Cell subpopulation composition and cytokine content in peripheral blood before spontaneous and induced labor
According to current concepts, immunological mechanisms play a fundamental role in the initiation of labor. The current literature lacks information related to the plasma cytokine content of pregnant women prior to spontaneous and induced labor. Studying the cytokine profile may contribute to the identification of its imbalance and determine prognostic criteria for failed labor preinduction.Tysyachnyi O.V., Inviyaeva E.V., Vtorushina V.V., Krechetova L.V., Baev O.R.
Objective: To investigate cell subpopulation composition and cytokine content in peripheral blood before spontaneous and induced labor.
Materials and methods: A prospective pilot study included 30 healthy primiparous women who were divided into 3 groups. Control group (n=8) included women with early signs of labor; patients in group 1 (n=14) required one stage of labor preparation to develop labor activity; patients in group 2 (n=8) required two stages of preparation.
Results: Women who required longer labor preparation had significantly higher TNF-α levels [2.9 (2.0;3.1) vs 2.9 (2.0;3.7) vs 5.4 (4.3;6.1) pg/ml in the control group, group 1 and group 2, respectively, p=0.025].
Conclusion: The highest level of TNF-α in women who required the second stage of labor preparation probably reflects slower cervical ripening.
Authors’ contributions: Tysyachny O.V. – material collection, manuscript drafting; Inviyayeva E.V. – statistical data processing; Vtorushina V.V. – material processing; Krechetova L.V. – conception and design of the study, manuscript drafting and editing; Baev O.R. – manuscript drafting and editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors’ Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Tysyachnyi O.V., Inviyaeva E.V., Vtorushina V.V.,
Krechetova L.V., Baev O.R. Cell subpopulation composition and cytokine content
in peripheral blood before spontaneous and induced labor.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; 1: 67-74 (in Russian)
https://dx.doi.org/10.18565/aig.2022.323.
Keywords
According to current concepts, immunological mechanisms play a fundamental role in the process of implantation, placentation, prolongation of pregnancy, and labor initiation [1–3]. The success of these processes is believed to depend on the balance of pro-inflammatory and anti-inflammatory cytokines, which changes throughout gestation [4].
The current literature is lacking sufficient coverage of the evidence related to blood plasma cytokine content in pregnant women before spontaneous labor [5] and before the preinduction of labor [6]. Considering that in 21% of cases the preinduction of labor fails [7], the study of cytokine profiles may help identify their imbalance and determine prognostic criteria for the failed preinduction of labor.
This study aimed to investigate the composition of the cell subpopulation and cytokine content in peripheral blood prior to spontaneous and induced labor.
Materials and methods
This pilot prospective study included 30 healthy primiparous pregnant women who gave birth at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology from January to June 2021.
The study inclusion criteria were age 18 to 40 years, spontaneous singleton pregnancy, cephalic fetal presentation, full-term pregnancy, the first spontaneous uncomplicated vaginal delivery, and the patient's informed consent to participate in the study.
The exclusion criteria were severe comorbidities, complicated pregnancy, labor, uterine abnormalities, fetal malformations, and laboratory-confirmed signs of intrauterine infection in the newborn.
The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
The patients were divided into 2 groups. The first group (control, n=8) included women with complaints of irregular pulling lower abdominal pain (early signs of labor), whose spontaneous labor activity occurred within 24 h after hospitalization. The second group (comparison group, n=22) included women who required labor preparation. The second group was divided into two subgroups: comparison group 1 (n=14), women who required a single dose of mifepristone to prepare for labor and went into labor, and comparison group 2 (n=8), women who required a second dose of mifepristone to prepare for labor and went into labor.
In all pregnant women, PB sampling was performed on admission to the emergency room using a 9-milliliter polypropylene vacutainer (S-Monovette, Germany). Blood samples were centrifuged at 23С for 10 min at 3000 rpm, after which serum was frozen and stored at -80С until assayed for cytokines.
PB lymphocytes were phenotyped by flow cytometry using monoclonal antibodies (mAbs), (Becton Dickinson and eBioscience, USA), labeled with FITC, PE, and APC. Lymphocyte gating, which allows exclusion of other blood cells from the analysis, was detected using mAt to CD45 (Dako, Denmark). The lymphocyte subpopulation composition was assessed using the following markers: CD3+, CD3+CD4+, CD3+CD8+, CD19+, CD3-CD56+CD16+, CD3+CD56+CD16+, CD19+CD5+, CD56+, CD56brCD16dim, CD56dimCD16br; T regulatory cells (Treg) were defined as cells with the CD4+CD25+CD127low/- phenotype. The analysis was performed on a Navios flow cytofluorimeter (Beckman Coulter, USA) using Kaluza software.
To study the serum cytokine (SC) content at different stages of labor preparation, the following indicators were assessed: IL-2, IL-4, IL-6, IL-8, IL-10, GM-CSF, IFN-γ, TNF-α. Cytokine concentrations were determined using standard multiplex methodology using a standard Bio-Plex Pro human cytokine 8-plex Assay (Bio-Rad, USA) on a Bio-Plex 200 flow laser immunoanalyzer (Bio-Rad, USA) and subsequent processing of the results using the Bio-Plex Manager 6.0 Properties application (Bio-Rad, USA). The studies were performed according to the manufacturer's instructions. The serum cytokine content in was expressed in picograms per milliliter (pg/ml).
Indications for pre-induction were pelvic anatomy, large fetal size, and a combination of these parameters. Cervical preparation for labor was performed using the clinical guidelines "Failed attempted labor stimulation (cervical preparation for labor and labor induction). 2021" [8]. Of note, the mean Bishop score in the control group was 5.4 (2.3) versus 5.5 (3.1) in comparison group 1, and 5.3 (4.2) in comparison group 2, respectively (p>0.05).
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 22 for Windows. The normality of the distribution was tested by the Shapiro–Wilk test. Continuous variables showing normal distribution presented as means (M) and standard deviation (SD) and compared using Student’s t-test. Data with non-normal distribution were reported as the median (Me) and interquartile range (Q1; Q3). The Kruskal-Wallis test was used to compare numerical data between three or more groups followed by pairwise comparison using the Mann–Whitney U test and Bonferroni correction; differences were considered significant at p<0.025. Categorical variables were presented as counts and percentages and compared with Pearson's χ2 test. Differences were considered significant at p<0.05. The correlation analysis was performed using Spearman's rank correlation coefficient and differences were considered significant at p<0.05.
Results
The study groups did not differ in terms of age, with mean age 30.3 (2.9) years of women in the study group and 30.1 (3.3) and 30.9 (3.4) years in the comparison groups 1 and 2 (p>0.05). The mean body mass index of the women also did not differ and was 27.7 (3.2) kg/m2 versus 26.1 (3.5) kg/m2 and 26.9 (4.3) kg/m2, respectively (p>0.05).
There were no differences between the groups in the rates and structure of somatic diseases, gynecological history, and pregnancy course, as shown in Table 1.
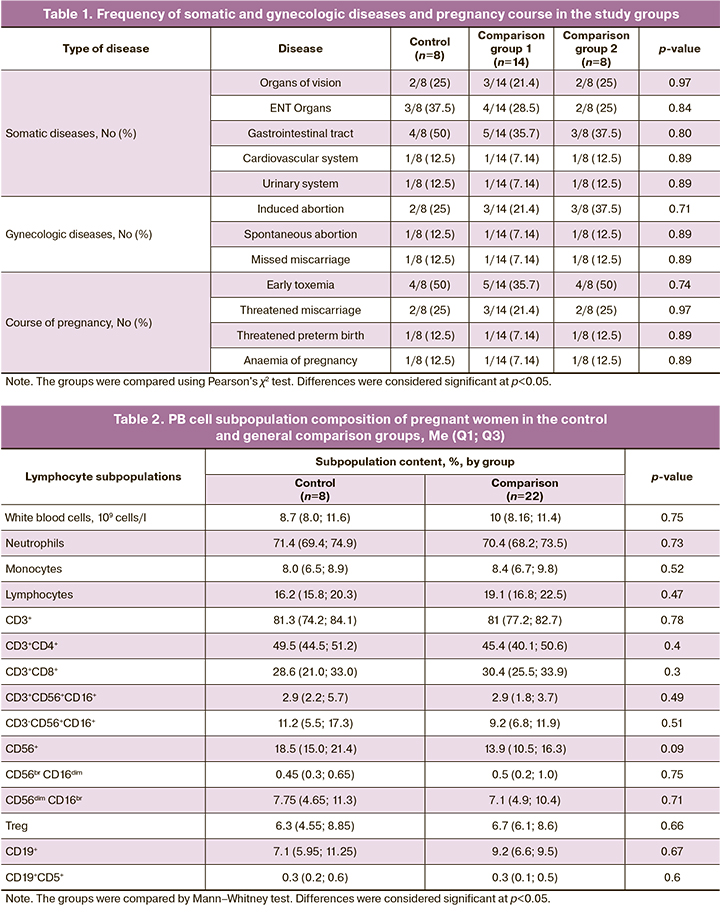
Gestational age at delivery did not differ between the groups and was 278 (2.6) days to 39 weeks 5 days in the control group versus 278.8 (3.5) days to 39 weeks 5 days in comparison group 1 and 279 (1.5) days to 39 weeks 6 days in comparison group 2, respectively (p=0.32).
In the first step, we analyzed the lymphocyte subpopulation composition in the control group (women without labor preparation) and the overall comparison group (women who required labor preparation), which is presented in Table 2, as well as considering the two comparison groups, which is presented in Table 3.
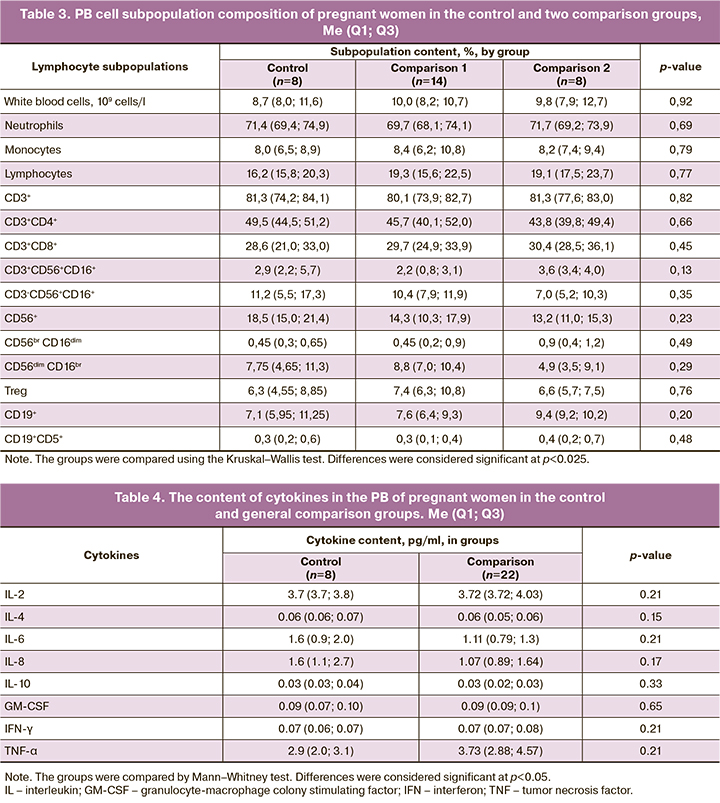
As follows from the results presented in Tables 2 and 3, no significant differences in the subpopulation composition of the PB cells of women who had spontaneous labor or underwent preinduction of labor were detected at the time of admission to the hospital.
Tables 4 and 5 present the analysis of cytokine content in the PB of the examined women, both in the general comparison group (Table 4) and considering two comparison groups (Table 5).
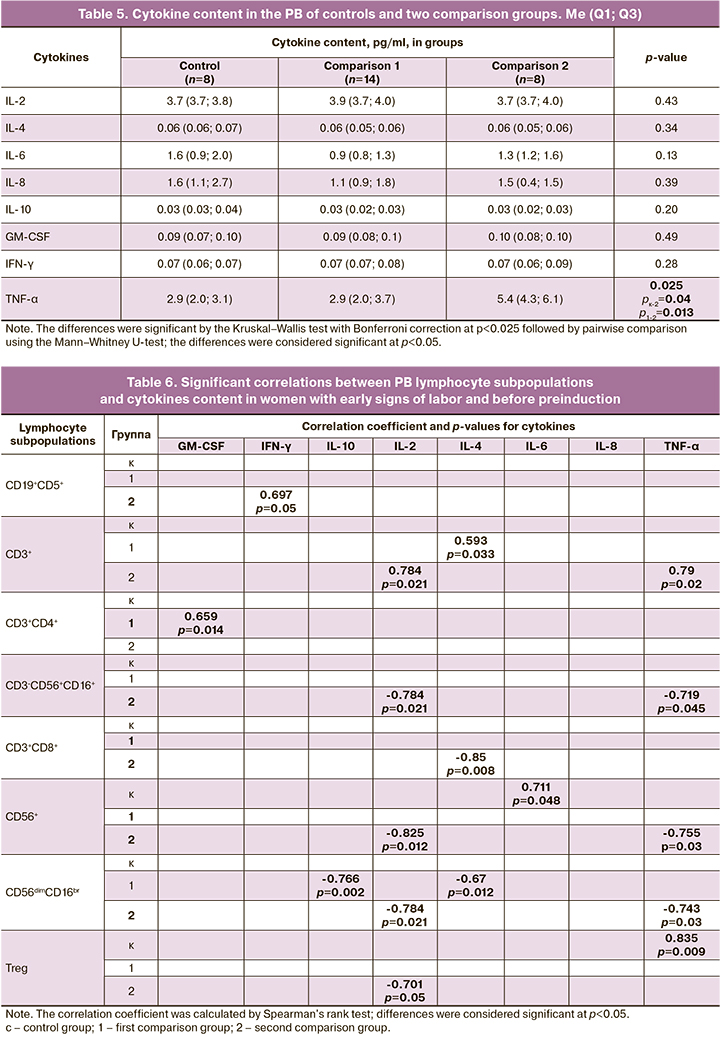
Women who required longer preparation for labor had a significantly higher level of TNF-α. However, there were no differences in the content of TNF- α content in those women who had spontaneous labor activity and those who required only one stage of labor preparation. However, women who required a second stage of labor preparation had higher TNF-α levels than women in the first group. No differences were found in the content of the other cytokines studied.
Further analysis involved assessing correlations between cell subpopulation composition and cytokine PB content of the cytokines in the women admitted for delivery. The results are presented in Table 6.
As shown in Table 6, only the control group showed strong significant correlations between the pro-inflammatory cytokine IL-6 and the subpopulation of natural killer cells (CD56+) in PB, between the proinflammatory TNF-α and Treg cells with natural regulatory activity. This correlation reflects the pro-inflammatory tendency of the immune system in women before labor and is associated with a reversal of tolerance formed during pregnancy to the antigens of the semi-allogeneic fetus. These women had spontaneous labor.
No such correlation was observed in any subgroup of women who required preinduction.
In the subgroup of women who required a second pre-induction procedure, cytokine content (IL-2, IL-4, TNF-α) was significantly inversely correlated with the content of subpopulations of natural killer cells (CD3-CD56+CD16+, CD56+, CD56dimCD16br), cytotoxic T lymphocytes (CD3+CD8+) and Treg, which was associated with an inactive cellular immune system prior to labor. However, strong significant direct associations between total T-lymphocyte count (CD3+) and the cytokines IL-2 and TNF-α indicate a very early stage of activation processes. Indirect confirmation can also be provided by a significant direct link between the content of IFN-γ and a subpopulation of B1-cells (CD19+CD5+), which reflects activation of the humoral link of the immune system.
In contrast to the second group, the women who underwent only one stage of preparation (comparison group 1) showed a significant inverse relationship between the content of anti-inflammatory cytokines IL-4 and IL-10 and the content of natural killer cells with predominant cytokine activity (CD56dimCD16br), as well as a direct relationship between IL-4 and total T-lymphocytes (CD3+), between GM-CSF and helper-active T-lymphocytes (CD3+CD4+), which can be explained by the activation of the innate immune system, followed by an activation of the adaptive immune system. These findings indicate less active immune reactions in women of group 1 compared to the control group, but more active compared to women in comparison group 2.
To confirm the above results, the analysis of the pro and anti-inflammatory cytokines in the PB of the examined women was carried out (Table 7).
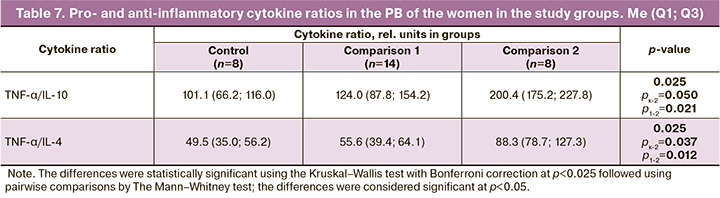
The high TNF-α/IL-10 and TNF-α/IL-4 ratios detected in the double dose preinduction group (Table 7) are probably not indicative of the intensity of inflammatory responses, but rather of tolerance the slower rate of withdrawal before labor in women who required a second dose of preinduction, or in other words of the slower rate of proinflammatory reactions before labor in this group.
Discussion
In this study, we compared the composition of the PB cell subpopulation and cytokine content in women with spontaneous onset of labor and those who needed preinduction of labor.
The proinflammatory cytokine TNF-α is known to be produced by activated macrophages of decidual and trophoblast cells, one of the key mediators of the inflammatory process and leukocyte activation. Being a biomarker of inflammation, TNF-α promotes labor activity by producing prostaglandins [9, 10]. In addition, TNF-α levels in the tissues of the lower uterine segment are known to increase with the onset of labor and continue to rise until lower uterine segment dilatation of 4–6 cm, after which a slight decrease is noted [11]. Tao Li et al. reported a negative correlation between TNF-α and the onset of spontaneous labor (r=-0.409) [12].
Based on these data, it is logical to assume that in our study, TNF-α levels should have been significantly higher in women with early signs of labor compared to those who underwent induction of labor. However, our study found that women with with early signs of labor compared to those hospitalized for labor preparation had lower TNF-α levels. At the same time, women who needed a second stage of pre-induction had the highest TNF-α levels.
Serum TNF-α in pregnant women is known to increase with gestational age (13). Besides, according to V.P. Rumyantseva et al. (2013), a maternal concentration of PB TNF-α concentration ≤0.946 pg/ml before the onset of labor with 80% sensitivity and 100% specificity allows the identification of patients at risk of prolonged pregnancy [14]. Our finding of higher TNF-α levels in women who were due to preinduction of labor probably reflects this relationship.
Furthermore, a longer duration of the first labor period correlates with an increase in serum TNF-α [15]. The higher level of TNF-α we found in women who required a second stage of labor preparation probably reflects a slower process of cervical ripening. Tesakova M.L. et al. who showed that women who were induced but subsequently delivered by emergency caesarean section had higher serum TNF-α levels compared to those who had natural childbirth, 4.3 (3.8–6.6) pg/ml versus 2.0 (1.0–2.8) pg/ml, respectively, p=0.0101 [16].
Conclusion
The highest level of TNF-α in women who required the second stage of labor preparation probably reflects slower cervical ripening. The results of our pilot study suggest that cytokine balance is an important component in the development of labor reflecting the intensity of proinflammatory responses. More studies are required to investigate the mechanisms underlying the role of cytokines in the initiation of labor.
References
- Левкович М.А. Современные представления о роли цитокинов в генезе физиологического и патологического течения беременности. Российский вестник акушера-гинеколога. 2008; 8(3): 37-40. [Levkovich M.A. Modern ideas about the role of cytokines in the genesis of the physiological and pathological course of pregnancy. Russian Bulletin of Obstetrician-Gynecologist. 2008; 8(3): 37-40. (in Russian)].
- Тысячный О.В., Павлова О.А., Вторушина В.В., Кречетова Л.В. Баев О.Р. Содержание цитокинов в периферической крови женщин в зависимости от фазы первого периода родов. Акушерство и гинекология. 2019; 2: 86-92. https://dx.doi.org/10.18565/aig.2019.2.86-92. [Tysyachnyi O.V., Pavlova O.A., Vtorushina V.V., Krechetova L.V. Baev O.R. Peripheral blood cytokine levels in women according to the phase of the first period of labor. Obstetrics and Gynecology. 2019; 2: 86-92. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.2.86-92.
- Сельков С.А., Павлов О.В., Лалаян Д.В. Цитокиновая сеть плаценты. Возможная роль в инициации родовой деятельности. Медицинская иммунология. 2003; 5(3-4): 341. [Selkov S.A., Pavlov O.V., Lalayan D.V. The cytokine network of the placenta. possible role in the initiation of labor. Medical immunology. 2003; 5(3-4): 341. (in Russian)].
- Thornton C.A. Immunology of pregnancy. Proc. Nutr. Soc. 2010; 69(3): 357-65. https://dx.doi.org/10.1017/S0029665110001886.
- Воскресенский C.Л., Федорков А.Ч., Мосько П.Л., Иванчик Г.И., Мельник Е.В. Содержание цитокинов в цервикальной слизи у беременных женщин накануне родов. Репродуктивное здоровье. Восточная Европа. 2012; 2: 19-26. [Voskresensky S.L., Fedorkov A.Ch., Mosko P.L., Ivanchik G.I., Melnik E.V. The content of cytokines in cervical mucus in pregnant women on the eve of childbirth. Reproductive Health. Eastern Europe. 2012; 2: 19-26. (in Russian)].
- Тесакова М.Л., Небышинец Л.М., Малолеткина О.Л., Мельник Е.В., Иванчик Г.И. Прогнозирование исхода индукции родов по уровням цитокинов в плазме крови. Репродуктивное здоровье. Восточная Европа. 2014; 1: 36-42. [Tesakova M.L., Nebyshinets L.M., Maloletkina O.L., Melnik E.V., Ivanchik G.I. Predicting the outcome of labor induction by the levels of cytokines in blood plasma. Reproductive Health. Eastern Europe. 2014; 1: 36-42. (in Russian)].
- Desta M., Duguma A. The magnitude of failed induction of labor and associated factors among women delivered at public hospitals of Arsi zone, Southeast Ethiopia, 2020: A cross-sectional study. Int. J. Gen. Med. 2021; 14: 6021-33. https://dx.doi.org/10.2147/IJGM.S318441.
- Российское общество акушеров-гинекологов. Клинические рекомендации "Неудачная попытка стимуляции родов (подготовка шейки матки к родам и родовозбуждение)" 2021. https://roag-portal.ru/recommendations_obstetrics. [Russian Society of Obstetricians-Gynecologists. Clinical guidelines "Unsuccessful attempt to induce labor (preparation of the cervix for labor and labor induction)". 2021. https://roag-portal.ru/recommendations_obstetrics. (in Russian)].
- Keelan J.A., Blumenstein M., Helliwell R.J.A., Sato T.A., Marvin K.W., Mitchell M.D. Cytokines, prostaglandins and parturition - a review. Placenta. 2003; 24(Suppl. 3): S33-46. https://dx.doi.org/10.1053/plac.2002.0948.
- Lim R., Barker G., Lappas M. TRADD, TRAF2, RIP1 and TAK1 are required for TNF-α-induced pro-labour mediators in human primary myometrial cells. Am. J. Reprod. Immunol. 2017; 78(1): e12664. https://dx.doi.org/10.1111/aji.12664.
- Winkler M. Role of cytokines and other inflammatory mediators. BJOG. 2003; 110(Suppl. 20): 118-23. https://dx.doi.org/10.1016/s1470-0328(03)00062-4.
- Li T., Yi T., Zhao J., Zhao X., He X. Combined proinflammatory biomarkers have better predictive value for term labor than single markers. Med. Sci. Monit. 2019; 25: 4513-20. https://dx.doi.org/10.12659/MSM.917298.
- Spence T., Allsopp P.J., Yeates A.J., Mulhern M.S., Strain J.J., McSorley E.M. Maternal serum cytokine concentrations in healthy pregnancy and preeclampsia. J. Pregnancy. 2021; 2021: 6649608. https://dx.doi.org/10.1155/2021/6649608.
- Румянцева В.П., Стрижаков А.Н., Баев О.Р., Донников А.Е., Рыбин М.В. Сухих Г.Т. Полиморфизм генов цитокинов при своевременных родах и перенашивании беременности. Акушерство и гинекология. 2013; 6: 34-40. [Rumyantseva V.P., Strizhakov A.N., Baev O.R., Donnikov A.E., Rybin M.V., Sukhikh G.T. Cytokine gene polymorphism during term labor and prolonged pregnancy. Obstetrics and Gynecology. 2013; 6: 34-40. (in Russian)].
- Cierny J.T., Unal E.R., Flood P., Rhee K.Y., Praktish A., Olson T.H. et al. Maternal inflammatory markers and term labor performance. Am. J. Obstet. Gynecol. 2014; 210(5): 447.e1-447.e6. https://dx.doi.org/10.1016/j.ajog.2013.11.038.
- Тесакова М.Л., Мельник Е.В., Малолеткина О.Л. Соотношение уровней цитокинов в слизи к их уровню в крови как прогностический критерий исхода индукции родов. Охрана материнства и детства. 2014; 1: 41-5. [Tesakova M.L., Melnik E.V., Maloletkina O.L. The ratio of the levels of cytokines in the mucus to their level in the blood as a prognostic criterion for the outcome of labor induction. Protection of motherhood and childhood. 2014; 1: 41-5. (in Russian)].
Received 23.12.2022
Accepted 12.01.2023
About the Authors
Oleg V. Tysyachnyi, Ph.D., Researcher at the 1st Maternity Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, o_tysyachny@oparina4.ru, https://orcid.org/0000-0001-9282-9817, 4, Academician Oparin str., Moscow, 117997, Russia.Lubov V. Krechetova, Dr. Med. Sci., Head of the Laboratory of Clinical Immunology, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
+7(495)438-11-83, https://orcid.org/0000-0001-5023-3476, l_krechetova@oparina4.ru, 4, Academician Oparin str., Moscow, 117997, Russia.
Valentina V. Vtorushina, PhD, Immunologist at the Laboratory of Clinical Immunology, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
v_vtorushina@oparina4.ru, https://orcid.org/0000-0002-8406-3206, 4, Academician Oparin str., Moscow, 117997, Russia.
Evgeniya V. Inviyaeva, PhD (Bio), Senior Researcher at the Laboratory of Clinical Immunology, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, e_inviyaeva@oparina4.ru, https://orcid.org/0000-0001-9878-3637, 4, Academician Oparin str., Moscow, 117997, Russia.
Oleg R. Baev, Dr. Med. Sci., Professor, Head of the 1st Maternity Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-11-88, o_baev@oparina4.ru, https://orcid.org/0000-0001-8572-1971, 4, Academician Oparin str., Moscow, 117997, Russia.



