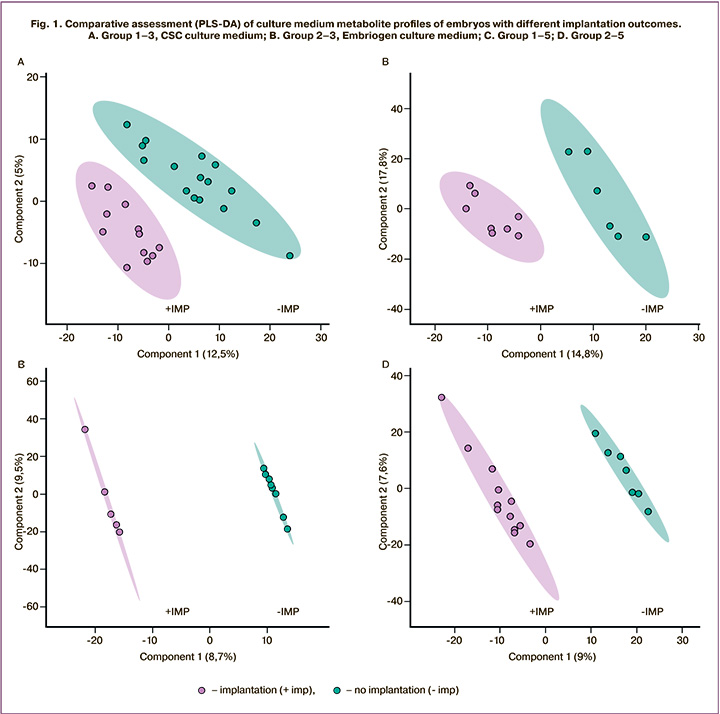
Using multivariate statistical analysis, for each of the four studied groups, lists of molecular ions were formed, which most of all determined the observed differences between the groups. Then, to determine the molecular ion mass matching to the potential metabolite, initial identification was performed using the HMDB database. As a result, lists of potential metabolites with different representation levels were obtained in groups with successful and failed implantations (Tables 1, 2). The multiplicity of changes for each metabolite was determined using the Progenesis QI package.
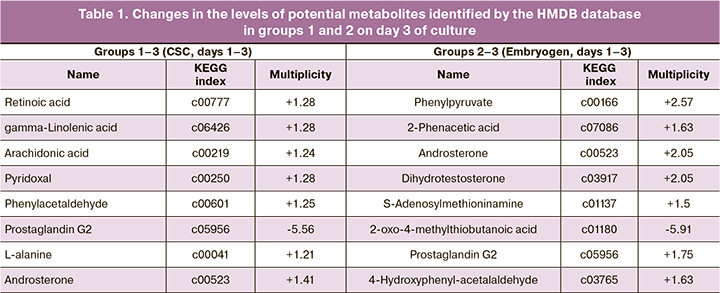
In groups 1–3, on day 3 of culture of implanted embryos in Irvine CSC medium, an increase in the levels of potential metabolites was observed, including retinoic acid (1.28 fold increase), arachidonic acid (1.24), gamma-linolenic acid (1.28), pyridoxal (1.28), phenylacetaldehyde (1.25), androsterone (1.41), and L-alanine (1.21). In groups 2–3, on day 3 of culture in Origio Embryogen medium supplemented with GM-CSF, the implanted embryos showed an increase in the levels of such potential metabolites as phenylpyruvate (2.57 fold), 2-phenyl acetate (1.63), dihydrotestosterone (2.05), androsterone (2.05), prostaglandin G2 (1.75), and 4-hydroxyphenylacetaldehyde (1.63) (Table 1).
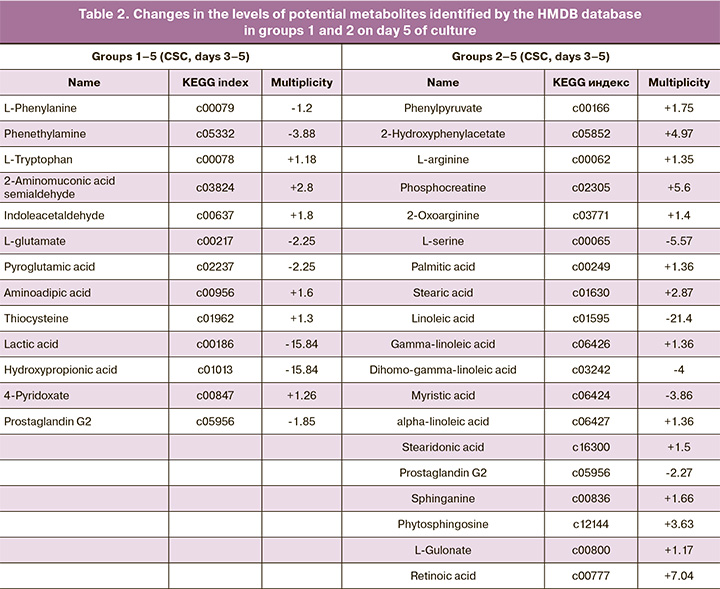
In groups 1–5, on day 5 of culture, the following changes in the levels of candidate substances in implanted embryos were observed: a decrease in L-phenylalanine (1.2 fold), phenethylamine (3.88), L-glutamate (2.25),pyroglutamic acid (2.25), hydroxypropionic acid (15.84), prostaglandin G2 (1.85) and an increase in L-tryptophan (1.18), 2-aminomuconic acid semialdehyde (2.8), indole acetaldehyde (1.8), thiocysteine, and 4-pyridoxate (1.26). In groups 2–5, where the embryos were cultured from 3 to 5 days in Irvine CSC medium, the implanted embryos showed an increase in phenylpyruvate (1.75 fold), L-arginine (1.35), hydroxyphenyl acetate (4.97 ), phosphocreatine (5.6), palmitic (1.36), gamma-linoleic (1.36), stearic (2.87), stearidonic (1.5) fatty acids, sphinganine (1.66), L-gulonate (1.17), phytosphingosine (3.63), retinoic acid (7.04) and a decrease in linoleic (21.4), dihomogamma-linolenic (4), and myristic (3.86) fatty acids, L-serine (5.57), and prostaglandin G2 (2.27), compared to culture media of non-implanted embryos.
Analysis of metabolic pathways involving the metabolites identified in KEGG showed similar metabolic pathways on day 3 both in the CSC Irvine medium and in the Origio Embryoge medium. They include vitamin B6, alanine, phenylalanine, glycine, serine, threonine, retinol, steroid hormones, and alpha-linolenic acid (Fig. 2).
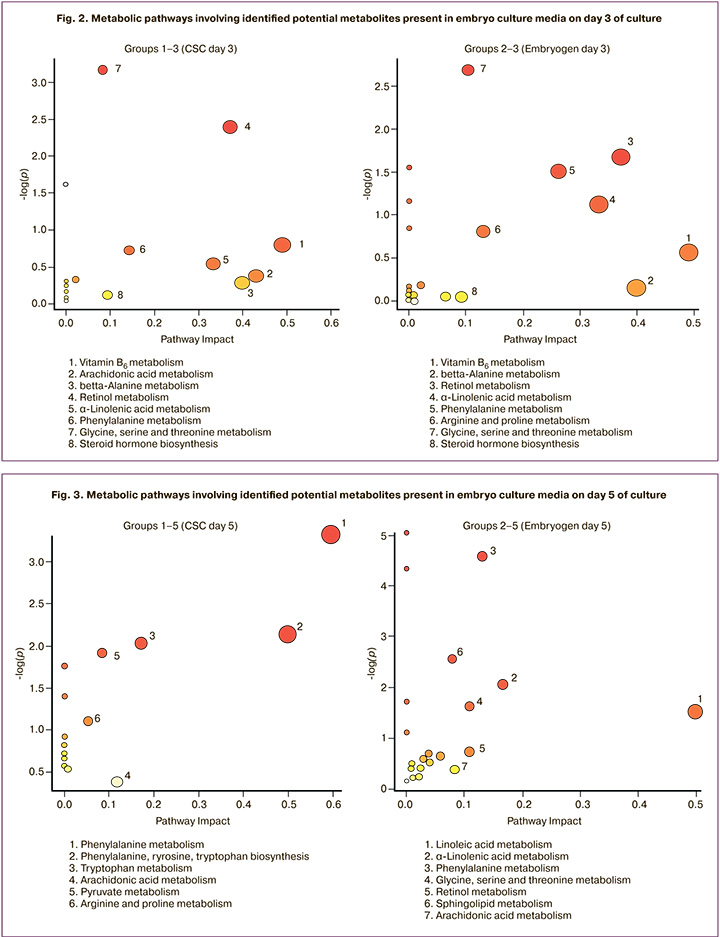
As can be seen from the data presented in Figure 3, on day 5, the presence of pathways associated with the biosynthesis of amino acids persisted in group 1. The pathways of fatty acids biosynthesis (linoleic, linolenic, arachidonic) and sphingolipids were seen in group 2. In Figures 2 and 3, the y-axis shows the values of -Log (p) – the statistical parameter of the validity of the findings on the participation of metabolites in the pathway (the higher the value, the higher the validity of participation), the x-axis shows the pathway impact, which depends on the number of identified metabolites and their significance in the pathway. Since various metabolites can be part of a variety of conjugated pathways, this representation allows assessment of the probable course of the transformation and the contribution of the identified metabolites to its implementation (a larger figure indicates a more preferable implementation of the corresponding pathway).
Discussion
Metabolomics is one of the omics techniques aimed at a comprehensive analysis of the metabolites present in biofluids, cells, and tissues. Unlike genomics and proteomics, metabolomics deals with low-molecular-weight compounds that participate in metabolic path-ways transforming the main classes of biologically significant organic compounds. In most of the earlier studies, metabolomic profiling of embryo culture media was investigated by near-infrared spectroscopy, which allows measurements in small volumes (less than 15 μL) and in a relatively short time. This method helps identify molecular functional groups, which can be used to compare the absorption spectra of samples and associate them with viability and implantation potential, though without identifying various metabolites [16–18].
Proton nuclear magnetic resonance spectroscopy has high accuracy in analyzing metabolic profiles, but this method requires large sample volumes and expensive and complicated equipment. To interpret the results, samples need to undergo lengthy processing and complex statistical analysis rendering this method impractical for routine clinical diagnosis [19].
HPLC and mass spectrometry (HPLC-MS) combination allows a highly accurate determination of their molecular weights by separating molecular components in the culture medium. Determination of chromatographic mobility parameters, areas of chromatographic peaks, and molecular weights of compounds allow identification of culture media components and their relative representation in the compared groups. This method has high specificity and sensitivity, comprehensively, qualitatively identify, and also quantify metabolites in a wide range of molecular weights.
In our study, we used spent embryo culture media on days 3 and 5 of culturing high-quality embryos based on Gardner’s morphological criteria that were obtained from patients included in the study and undergoing the IVF (ICSI).
It is noteworthy that the PLS-DA of HPLC-MS data showed significant differences in metabolite profiles between the study groups, depending on the type of culture medium and the outcomes after embryo transfer. In all cases, there was a clear clustering of the samples depending on implantation outcomes, which indicates the presence of molecular components in the media, which had significantly different concentrations in the compared groups. These differences were observed both in a medium without GM-CSF (Irvine CSC) and that cultured sequentially in a medium with GM-CSF (Origio Embryogen), and then on Irvine CSC medium. Using the HMDB databases, we identified potential metabolites contributing to the differences between the positive and negative implantation groups. We also analyzed metabolic pathways that may involve potential metabolites. There was a change in some amino acid concentrations, including alanine, methionine, tryptophan, serine, and arginine, and a number of their derivatives (Tables 1, 2). Previous studies have shown characteristic changes of amino acid profiles during human embryo development. According to the authors, the assessment of these changes of amino acid profiles could be used as predictors of their ability to achieve blastocyst stage [7]. Also noteworthy is the change in the concentration of the amino acid phenylalanine and its derivatives, including phenylacetaldehyde, 4-hydroxyphenylacetaldehyde, phenylacetic acid, and phenylpyruvate in groups 1 and 2 on both days 3 and 5 of culture. This observation may suggest the critical role of phenylalanine metabolic transformations in embryonic development. Besides participating in protein synthesis, this amino acid is a precursor for catecholamine synthesis, a co-substrate for other amino acids' interconversion, and can participate in energy metabolism.
On day 3, both in Irvine and Origio Embryogen culture media, we observed similar metabolic transformations associated with the metabolism of vitamin B6, retinol, alanine, phenylalanine, linoleic acid, and steroids (Fig. 1). These transformations may indicate their significant contribution to embryo development at a given culture period. These findings support studies reporting an association of vitamins and amino acid metabolic processes with the normal embryo phenotype [20, 21].
On day 5 of culture, there was a shift in metabolic activity towards the metabolism of monosaccharides and amino acids in embryos incubated in Irvine CSC culture medium (group 1) for the first three days. This observation is related to the fact that the medium used contains a complete set of nonessential and essential amino acids and glucose. In group 2 (Origio Embryogen medium), enrichment in amino acid conversion pathways was also observed. However, of note is the enrichment in bio-transformation pathways of fatty acids (Fig. 2). It can be assumed that culturing embryos for the first three days in a medium with limited composition and low concentrations of amino acids (Origio Embryogen) and supplementation of GM-CSF switches metabolic pathways to the synthesis and conversion of fatty acids, which may be a positive factor for embryo implantation potential. The supplementation of GM-CSF in culture medium stimulates proliferation and leads to an increase in the number of trophectoderm cells and internal cell mass, and increases the likelihood of developing a human embryo to the blastocyst stage [22]. Nevertheless, the effect of this cytokine on human embryo metabolism remains unexplored. In immune cells, in particular, in macrophages, GM-CSF stimulates glycolysis and lipogenesis by increasing the activity of glucose transporters and lipid biosynthesis enzymes, which in turn is a prerequisite for the development of a proinflammatory phenotype [23].
Fatty acids are essential structural components of lipids. Besides their role as a source of energy, they are also precursors of mediators, particularly prostaglandins, involved in the processes of development and differentiation. Recently, GM-CSF has been shown to promote T-lymphocytes' differentiation by regulating the activity of cyclooxygenase-2 and the production of prostaglandin E2 [24].
Our study findings showed a predominant decrease in the concentration of prostaglandin G. A decrease in this eicosanoid might be associated with both a reduction of its production and an increase in its utilization. It is known that after formation, prostaglandin G is rapidly converted by cyclooxygenases to prostaglandin H, which is the precursor of most prostaglandin family members. Thus, an increase in prostaglandin G levels may indicate a decrease in cyclooxygenase activity and a possible decline in prostaglandin synthesis. Previously, it was shown that prostaglandins are synthesized by blastocyst cells and that impaired production of these eicosanoids leads to a decrease in implantation potential [25]. Thus, reducing the utilization of a common prostaglandin precursor, prostaglandin G, and an increase in its level in culture media at the blastocyst stage may be one of the potential markers of implantation outcome. In recent work A. Yagi et al. reported the feasibility of analyzing fatty acid composition to assess the quality of the embryo. Using HPLC-MS, they analyzed free fatty acids in the culture media of 5-day embryos of different morphological groups. The authors showed a significant reduction in some free fatty acids in the media of embryos with better morphological characteristics. However, this work did not assess the relationship between fatty acid levels in the culture medium and cultured embryos' implantation potential [26]. Thus, the analysis of fatty acids and their metabolites can help assess the quality and implantation potential.
It should be noted that the lists of identified metabolites differed between the analyzed groups. However, the analysis of metabolic pathways showed a significant number of overlapping biochemical processes (Fig. 2, 3). This observation indicates that the identified metabolites in the groups of embryos cultured in different culture media are involved in the same pathways of biochemical transformations. This, in turn, testifies to the significance of these pathways in forming an embryonic phenotype with an increased implantation potential. These findings show that to identify the list of specific markers, it is necessary to consider the composition of the medium used for culturing. The results of metabolomic profiling indicate that, regardless of the type of culture media, there are significant differences in their molecular composition, allowing discriminating implanted and non-implanted embryos, both on days 3 and 5 of culture. These findings emphasize the need for further studies to identify molecular ions and determine quantitative parameters of changes in their spent culture media levels. Such research will make it possible to compile a list of the most significant metabolites, which change their concentrations in culture media, thus aiding the selection of a viable embryo with high implantation potential.
Conclusion
Metabolic profiling can simultaneously identify many biomarkers, which can be used to develop a model of an objective embryo scoring system. In the future, identifying predictive biomarkers using HPLC-MS on days 3 and 5 of culture may result in the development of simple, faster, and cheaper test systems that could be used in ART, regardless of spent culture media. Metabolomic profiling of embryo culture media constituents offers a more accurate selection for elective single-embryo transfer to reduce the risks associated with multiple pregnancies.
References
- Malina A., Pooley J.A. Psychological consequences of IVF fertilization. Review of research. Ann. Agric. Environ. Med. 2017; 24(4): 554-8. https://dx.doi.org/10.5604/12321966.1232085.
- Practice Committee of the American Society for Reproductive Medicine. Multiple gestation associated with infertility therapy. Fertil. Steril. 2012; 97(4): 825-34. https://dx.doi.org/10.1016/j.fertnstert.2011.11.048.
- Sullivan E.A., Wang Y.A., Hayward I., Chambers G.M., Illingworth P., McBain J.,Norman R.J. Single embryo transfer reduces the risk of perinatal mortality, a population study. Hum. Reprod. 2012; 27(12): 3609-15. https://dx.doi.org/10.1093/humrep/des315.
- Wang Q., Sun Q.Y. Evaluation of oocyte quality: mor-phological, cellular and molecular predictors. Reprod. Fertil. Dev. 2007; 19: 1-12. https://dx.doi.org/10.1071/rd06103.
- Cimadomo D., Capalbo A., Ubaldi F.M., Scarica C., Palagiano A., Canipari R., Rienzi L. The impact of biopsy on human embryo developmental potential during preimplantation genetic diagnosis. Biomed. Res. Int. 2016; 2016: 7193075. https://dx.doi.org/10.1155/2016/7193075.
- Chen M., Wei S., Hu J., Quan S. Can comprehensive chromosome screening technology improve IVF/ICSI outcomes? A meta-analysis. PLoS One. 2015; 10(10): e0140779. https://dx.doi.org/10.1371/journal.pone.0140779.
- Houghton F.D., Hawkhead J.A., Humpherson P.G., Hogg J.E., Balen A.H., Rutherford A.J., Leese H.J. Non-invasive amino acid turnover predicts human embryo developmental capacity. Hum. Reprod. 2002; 17(4): 999-1005. https://dx.doi.org/10.1093/humrep/17.4.999.
- Gardner D.K., Lane M., Stevens J., Schoolcraft W.B. Noninvasive assessment of human embryo nutrient consumption as a measure of developmental potential. Fertil. Steril. 2001; 76(6): 1175-80. https://dx.doi.org/10.1016/s0015-0282(01)02888-6.
- Vergouw C.G., Botros L.L., Judge K., Henson M., Roos P., Kostelijk E.H. et al. Non-invasive viability assessment of day-4 frozen-thawed human embryos using near infrared spectroscopy. Reprod. Biomed. Online. 2011; 23(6): 769-76. https://dx.doi.org/10.1016/j.rbmo.2011.08.015.
- Uyar A., Seli E. Embryo assessment strategies and their validation for clinical use: a critical analysis of methodology. Curr. Opin. Obstet. Gynecol. 2012; 24(3): 141-50. https://dx.doi.org/10.1097/GCO.0b013e328352cd17.
- Robertson S.A., Chin P.Y., Femia J.G., Brown H.M. Embryotoxic cytokines – Potential roles in embryo loss and fetal programming. J. Reprod. Immunol. 2018; 125: 80-8. https://dx.doi.org/10.1016/j.jri.2017.12.003.
- Kawamura K., Chen Y., Shu Y., Cheng Y., Qiao J., Behr B. et al. Promotion of human early embryonic development and blastocyst outgrowth in vitro using autocrine/paracrine – growth factors. PLoS One. 2012; 7(11): e49328. https://dx.doi.org/10.1371/journal.pone.0049328.
- Sjöblom C., Wikland M., Robertson S.A. Granulocyte-macrophage colony-stimulating factor (GM-CSF) acts independently of the beta common subunit of the GM-CSF receptor to prevent inner cell mass apoptosis in human embryos. Biol. Reprod. 2002; 67(6): 1817-23. https://dx.doi.org/10.1095/biolreprod.101.001503.
- Morbeck D.E., Krisher R.L., Herrick J.R., Baumann N.A., Matern D., Moyer T. Composition of commercial media used for human embryo culture. Fertil. Steril. 2014; 102(3): 759-66. https://dx.doi.org/10.1016/j.fertnstert.2014.05.043.
- Sunde A., Balaban B. The assisted reproductive technology laboratory: toward evidence-based practice. Fertil. Steril. 2013; 100(2): 310-18. https://dx.doi.org/10.1016/j.fertnstert.2013.06.032.
- Vergouw C.G., Botros L.L., Roos P., Lens J.W., Schats R., Hompes P.G. et al. Metabolomic profiling by near-infrared spectroscopy as a tool to assess embryo viability: a novel, noninvasive method for embryo selection. Hum. Reprod. 2008; 23(7): 1499-504. https://dx.doi.org/10.1093/humrep/den111.
- Seli E., Sakkas D., Scott R., Kwok S.C., Rosendahl S.M., Burns D.H. Noninvasive metabolomic profiling of embryo culture media using; Raman and near-infrared spectroscopy correlates with reproductive potential of embryos in women undergoing in vitro fertilization. Fertil. Steril. 2007; 88(5): 1350-7. https://dx.doi.org/10.1016/j.fertnstert.2007.07.1390.
- Scott R., Seli E., Miller K., Sakkas D., Scott K., Burns D.H. Fertil. Steril. 2008; 90(1): 77-83. https://dx.doi.org/10.1016/j.fertnstert.2007.11.058.
- Rinaudo P., Shen S., Hua J., Qian S., Prabhu U., Garcia E. et al. H-1 NMR based profiling of spent culture media cannot predict success of implantation for day 3 human embryos. J. Assist. Reprod. Genet. 2012; 29(12): 1435-42. https://dx.doi.org/10.1007/s10815-012-9877-9.
- Gode F., Akarsu S., Gunnur Dikmen Z., Tamer B., Isik A.Z. The effect follicular fluid vitamin A, E, D and B6 on embryo morphokinetics and pregnancy rates in patients receiving assisted reproduction. Gynecol. Obstet. Reprod. Med. 2019; 25(2): 89-95. https://dx.doi.org/10.21613/GORM.2018.860.
- Picton H.M., Elder K., Houghton F.D., Hawkhead J.A., Rutherford A.J., Hogg J.E. et al. Association between amino acid turnover and chromosome aneuploidy during human preimplantation embryo development in vitro. Mol. Hum. Reprod. 2010; 16(8): 557-69. https://dx.doi.org/10.1093/molehr/gaq040.
- Richter K.S. The importance of growth factors for preimplantation embryo development and in-vitro culture. Curr. Opin. Obstet. Gynecol. 2008; 20(3): 292-304. https://dx.doi.org/10.1097/GCO.0b013e3282fe743b.
- Na Y.R., Gu G.J., Jung D., Kim Y.W., Na J., Woo J.S. et al. GM-CSF induces inflammatory macrophages by regulating glycolysis and lipid metabolismт. J. Immunol. 2016; 197(10): 4101-9. https://dx.doi.org/10.4049/jimmunol.1600745.
- Kim I.K., Koh C.H., Jeon I., Shin K.S., Kang T.S., Bae E.A. et al. GM-CSF promotes antitumor immunity by inducing Th9 cell responses. Cancer Immunol. Res. 2019; 7(3): 498-509. https://dx.doi.org/10.1158/2326-6066.CIR-18-0518.
- Kennedy T. Interactions of eicosanoids and other factors in blastocyst implantation. In: Hillier K., ed. Eicosanoids and reproduction. Springer; 2012: 73-88. https://dx.doi.org/10.1007/978-94-009-3215-9.
- Yagi A., Miyanaga S., Shrestha R., Takeda S., Kobayashi S., Chiba H. et al. A fatty acid profiling method using liquid chromatography-high resolution mass spectrometry for improvement of assisted reproductive technology. Clin. Chim. Acta. 2016; 456: 100-6. https://dx.doi.org/10.1016/j.cca.2016.03.001.
Received 21.04.2020
Accepted 02.07.2020
About the Authors
Svetlana A. Yarygina, Ph.D. Student at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel: +7(985)369-07-06. E-mail: s.a.iarygina@yandex.ru. 4 Academica Oparina str, 117997, Moscow, Russia.Veronika Yu. Smolnikova, Dr. Med. Sci., Leading Researcher at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. E-mail: veronika.smolnikova@mail.ru. 4 Academica Oparina str, 117997, Moscow, Russia.
Elena A. Kalinina, Dr. Med. Sci., Professor, Head of the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(495)438-13-41. E-mail: e_kalinina@oparina4.ru. 4 Academica Oparina str, 117997, Moscow, Russia.
Chupalav M. Eldarov, Ph.D. in Chemistry, Senior Researcher at the Laboratory of Molecular Pathophysiology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
4 Academica Oparina str, 117997, Moscow, Russia.
Alina M. Gamisoniya, Researcher at the Laboratory of Molecular Pathophysiology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
4 Academica Oparina str, 117997, Moscow, Russia.
Natalia P. Makarova, Dr. Biol. Sci., Senior Researcher at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. 4 Academica Oparina str, 117997, Moscow, Russia.
Mikhail Yu. Bobrov, Ph.D. in Chemistry, Head of the Laboratory of Molecular Pathophysiology, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia. E-mail: mbobr@mail.ru. 4 Academica Oparina str, 117997, Moscow, Russia.
For citation: Yarygina S.A., Smolnikova V.Yu., Kalinina E.A., Eldarov Ch.M., Gamisonia A.M., Makarova N.P., Bobrov M.Yu. Analysis of human embryo culture medium metabolites.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 11: 114-123 (in Russian).
https://dx.doi.org/10.18565/aig.2020.11.114-123



