Comparison of outpatient with inpatient mifepristone usage for cervical ripening: study protocol for a randomized controlled trial
Background: The proportion of women who undergo labor induction steadily increases due to the increasing number of indications. Most inductions of labor are carried out in inpatient settings. However, outpatient induction may be more convenient to women and cheaper for health service providers. Mifepristone as a cervical ripening agent is rapidly gaining popularity. However, the current literature is lacking regarding the outcomes of its use in outpatient settings. It is also essential to compare clinical outcomes from outpatient with inpatient mifepristone for induction of labor. The present study aimed to evaluate the efficacy and safety of outpatient versus inpatient mifepristone for labor induction.Baev O.R., Karapetyan A.O., Babich D.A., Sukhikh G.T.
Materials and methods: This is a protocol for a randomized, prospective, open-label clinical trial. Primiparous and multiparous women (n=300) with a singleton pregnancy with fetus in the cephalic presentation at the gestational age between 39 and 41+6 weeks, cervical Bishop’s score 6 or less, indications for labor induction, who meet all inclusion criteria, none of the exclusion criteria, and sign an informed consent form will be randomly allocated to one of two groups (outpatient and inpatient). The two groups will receive a standard protocolized cervical ripening with mifepristone (one or two doses).
Discussion: We expect outpatient management with mifepristone to be as effective and safe as an inpatient while increasing economic benefit and patients’ compliance during cervical ripening.
Trial registration: isrctn.com. ISRCTN26164110. Registered on February 21th, 2020.
Keywords
In recent years, the proportion of women who undergo labor induction has increased, accounting for about 20% of pregnancies. Main indications include prolonged pregnancy, prelabor rupture of membranes, hypertension and preeclampsia, and diabetes [1]. For the unfavorable cervix, several pharmacologic and mechanical methods are available for cervical ripening and induction of labor. The most commonly used cervical ripening agents and methods are prostaglandins, oxytocin, balloon catheter, and osmotic dilators [2].
In 1980 the first cervical ripening agent, mifepristone with anti-progestational and anti-glucocorticoid properties, was developed and licensed at the Russel Uclaf laboratory. Mifepristone, a synthetic steroid compound that antagonizes progesterone action at the receptor level, has been shown to increase uterine activity and cervical maturation [3]. Initially, it was used for medically induced abortion, but later studies have emerged on cervical ripening with mifepristone before labor induction at term pregnancy [4]. Myometrium's sensitivity to contraction-inducing prostaglandins activity markedly increased after mifepristone administration, and labor sometimes starts without additional inductors. Several studies have shown improvement in cervical Bishop's score, reduction in cesarean delivery rates and oxytocin usage to augment labor, and fewer NICU admissions after cervical ripening with mifepristone [5–7].
Most inductions of labor are carried out in inpatient settings. An alternative is allowing women to go home after starting induction. As shown in studies comparing inpatient with outpatient mechanical cervical ripening for induction of labor, women preferred outpatient care. They did not experience additional anxiety upon randomization to the outpatient group [8, 9]. The economic evaluation was favorable for outpatient care.
A Cochrane review on mechanical interventions for outpatient induction found insufficient evidence to draw conclusions on the efficacy, safety, and cost-effectiveness of home induction of labor [10]. This review also included studies of outpatient mifepristone versus placebo (Elliott 1998; Frydman 1992; Giacalone 1998; Lelaidier 1994; Stenlund 1999) [10]. There was no information on the proportion of vaginal birth not achieved within 24 hours, length of hospital stay, use of emergency services, maternal or caregiver satisfaction, or maternal severe morbidity or death, vaginal birth not achieved within 48 and 72 hours, and time from randomization to delivery was not reported [10]. Also, there was only limited evidence on the impact of mifepristone on maternal and neonatal health [10, 11, 12]. Therefore, further studies investigating outpatient cervical ripening with mifepristone are needed. Moreover, we found no studies comparing outpatient with inpatient mifepristone for cervical ripening.
Study hypothesis
- Is outpatient cervical ripening with mifepristone as efficient as an inpatient? (noninferiority)
- Is outpatient cervical ripening with mifepristone as safe as an inpatient? (noninferiority)
- Is outpatient cervical ripening with mifepristone more favorable than inpatient in terms of economic benefit? (superiority).
Materials and Methods
Study design
A randomized, prospective, open-label clinical trial.
Sample size calculation
A sample size of the study group was calculated based on previous results, that the expected success rate (number of women going into spontaneous labor or reaching Bishop score of 8 within 48-h) was 50% with placebo and 75% with mifepristone. On this basis, with a 2-sided level of 5%, 135 patients per group were required to detect a difference with a power of 95%. Considering potential dropouts, it was decided to include 150 patients per group, thus, a total of 300 patients.
Recruitment procedure
Eligible participants will be recruited for this clinical trial at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia between January 2020 and August 2021.
The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P 12.04.2018.
Women will be considered for recruitment into the study after a detailed review of the clinical history, examination by an obstetrician, and vaginal assessment of the cervix to establish the Bishop score. A modified Bishop’s score suggested by the Royal College of Obstetricians & Gynecologists will be used [13]. Also, all patients must undergo laboratory and instrumental tests before offering them to participate in the study.
The inclusion criteria will be age 18–45 years, gestational age of 39–41 weeks, 6 days, singleton baby in a cephalic presentation, Bishop score 6 or less, indications for labor induction (of which the induction can be postponed for 24–48 h), normal cardiotocography (CTG) tracing. Gestational age will be confirmed by the first-trimester ultrasound biometric measurements.
The exclusion criteria will be transverse fetal position or presentation other than cephalic, and ultrasound estimated fetal weight more than 4500 g or other evidence of cephalopelvic disproportion, placenta previa or another unexplained vaginal bleeding, previous cesarean delivery or history of uterine surgery, severe preeclampsia, evidence of chorioamnionitis, a severe form of any preexisting medical condition.
Primiparous and multiparous women with a singleton pregnancy with fetus in a cephalic presentation at a gestational age between 39 and 41+6 weeks, cervical Bishop’s score 6 or less, indications for labor induction (of which the induction can be postponed for 24–48 h), who meet all inclusion criteria and none of the exclusion criteria will be invited to participate in the trial and explicitly provided with all the relevant information verbally and in writing. They will be told that they will be randomly allocated into the two study groups, each group will be offered to take mifepristone, and that the objective of the study is to determine the efficacy and safety of outpatient labor induction with mifepristone in comparison to an inpatient. If they decide to participate, they will be asked to sign an informed consent form.
Randomization of participants
A list of consecutive numbers (number of 300) will be generated using computer-generated random numbers (Excel Microsoft software) prior to recruitment. Each of these numbers will be randomly assigned to inpatient or outpatient groups using opaque sealed envelopes.
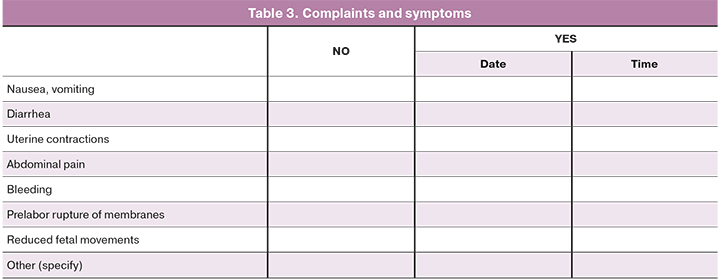
Before taking mifepristone, fetal wellbeing will be evaluated using CTG. If the fetal heart is normal, the woman will be given the drug and allowed home. The woman will receive detailed verbal information about what to expect, to return to the hospital at any time if she has a spontaneous rupture of membranes, contractions, bleeding, or reduced fetal movements. If she does not have any of the symptoms mentioned above, she will be asked to come after 24 hours from taking the first dose of mifepristone to determine fetal wellbeing and cervical reassessment. Women with Bishop score 8 and more will be hospitalized to the maternity unit for further labor induction, including amniotomy and oxytocin infusion if uterine contractions are absent within 4 hours. If the Bishop’s score is less than 8 and the fetal condition is normal, the woman will receive the second tablet of mifepristone and be sent home for the next 24 hours with the same recommendations. After 24 hours participant will be admitted to the maternity unite.
Further induction plan will be determined depending on Bishop’s score, according to local induction protocol. If Bishop’s score is 7 and less, osmotic dilators and/or intracervical prostaglandin E2 gel (maximum three times with 6 hours intervals) will be administered. If Bishop’s score is 8 and more, the patient will receive amniotomy and, if uterine contractions are absent within 4 hours, oxytocin infusion. Before and after interventions, the fetal condition will be checked. For inpatient women, the pre-induction plan by mifepristone and further management is similar to outpatient.
The studied variables
The following variables will be analyzed: maternal age, height, weight, body mass index (BMI), race, gynecology, and obstetrics history will be recorded at the beginning of the study. Table 1 summarizes the conditions that will serve as indications for labor induction.
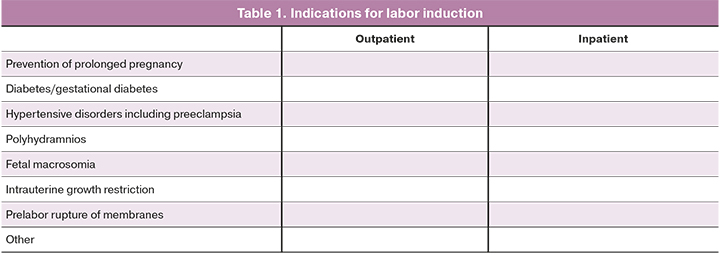
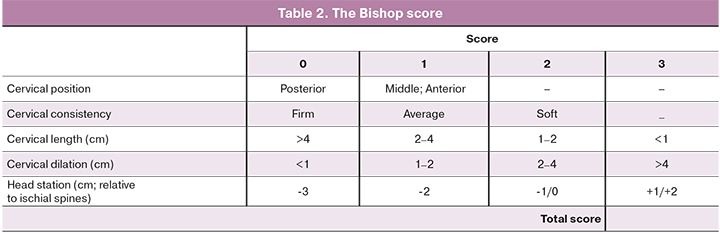
Table 2 represents the components of the Bishop score. Vaginal examination to determine the Bishop score will be performed by a single obstetrician.
After taking mifepristone, the occurrence of specific complaints and symptoms will be studied (Table 3).
As per the primary outcomes, we will assess the Bishop score improvement (increase in the Bishop score) after 24 and 48 h of the mifepristone administration, additional use of prostaglandin E2 and/or mechanical methods for cervical ripening, additional use of oxytocin, and operative delivery rate.
Secondary outcomes will be time from administering mifepristone to the onset of labor, adverse events rate, and perinatal outcomes.
Intervention
Before inductions of labor, participants will undergo a vaginal examination to determine the Bishop score by one obstetrician-gynecologist (dr. med. sci., professor, head of the maternity ward), who will be the only person with access to the random allocation list and implemented the treatment according to the group assigned to the participant’s number by the computer program.
The two groups (outpatient and inpatient) will receive a standard protocolized cervical ripening with mifepristone (one or two doses).
Statistical analysis
Statistical analysis will be performed using SPSS Statistics 21 software after obtaining a complete dataset of all patients. The critical level of significance when interpreting the statistical analysis results will be considered at p<0.05.
Discussion
Induction of labor has been on the rise over recent decades, with significant variation within countries and between hospitals. It is generally undertaken to decrease maternal and/or fetal morbidity or mortality.
Currently, available labor induction methods are not considered specific, as none guarantees success and is not considered entirely safe. Among pharmacologic methods for cervical ripening and induction of labor, oxytocin and prostaglandins remain the most popular and widespread induction agents. Mifepristone is also used to induce labor in late pregnancy through its actions in antagonizing progesterone, thus increasing uterine contractility and the uterus's sensitivity to the actions of prostaglandins.
Thus, this study's first question is whether outpatient cervical ripening with mifepristone is efficient for labor induction.
An equally important aspect is the safety of the method used for the woman and fetus. Therefore, the second question to be answered as a result of this study is whether outpatient cervical ripening with mifepristone is as safe as an inpatient.
Compared to spontaneous labor, induction of labor is associated with a prolonged length of hospital stay and increased rates of epidural analgesia, cesarean section, and post-partum hemorrhage. Induction of labor affects the woman’s labor and delivery experience, sometimes leading to changing their birth plans and planned place of birth. Women reported a greater need for pain relief during labor, are less optimistic about their birth experience, and consider the process of induction of labor a challenge. According to research evidence, a notable proportion of laboring women admitted in early labor are more likely to undergo several medical procedures, neonatal resuscitation and admission to special care nurseries, and more extended hospital stay. Therefore, many women would prefer and would be more satisfied with the outpatient option. Outpatient induction of labor enables a woman to enter labor at home and come to the hospital in the active phase of labor.
Induction of labor also impacts hospital and staff resources, with a significant occupancy of acute beds and allocation of clinical resources for the obstetric unit. Hence, the third question this study aims to answer is whether outpatient cervical ripening with mifepristone is more favorable than inpatient in terms of economic benefit.
The study will include 300 pregnant women, randomly allocated to two groups (outpatient or inpatient) to answer the above questions.
If this study provides positive results, it will imply the possibility of including outpatient cervical ripening with mifepristone in future guidelines.
References
- Coates D., Makris A., Catling C., Henry A., Scarf V., Watts N. et al. A systematic scoping review of clinical indications for induction of labour. PLoS One. 2020; 15(1): e0228196. https://dx.doi.org/10.1371/journal.pone.0228196.
- Penfield C.A., Wing D.A. Labor induction techniques: which is the best? Obstet. Gynecol. Clin. North Am. 2017; 44(4): 567-82. https://dx.doi.org/10.1016/j.ogc.2017.08.011.
- Schatz F., Papp C., Aigner S., Krikun G., Hausknecht V., Lockwood C.J. Biological mechanisms underlying the clinical effects of RU 486: modulation of cultured endometrial stromal cell stromelysin-1 and prolactin expression. J. Clin. Endocrinol. Metab. 1997; 82(1): 188-93. https://dx.doi.org/10.1210/jcem.82.1.3677.
- World Health Organization. Medical management of abortion. Geneva: World Health Organization; 2018.
- Athawale R., Acharya N., Samal S., Hariharan C. Effect of mifepristone in cervical ripening for induction of labor. Int. J. Reprod. Contracept. Obstet. Gynecol. 2013; 2(1): 35-8. https://dx.doi.org/10.5455/2320-1770.ijrcog20130206.
- Yelikar K., Deshpande S., Deshpande R., Lone D. Safety and efficacy of oral mifepristone in pre-induction cervical ripening and induction of labour in prolonged pregnancy. J. Obstet. Gynaecol. India. 2015; 65(4): 221-5. https://dx.doi.org/10.1007/s13224-014-0584-6.
- Hapangama D., Neilson J.P. Mifepristone for induction of labour. Cochrane Database Syst. Rev. 2009; (3): CD002865. https://dx.doi.org/10.1002/14651858.CD002865.pub2.
- Wise M.R., Marriott J., Battin M., Thompson J.M.D., Stitely M., Sadler L. Outpatient balloon catheter vs inpatient prostaglandin for induction of labour (OBLIGE): a randomised controlled trial. Trials. 2020; 21(1): 190. https://dx.doi.org/10.1186/s13063-020-4061-5.
- Wilkinson C., Adelson P., Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy Childbirth. 2015; 15: 126. https://dx.doi.org/10.1186/s12884-015-0550-z.
- Vogel J.P., Osoti A.O., Kelly A.J., Livio S., Norman J.E., Alfirevic Z. Pharmacological and mechanical interventions for labour induction in outpatient settings. Cochrane Database Syst. Rev. 2017; (9): CD007701. https://dx.doi.org/10.1002/14651858.CD007701.pub3.
- Баев О.Р., Бабич Д.А. Сравнение эффективности индукции родов при беременности «Full term» и «Late term». Акушерство и гинекология. 2020; 2: 97-103. [Baev O.R., Babich D.A. Comparison of the efficiency of labor induction in full term and late term pregnancies. Akusherstvo i ginekologiya/ Obstetrics and Gynecology. 2020;( 2): 97-103. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.2.97-103.
- Тысячный О.В., Баев О.Р., Кречетова Л.В. Течение и исходы родов в зависимости от тактики ведения при пролонгированной беременности. Акушерство и гинекология. 2016; 7: 28-33. [Tychychnyi O.V., Baev O.R, Krechetova L.V. The course and outcomes of labor in relation to management tactics during prolonged pregnancy. Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2016; (7): 28-33. (in Russian)]. https://dx.doi.org/10.18565/aig.2016.7.28-33.
- Royal College of Obstetricians and Gynaecologists; RCOG Clinical Effectiveness Support Unit. Induction of labour. Evidence-based Clinical Guideline Number 9. London: RCOG Press; 2001.
Received 17.08.2021
Accepted 02.09.2021
About the Authors
Oleg R. Baev, Dr.Med.Sci., Professor, Head of the 1st Maternity Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia; Professor at the Department of Obstetrics, Gynecology, Perinatology and Reproductology, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University), +7(916)389-75-22,metod_obsgyn@hotmail.com, https://orcid.org/0000-0001-8572-1971, 117997, Russia, Moscow, Ac. Oparina str. 4.
Anna О. Karapetyan, Ph.D., Obstetrician Gynecologist at the1st Maternity Department, V.I. Kulakov NMRC, Ministry of Health of Russia, +7(905)706-84-81, anne-89@mail.ru, https://orcid.org/0000-0001-8555-144X, 117997, Russia, Moscow, Ac. Oparina str. 4.
Dmitry A. Babich, Ph.D., Obstetrician Gynecologist at the1st Maternity Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(903)295-05-05,
babich.d@rambler.ru, https://orcid.org/0000-0002-3264-2038, 117997, Russia, Moscow, Ac. Oparina str. 4.
Gennady T. Sukhikh, Dr. Med. Sci., Professor, Academician of the RAS, Director of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia; Head of the Department of Obstetrics, Gynecology, Perinatology and Reproductology, Faculty of Postgraduate Professional Training of Physicians, I.M. Sechenov First MSMU, Miistry of Health of Russia (Sechenov University), +7(495)438-18-00, g_sukhikh@oparina4.ru, https://orcid.org/0000-0002-7712-1260, 117997, Russia, Moscow, Ac. Oparina str. 4.
Corresponding authors: Oleg R. Baev, o_baev@oparina4.ru; Dmitry A. Babich, d_babich@oparina4.ru.
Authors' contributions: Baev O.R., Sukhikh G.T. – concept and design of the study, manuscript editing; Baev O.R., Karapetyan A.O., Babich D.A. – data collection, statistical analysis, manuscript preparation, and editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Patient Consent for Publication: All patients will provide informed consent for the publication of their data. They will be notified that all records containing names or other personal identifiers will not be used in any written reports or publications.
Authors' Data Sharing Statement: The data supporting the findings of this study will be available on request from the corresponding author after approval from the principal investigator. It is also planned to create an online database. Web link to datasets will be provided.
For citation: Baev O.R., Karapetyan A.O., Babich D.A., Sukhikh G.T. Comparison of outpatient with inpatient mifepristone usage for cervical ripening: study protocol for a randomized controlled trial.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 9: 66-71 (in Russian)
https://dx.doi.org/10.18565/aig.2020.9.66-71



