Obstetric and neonatal outcomes of different management strategies in fetal macrosomia
Fetal macrosomia is important to study given the high incidence of obstetric and neonatal complications. The induction of labor can reduce the incidence of adverse outcomes in complicated pregnancies. However, the effectiveness of labor induction in reducing the incidence of obstetric and neonatal complications in fetal macrosomia remains controversial.Tysyachnyi O.V., Baev O.R., Chausov A.A., Edilberg I.V., Gaidarova A.R.
Objective: To investigate obstetric and neonatal outcomes of different management strategies for fetal macrosomia (expectant management versus labor induction).
Materials and methods: This retrospective cohort study analyzed birth outcomes in 626 healthy primiparous women with fetal macrosomia. The patients were divided into a study group (n=334) with labor induction and a control group (n=295) with expectant management. Each group was divided into gestational age subgroups 1, 2, 3, and 4 of 37–38, 39, 40, and 41 weeks, respectively.
Results: The caesarean section rate for induction of labor at 37-39 weeks was not different from that in the expectant management group, whereas it was significantly higher at 40 weeks (p=0.02). However, at 41 weeks, the situation changed, and the rate of caesarean sections was significantly higher in the expectant management group (p=0.04). There were no differences in the rates of vaginal delivery, fetal shoulder dystocia, or perinatal outcomes.
Conclusion: With the development of fetal macrosomia, it is too late to count on the effectiveness of labor induction, which is the traditional approach to preventing complications. Preventing macrosomia and developing other approaches to prevent its adverse consequences are important.
Authors' contributions: Tysyachnyi O.V. – conception and design of the study, data collection and manuscript drafting; Chausov A.A. – statistical analysis; Edilberg I.V., Gaidarova A.R. – data analysis; Baev O.R. – manuscript drafting, data editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Tysyachnyi O.V., Baev O.R., Chausov A.A., Edilberg I.V., Gaidarova A.R. Obstetric and neonatal outcomes of different management strategies in fetal macrosomia.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (2): 46-52 (in Russian)
https://dx.doi.org/10.18565/aig.2022.275
Keywords
Fetal macrosomia is defined as a birth weight of 4000 g or more [1]. The prevalence of fetal macrosomia ranges from 5 to 20% [2], and there has been an increase in the incidence of macrosomia in recent years, which has been associated with an increase in maternal obesity and gestational diabetes mellitus [3]. The incidence of fetal macrosomia ranges from 6.5% to 7.5%, according to the V.I. Kulakov NMRC for OG&P.
The importance of studying fetal macrosomia is due to the high incidence of obstetric and neonatal complications [4]. For example, there is a two-fold increase in the risk of caesarean section (OR 1.98 (95% CI 1.80–2.18) with a fetal weight of 4000g or more, and a three-fold increase in the risk with a fetal weight of 4500g or more (OR 2.55 (95% CI 2.33–2.78). There was an increased risk of shoulder dystocia in pregnancies with a birth weight > 4000 g [OR 9.54 (95% CI 6.76–13.46)]; the risk was even higher in pregnancies with a birth weight > 4500 g [OR 5.64 (95% CI 11.31–21.64)] [5].
Current literature considers fetal macrosomia to be a birth weight greater than 4000 g, regardless of gestational age [1]. However, owing to racial differences, a percentile scale is recommended for dynamic growth control and standardized estimation of normal and abnormal anthropometric data. Therefore, a birth weight above the 90th percentile for each gestational age is defined as large for gestational age [6–8].
Labor induction is now widely used in obstetric practice because it reduces the incidence of adverse outcomes in complicated pregnancies [9–12]. However, the effectiveness of labor induction in reducing the incidence of obstetric and neonatal complications in fetal macrosomia remains controversial.
This study aimed to investigate the obstetric and neonatal outcomes of different management strategies for fetal macrosomia (expectant management versus labor induction).
Materials and methods
The study was conducted at the Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. This retrospective analysis included 36,000 delivery case notes from electronic birth records from January 2018 to June 2022. During the analysis, the following data were obtained from pregnancy and delivery case notes: characteristics of the pregnancy course, delivery, and the condition of the newborn. Women who met certain criteria were included in the study.
The study inclusion criteria were age 18–40 years, spontaneous singleton pregnancy, nullipara, fetus in cephalic presentation, full-term pregnancy, and fetal macrosomia.
The exclusion criteria were severe non-obstetric comorbidities, complicated pregnancy (pre-eclampsia, gestational arterial hypertension, gestational diabetes mellitus, carbohydrate metabolism disorders), elective caesarean section, uterine anomalies, fetal malformations, and laboratory-confirmed signs of intrauterine infection in the newborn.
A total of 626 women with fetal macrosomia met the inclusion criteria. The primary outcome was the rate of caesarean sections. The secondary outcomes were the incidence of vaginal delivery, fetal shoulder dystocia, and perinatal outcomes.
The patients were divided into a study group (n=334) with labor induction and a control group (n=295) with expectant management.
In the study group, the indication for labor induction was an unfavorable cervix in women with fetal macrosomia at full-term according to ultrasound examination. Preinduction/induction of labor was carried out according to the 2021 Clinical Guidelines "Failed attempted induction of labor (cervical preparation and induction of labor)" [13]. The control group included pregnant women who were diagnosed with fetal macrosomia at the aforementioned time points but who did not undergo preinduction/induction of labor (expectant management).
In the induction of labor group, the Bishop score ranged from 1 to 5 and averaged 4.2 (1.5). This information has been added to the Materials and Methods.
Labor was induced according to clinical guidelines [13]. A Bishop score of 8 or higher was a prerequisite for labor induction in all cases, allowing amniotomy to be performed. Oxytocin induction after amniotomy was required in 11% of the cases (37 women) and was administered 4 h later in the absence of active labor. Oxytocin induction was stopped when the active phase of labor was reached according to clinical guidelines [13].
Each group was divided into gestational age subgroups: 1 (gestational age 37–38 weeks, n=15 in the study group and n=21 in the control group), 2 (gestational age 39 weeks, n=55 and n=91), 3 (gestational age 40 weeks, n=183 and n=152), and 4 (gestational age 41 weeks, n=81 and n=31).
The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 22 for Windows. The normality of the distribution was tested using the Shapiro–Wilk test. Quantitative variables that showed a normal distribution were expressed as mean (M) and standard deviation (SD) and presented as M (SD); otherwise, the median (Me) with the interquartile range (Q1; Q3) was reported. Continuous variables were compared using the nonparametric Mann–Whitney U test. Categorical variables are presented as counts and percentages. For categorical variables, absolute and relative values were calculated to compare groups using Pearson's chi-square test. Differences between the groups were considered statistically significant at p<0.05.
Results
There were no significant differences in age between study groups. The mean age of the women in the study and control groups was 32.6 (2.8) vs. 32.5 (3.2) years, respectively (p=0.44). The mean BMI also did not differ between groups and was 25.3 (3.9) vs. 25 (3) kg/m2, respectively (p=0.58).
There were no differences between the groups in the incidence and pattern of somatic diseases, gynecological history, or course of pregnancy, as shown in Table 1.
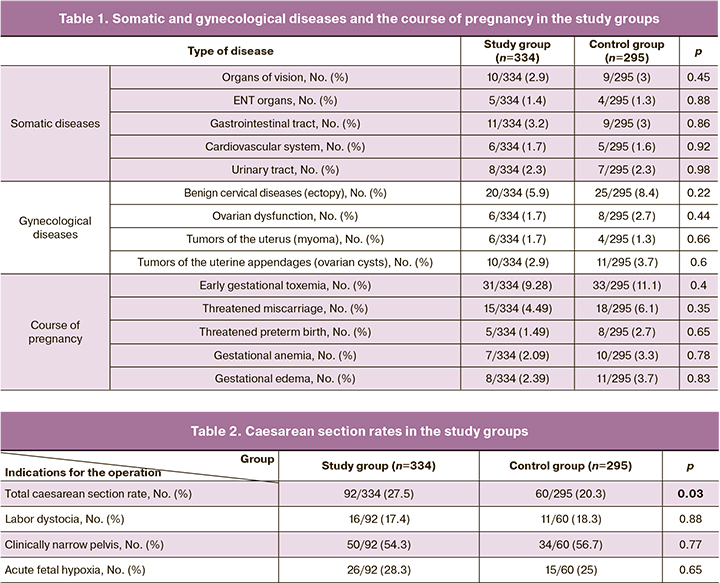
The gestational age at the time of delivery was 282.8 (3.8) to 40 weeks 2 days in the study group versus 280.1 (4.0) to 40 weeks 1 d in the control group (p<0.001).
The overall rate of caesarean section was significantly higher in the study group and was 92/334 (27.5%) vs. 60/295 (20.3%) in the control group (p=0.03). There were no differences in the patterns of indications for abdominal deliveries (Table 2). The indication for caesarean section in both groups was a clinically narrow pelvis in one in two cases, fetal hypoxia in one in four cases, and labor dystocia that could not be treated conservatively was a rare indication.
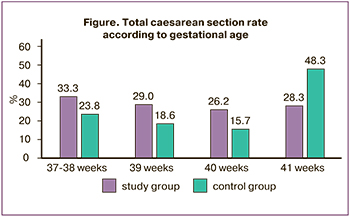
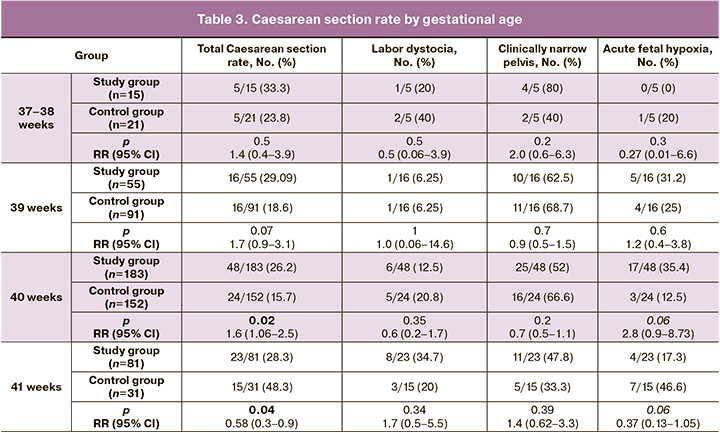
However, the analysis of caesarean section rates by gestational age at the time of delivery revealed fundamental differences. For example, the results showed that the caesarean section rate for labor at 37–39 weeks did not differ from that in the expectant management group, whereas it was significantly higher at 40 weeks (Figure). However, after 41 weeks, the situation had changed dramatically. The rate of caesarean section was significantly higher in the expectant management group. This increase was due to acute fetal hypoxia (Table 3).
Vaginal delivery was less common in the induction group, but the difference was not statistically significant [5/334 (1.4 %) vs. 8/295 (2.7 %), respectively; p=0.28]. Fetal shoulder dystocia was slightly less common in the study group, but the difference was not statistically significant [1/334 (0.29 %) vs. 1/295 (0.34 %), respectively; p=0.92].
In all the observations, the babies were born alive. The Apgar score at minute 1 was not significantly different and was in the study group 7.88 (0.21) vs 7.84 (0.27) in the control group (p=0.66). At the fifth minute, there was also no difference between the groups, with an Apgar score of 8.78 (0.35) vs. 8.77 (0.36), p=0.89).
The rate of newborns with Apgar scores of seven or less at minute 1 was slightly lower in the induction group, 33/334 (9.8%) vs 32/295 (10.8%), p=0.65, and 7/334 (2.09%) vs. 7/295 (2.3%), p=0.80 at minute 5.
Evaluation of the anthropometric data of the newborns showed no differences between the groups. The mean birth weight of the newborns in the study group was 4209.7 (154.0) g vs 4175.9 (113.2) g, respectively (p=0.34). Mean body length was 55.6 (1.33) cm vs. 55.5 (1.17) cm, respectively, p=0.31.
The characteristics of the newborn groups according to gestational age are shown in Table 4.
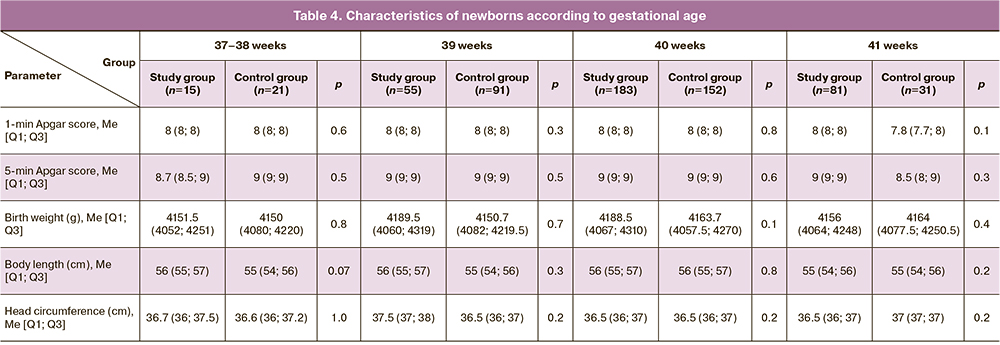
Analysis of neonatal outcomes in the study groups showed no differences in the mean Apgar scores at 1 and 5 minutes and neonatal anthropometric characteristics.
Discussion
In our study, we examined the outcomes of birth in nulliparous women with fetal macrosomia who underwent different management strategies (expectant management versus induction of labor).
A 2016 Cochrane review showed that induction of labor for suspected macrosomia did not reduce the risk of caesarean section (RR, 0.91; 95% CI 0.76–1.09) and operative vaginal delivery, RR 0.86 (95% CI 0.65–1.13) [7]. A study by Odinokova V.A. et al. (2022) showed an increased incidence of caesarean section when using an active strategy (induction of labor) in established macrosomia [14]. According to Vitner D. et al. (2018), induction of labor compared to expectant management did not increase the rate of caesarean section regardless of gestational age, but induction performed beyond 39 weeks was associated with an increase in the number of maternal and perinatal complications [15].
Our study also found no difference in caesarean section rates between labor induction and expectant management. However, this finding was only true at 37–39 weeks. At 40 weeks, caesarean section was performed less frequently with the expectant management approach, whereas at 41 weeks, it was performed with induction of labor. The increased rate of caesarean section at 41 weeks' gestation with expectant management strategy was due to fetal hypoxia, indicating a protective effect of labor induction.
We found no differences in operative vaginal delivery rates between the groups. Similar data were reported in a study by Boulvain M. et al., who reported a rate of operative vaginal delivery of 13% and 17%, RR 0.80 (0.58–1.12) [16].
There is evidence in the literature that the incidence of shoulder dystocia and clavicle fracture is lower with labor induction, RR 0.60 (95% CI 0.37–0.98) [7]. In our study, no significant differences were observed in this outcome. Analysis of perinatal outcomes did not show any differences between the groups.
Our results showed that when fetal macrosomia was already present, labor at 37-40 weeks did not reduce the rate of caesarean section. We also did not find a significant reduction in the incidence of vaginal delivery. Perinatal outcomes did not differ between the induction of labor and expectant management groups.
Conclusion
Therefore, when fetal macrosomia is already present, it is too late to determine the efficacy of labor induction, which is the traditional approach to prevent complications. Therefore, it is important to prevent macrosomia and to develop other approaches to prevent adverse outcomes. It is of interest to determine the feasibility of labor induction at 37–40 weeks of gestation in a group of women with large-for-gestational-age fetuses who have not yet reached the traditional criteria for macrosomia (weight 4000 g). As macrosomia formation is a dynamic process of progressive overgrowth, it can be suggested that delivery one to two weeks earlier may prevent the birth of a macrosomic neonate and reduce the incidence of intrapartum complications.
A limitation of this study was its retrospective design. First, despite the induction of labor, the delivery time was significantly longer in the study group, indicating more favorable conditions in the control group. Second, it was not possible to ensure full compliance with the principle of expectant management strategy, as the comparison was made according to gestational age at delivery. A more accurate result is only possible in a prospective trial, preferably a randomized trial in which the starting point is the time of enrollment. Then, from the time of enrollment, a division will be made into a group with immediate induction of labor and an expectant management strategy group, which will continue pregnancy until spontaneous delivery at other gestational ages.
Therefore, it is important to continue research aimed at the prevention of fetal macrosomia and its associated complications.
References
- Salihu H.M., Dongarwar D., King L.M., Yusuf K.K., Ibrahimi S., Salinas-Miranda A.A. Trends in the incidence of fetal macrosomia and its phenotypes in the United States, 1971-2017. Arch. Gynecol. Obstet. 2020; 301(2): 415-26. https://dx.doi.org/10.1007/s00404-019-05400-9.
- Henriksen T. The macrosomic fetus: a challenge in current obstetrics. Acta Obstet. Gynecol. Scand. 2008; 87(2): 134-45. https://dx.doi.org/10.1080/00016340801899289.
- Moldéus K., Cheng Y.W., Wikström A.-K., Stephansson O. Induction of labor versus expectant management of large-for-gestational-age infants in nulliparous women. PLoS One. 2017; 12(7): e0180748. https://dx.doi.org/10.1371/journal.pone.0180748.
- Никифоровский Н.К., Покусаева В.Н., Стась Л.И. Акушерские и перинатальные исходы при крупном плоде. Российский вестник акушера-гинеколога. 2010; 10(1): 55-8. [Nikiforovskiĭ N.K., Pokusaeva V.N., Stas' L.I., Gul'chenko O.V. Obstetric and perinatal outcomes in a large fetus. Russian Bulletin of Obstetrician-Gynecologist. 2010; 10(1): 55 8. (in Russian)].
- Beta J., Khan N., Khalil A., Fiolna M., Ramadan G., Akolekar R. Maternal and neonatal complications of fetal macrosomia: systematic review and meta‐analysis. Ultrasound Obstet. Gynecol. 2019; 54(3): 308-18.https://dx.doi.org/10.1002/uog.20279.
- Aviram A., Yogev Y., Ashwal E., Hiersch L., Danon D., Hadar E. et al. Different formulas, different thresholds and different performance—the prediction of macrosomia by ultrasound. J. Perinatol. 2017; 37(12): 1285-91.https://dx.doi.org/10.1038/jp.2017.134.
- Boulvain M., Irion O., Thornton J.G. Induction of labour at or near term for suspected fetal macrosomia. Cochrane Database Syst. Rev. 2016; 2022(8): CD000938. https://dx.doi.org/10.1002/14651858.CD000938.pub2.
- Баева И.Ю. Клиническая ценность дородовой диагностики крупного плода по данным ультразвуковых исследований. Журнал акушерства и женских болезней. 2014; 63(3): 12-20. [Bayeva I.Yu. Clinical significance of prenatal diagnosis of macrosomia by ultrasound. Journal of Obstetrics and Women's Diseases. 2014; 63(3): 12-20.(in Russian)].
- Knight M., Chiocchia V., Partlett C., Rivero-Arias O., Hua X., Hinshaw K. et al. Prophylactic antibiotics in the prevention of infection after operative vaginal delivery (ANODE): a multicentre randomised controlled trial. Lancet. 2019; 393(10189): 2395-403. https://dx.doi.org/10.1016/S0140-6736(19)30773-1.
- Cluver C., Novikova N., Koopmans C.M., West H.M. Planned early delivery versus expectant management for hypertensive disorders from 34 weeks gestation to term. Cochrane Database Syst. Rev. 2017; 1(1): CD009273.https://dx.doi.org/10.1002/14651858.CD009273.
- Wood A.M., Livingston E.G., Hughes B.L., Kuller J.A. Intrahepatic cholestasis of pregnancy: a review of diagnosis and management. Obstet. Gynecol. Surv. 2018; 73(2): 103-9. https://dx.doi.org/10.1097/OGX.0000000000000524.
- Сорокина А.В. Крупный плод: мифы и реальность. Российский вестник акушера-гинеколога. 2013; 13(4): 86-8. [Sorokina A.V. A large fetus: myths and realities. Russian Bulletin of Obstetrician-Gynecologist. 2013; 13(4): 86 8. (in Russian)].
- Российское общество акушеров-гинекологов (РОАГ). Клинические рекомендации "Неудачная попытка стимуляции родов (подготовка шейки матки к родам и родовозбуждение)". 2021. [Russian Society of Obstetricians and Gynecologists. Clinical guidelines "Failed attempt at labor stimulation (cervical preparation and labor induction)". 2021. (in Russian)]. Available at: https://roag-portal.ru/recommendations_obstetrics
- Одинокова В.А., Шмаков Р.Г. Исходы родов у первородящих с фетальной макросомией при активной и выжидательной тактике. Акушерство и гинекология. 2022; 1: 72-9. [Odinokova V.A., Shmakov R.G. Birth outcomes in primiparous women diagnosed with fetal macrosomia and managed with active surveillance and watch-and-wait approach. Obstetrics and Gynecology. 2022; (1): 72-9. (in Russian)].
- Vitner D., Bleicher I., Kadour-Peero E., Borenstein-Levin L., Kugelman A., Sagi S. et al. Induction of labor versus expectant management among women with macrosomic neonates: a retrospective study. J. Matern. Neonatal Med. 2020; 33(11): 1831-9. https://dx.doi.org/10.1080/14767058.2018.1531121.
- Boulvain M., Senat M.-V., Perrotin F., Winer N., Beucher G., Subtil D. et al. Induction of labour versus expectant management for large-for-date fetuses: a randomised controlled trial. Lancet. 2015; 385(9987): 2600-5.https://dx.doi.org/10.1016/S0140-6736(14)61904-8.
Received 15.11.2022
Accepted 13.01.2023
About the Authors
Oleg V. Tysyachnyi, PhD, Junior Researcher at the 1st Maternity Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,o_tysyachny@oparina4.ru, https://orcid.org/ 0000-0001-9282-9817, 117997, Russia, Moscow, Oparina str., 4.
Oleg R. Baev, Dr. Med. Sci., Head of the Maternity Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia; Professor at the Department
of Obstetrics, Gynecology, Perinatology, and Reproductology, I.M. Sechenov First MSMU, Ministry of Health of Russia, +7(495)438-11-88, o_baev@oparina4.ru,
https://orcid.org/0000-0001-8572-1971, 117997, Russia, Moscow, Ac. Oparina str. 4.
Andrey A. Chausov, Head of the Information and Analytical Center of the Department of Regional Cooperation and Integration, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, a_chausov@oparina4.ru, https://orcid.org/0000-0002-3094-7209, 117997, Russia, Moscow, Oparina str., 4.
Irina V. Edilberg, PhD Student, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, i_edilberg@oparina4.ru,
https://orcid.org/ 0000-0003-4194-8730, 117997, Russia, Moscow, Oparina str., 4.
Asiyat R. Gaidarova, PhD Student, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, a_gadzhieva@oparina4.ru,
https://orcid.org/ 0000-0003-1415-3318, 117997, Russia, Moscow, Oparina str., 4.



