Predictors of successful labor preinduction with mifepristone
Gaidarova A.R., Baev O.R., Gusar V.A., Edilberg I.V., Tysyachnyi O.V., Alekseev A.A.
Relevance: Labor induction rates have increased worldwide in recent years. To make informed decisions, optimize resources, and minimize the risk of failure, it is important to develop new predictive tools for the preinduction of labor.
Objective: To determine predictors of successful labor preinduction/induction with mifepristone.
Materials and methods: We analyzed 5000 electronic delivery case records from 2016 to 2023. Of the total number of records, 236 were included in the study and divided into two groups. Group 1 consisted of pregnant women who reached full cervical ripening (Bishop scale score of 8 or higher) or developed regular labor contractions within 24 h of a single 200 mg dose of oral mifepristone (n=116). Group 2 comprised pregnant women who required additional labor preparation with a second dose of mifepristone and/or other labor preinduction techniques (n=120).
Results: Significant differences were found between the two groups in parity (p=0.029), history of induced abortion (p=0.031), acute respiratory viral infections (p=0.010), novel coronavirus infection (p=0.039), use of dydrogesterone (p=0.013), and presence of polyhydramnios in the third trimester of pregnancy (p=0.012). The Bishop scale score before preinduction was higher in Group 1, with a score 4 (3; 4), than in Group 2, with a score 3 (2; 3) (p<0.001). A predictive model with a sensitivity of 64.2% and specificity of 75.8% was developed to predict successful preinduction of labor.
Conclusion: The predictors of successful and rapid labor preinduction with mifepristone were the initial Bishop score, parity, and the presence of polyhydramnios. The relationship between previous viral infections and the use of progesterone preparations during pregnancy remains debatable.
Authors' contributions: Baev O.R., Gaidarova A.R. – conception and design of the study; Gaidarova A.R., Baev O.R. – data collection and analysis; Gaidarova A.R., Alekseev A.A. – statistical analysis; Gaidarova A.R., Gusar V.A., Edilberg I.V. – drafting of the manuscript; Baev O.R., Tysyachnyi O.V. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Gaidarova A.R., Baev O.R., Gusar V.A., Edilberg I.V., Tysyachnyi O.V., Alekseev A.A. Predictors of successful labor preinduction with mifepristone. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (11): 104-114 (in Russian)
https://dx.doi.org/10.18565/aig.2023.137
Keywords
Preinduction of labor (PL) is an obstetric intervention designed to stimulate cervical ripening when the cervix is not sufficiently ripe for labor. Induction of labor (IL) is the stimulation of the onset of labor upon achieving cervical ripening, with the aim of having vaginal birth [1].
The rate of IL has increased worldwide in recent years, reaching 29.4% in the United States in 2019 [2] and 6.8% to 33% in Europe [3].
Despite its widespread use, there are still many questions for which there is no consensus in the scientific literature. The modern scientific community cannot provide a clear definition of successful and unsuccessful IL.
Thus, when discussing the concept of IL failure, some authors use the duration of the latent phase of labor as a criterion, using an interval of 15 h as a threshold [4]. Other studies have suggested that reaching the active phase of labor should be considered a measure of successful IL [5].
According to Lin et al., a failed induction is defined as the failure to achieve a cervical opening >4 cm within 12–18 hours from the start of oxytocin administration after the opening of the fetal membranes [6]. Nevertheless, most authors consider it more appropriate to focus on the outcome rather than the process and suggest that vaginal delivery is the main measure of successful IL [7, 8].
Spong C.Y. et al., in their publication, define unsuccessful IL in the form of the failure to achieve regular labor contractions (contractions every 3 minutes) and cervical changes after 24 hours from the administration of oxytocin [9].
A known factor for successful PL is an initially favorable birth canal, which is usually assessed using the Bishop scale [10, 11]. The components of the Bishop scale reflect the physiological changes that occur in the cervix shortly before labor. The cervix, which is composed of fibrous connective tissue, undergoes extensive remodeling during pregnancy to provide greater elasticity before delivery. Multiple factors, including matrix metalloproteinases, hyaluronic acid, prostaglandins, and mechanical pressure from the fetal vestibule, increase water content, decrease collagen concentration, and restructure the collagen and extracellular matrix to provide softening, flattening, and dilation of the cervix [12].
Parity is a strong predictor of a successful PL. Feghali M. et al. showed in their study that multiparous women undergoing induction of labor have a higher rate of vaginal delivery compared to primiparous mothers [13, 14]. The body mass index (BMI) of the pregnant women, correlating with the success of preinduction, was equally significant. Obesity is known to decrease the effectiveness of preinduction and also increase the duration of labor [14]. Numerous retrospective studies have consistently shown that the incidence of cesarean section in obese women after IL is 40% [15].
Transvaginal ultrasound measurement of cervical length before induction has been widely studied to predict successful preinduction. Pandis G.K. et al. reported in their study that this method is a useful predictor of vaginal birth within 24 hours after initiation of preinduction [16]. Studies indicating the superiority of cervical length ultrasonography over the Bishop scale in predicting PL success have been described in the literature [17–20].
In addition to transvaginal measurement of cervical length, elastography has been proposed to quantify the elastic properties or softness of the cervix in predicting labor outcomes [21, 22].
Several previous studies (Lockwood et al. [23], Garite T.J. et al. [24], Crane J.M.G. et al. [25]) evaluated the role of fetal fibronectin in predicting IL success and reported conflicting results. Fetal fibronectin is a glycoprotein found in the extracellular matrix surrounding the extravillous trophoblast. The detection of fetal fibronectin in the vagina prior to labor is thought to be due to fetal membrane changes in the lower uterine segment that occur concurrently with cervical remodeling processes prior to the onset of labor. Disruption of the choriodecidual layer leads to leakage of fetal fibronectin through the cervix into the vagina. Consequently, the presence of this protein in cervical secretions is linked to the approach of labor.
Kiss H. et al. concluded in their study that fetal fibronectin is a marker of changes in the cervix and fetal membranes, so it can be used as a predictor of IL success [26]. Similar results were obtained by Garite et al. [24] and Adedeji et al. (2023) [27], who reported that the detection of fetal fibronectin in the vagina predicts successful induction of labor, independent of the cervical maturity score on the Bishop scale. However, several studies (Sciscione et al. [28], A. Droulez et al. [29], Ojutiku et al. [30], and Roman et al. [31]) obtained opposite results and found no prognostic value in the determination of fetal fibronectin.
Phosphorylated insulin-like growth factor binding protein-1 (phIGFBP-1) is synthesized in the decidual membrane. High levels of this protein in cervicovaginal fluid are associated with cervical ripening and an increased risk of preterm labor; therefore, it is postulated that phIGFBP-1 may also help in predicting successful IL [32], as demonstrated by studies conducted by Riboni et al. (2012) [33] and Rathore et al. (2021) [34].
Activin A glycoprotein regulates biological functions, such as cell differentiation, proliferation, remodeling, and morphogenesis. Activin A stimulates the release of prostaglandins and interleukin-6 from amnion cells, confirming its role in labor [35]. Funghi L. et al. evaluated the level of activin A in maternal serum and placental mRNA expression in 64 women with spontaneous onset of labor and induced labor. They found that maternal serum activin A at a threshold value of >4643 ng/mL could predict successful IL with an area under the ROC curve of 0.858 (95% CI 0.714-0.947) [36]. Özdemir B.G. et al., based on a study that found a correlation between fetal adrenal volume and preterm labor, suggested that this method could be one of the predictors of IL success, which was confirmed in their study. The authors showed that adrenal volume was significantly higher in the successful than in the unsuccessful induction group [37].
In addition to the above factors that help predict the success of preinduction, there is the factor of time. In women undergoing IL, the process of cervical ripening and establishing regular labor contractions may take longer, especially if induction begins with an immature cervix than in women whose labor starts spontaneously. Based on this, it is important to consider that giving more time and having more patience in preparing women for labor induction increases the chances of successful PL [14].
Studies on the predictors of successful PL are based on different methods; therefore, they are often not comparable. The disadvantages of the available predictors are data inconsistency, low predictive value, and the need to involve related specialists. Therefore, there is still a need to develop new predictive tools to facilitate informed decision making, optimize resources, and minimize the potential risks of failure.
Preinduction with mifepristone is widely used in Russia, but there is a lack of research on the factors that predict its success.
Therefore, this study aimed to determine predictors of successful labor preinduction/induction with mifepristone.
Materials and methods
This retrospective study was conducted at V.I. Kulakov NMRC for OG&P of the Ministry of Health of Russia. We analyzed 5000 electronic delivery case records from 2016 to 2023.
The inclusion criteria were age of women from 18 to 45 years, singleton pregnancy, cephalic presentation, full-term pregnancy, unfavorable cervix, and PL/IL using mifepristone.
The non-inclusion criteria were multiple pregnancies, presence of severe non-obstetric comorbidities and pregnancy complications (preeclampsia, HELLP syndrome, antiphospholipid syndrome), fetal malformations, planned cesarean section, and spontaneous onset of labor.
Of the total number of records, 236 were included in the study and divided into two groups. Group 1 consisted of pregnant women who achieved full cervical ripening (Bishop scale score of 8 or higher) or developed regular labor contractions within 24 h of a single 200 mg dose of oral mifepristone (n=116). Group 2 comprised pregnant women who required additional labor preparation with a second dose of mifepristone and/or other labor preinduction techniques (n=120).
To identify predictors of success, we studied the characteristics of the onset and course of pregnancy (toxemia, threatened termination of pregnancy and preterm birth, asymptomatic bacteriuria, anemia, gestational thrombocytopenia, edema of pregnancy, acute respiratory viral infections, novel coronavirus infection (SARS-CoV-2), polyhydramnios, oligohydramnios, smoking during pregnancy, intrahepatic cholestasis, gestational arterial hypertension, gestational diabetes mellitus), anthropometric data of the patients (height, weight, BMI), medication intake (dydrogesterone, micronized progesterone, low molecular weight heparins, acetylsalicylic acid), cervical ripeness on the Bishop scale, course of labor, postpartum period (duration of labor, method of delivery, hemorrhage), and neonatal outcomes (Apgar score, birth weight, body length, and head circumference of the newborn).
All patients were screened before PL. Indications for preinduction were anatomically narrow pelvis (n=69), tendency to postterm pregnancy (n=80), gestational diabetes mellitus (GDM) (n=36), chronic arterial hypertension (CAH) (n=5), gestational arterial hypertension (GAH) (n=10), low birth weight (n=5), tendency to large fetus (n=27), large fetus (n=25), low birth weight by gestational age (n=3), intrahepatic cholestasis of pregnant women (n=2). In some cases, the same patient had more than one indication at the time of PL.
Preinduction and subsequent IL were performed according to the clinical guidelines [1].
Preparation of the birth canal for labor was started when the cervix was unripe (Bishop score 0–5); induction of labor was performed when the birth canal reached cervical ripening (Bishop score ≥8).
In the case of an unripe cervix, preinduction was started with one 200 mg mifepristone tablet. In case of a response to the first tablet in the form of reaching cervical ripening (Bishop score ≥8) or the appearance of regular labor activity, this category of women was referred to as Group 1. If further PL was necessary to achieve cervical ripening, additional methods of preinduction were used, which, depending on the clinical situation, included administration of a second tablet of mifepristone 200 mg, intracervical placement of a balloon dilator, use of Dilapan S hygroscopic cervical dilators, and intracervical administration of dinoprostone 0.5 mg. Amniotomy was performed to induce labor. Written informed consent was obtained from all the patients for all methods and stages of PL/IL.
Achievement of full cervical ripening (Bishop score 8) and onset of labor within 24 h of mifepristone administration were defined as the primary study outcomes. The secondary outcomes were defined as the percentage of cesarean sections, vaginal deliveries in the study groups, and perinatal outcomes.
The study was reviewed and approved by the Research Ethics Committee of V.I. Kulakov NMRC for OG&P of the Ministry of Health of Russia.
Statistical analysis
Statistical analysis was performed using StatTech v. 3.1.4 (StatTech LLC, Russia).
The normality of the distribution was tested using the Shapiro–Wilk test (when the number of subjects was < 50) and the Kolmogorov–Smirnov test (when the number of subjects was > 50). Quantitative variables showing normal distribution were expressed as mean (M) and standard deviation (SD) and 95% confidence interval (95% CI); otherwise, the median (Me) with interquartile range (Q1; Q3) was reported. Categorical variables were reported as frequencies and percentages. Normally distributed continuous variables with equal variances were compared between the two groups using Student’s t-test and Welch-t test (unequal variance t-test). Variables that did not meet normality assumptions were compared using a nonparametric Mann–Whitney test. Comparisons of percentages were performed using Pearson’s chi-square test with 2×2 contingency tables (when the expected frequencies were > 10) and Fisher's exact criterion (when the expected frequencies were < 10). Comparisons of percentages in the analysis of multi-way contingency tables were performed using Pearson's chi-squared (χ2) test. A predictive model for the probability of a certain outcome was constructed using logistic regression. Nagelkerke's R2 coefficient served as a measure of certainty, indicating part of the variance that can be explained by logistic regression.
ROC curve analysis was used to assess the diagnostic significance of quantitative features in predicting a certain outcome. The separating value of a quantitative trait at the cutoff point was determined by the highest value of the Youden index.
Results
The study groups were comparable in terms of age, 31 (5.1) years in both groups (p=0.32), weight, 73.0 (64.0; 79.0) kg vs. 73.0 (65.8; 81.0) kg (p=0.39) and height 167 (163; 170) cm vs. 168 (161; 170) cm (p=0.46). The median BMI values also did not differ between the two groups 26 (23;29) kg/m2 and 26 (24;28) kg/m2 (p=0.42). There were statistically significant differences in parity: the median of Group 1 was 0 (0;1) and Group 2 was 0 (0;0) (p=0.029).
The two groups were compared in terms of non-obstetric comorbidities (CAH, obesity, subclinical hypothyroidism, and gastrointestinal and genitourinary diseases) and gynecologic history (history of miscarriages, missed miscarriages, and infertility), which did not reveal statistically significant differences. The presence of history of induced abortion showed significant differences between the two groups, 14/116 (12.1%) vs. 5/120 (4.2%) (p=0.031; 95% CI 1.099–9.070) (Table 1).
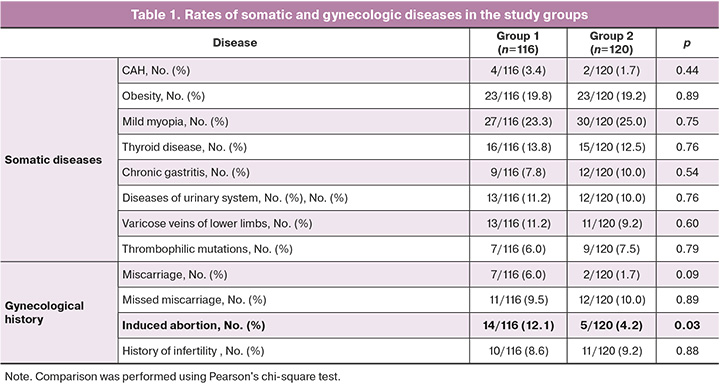
During the index pregnancy, there were statistically significant differences between the groups with respect to a history of acute respiratory infections, 24/116 (20.7%) in Group 1 and 43/120 (35.8%) in Group 2 (p=0.010) (OR=0.467; 95% CI 0.261–0.838); novel coronavirus infection, 16/116 (13.8%) vs. 7/120 (5.8%) (p=0.039; 95% CI 1.021–6.534); taking dydrogesterone, 10/116 (8.6%) in Group 1 and 24/120 (20.0%) in Group 2 (p=0.013; RR=0.377; 95% CI 0.172–0.830); as well as the presence of polyhydramnios in the third trimester of pregnancy, 17/116 (14.7%) vs. 6/120 (5.0%) (p=0.012; 95% CI 1.238–8.597) (Table. 2).
Comparison of the two groups regarding indications for PL showed differences with respect to tendency to postterm pregnancy in Group 1 – 42/116 (35%), in Group 2 – 38/120 (32.8%); anatomically narrow pelvis – 36/116 (30.0%) vs. 33/120 (28.4%); GSD – 18/116 (15.0%) vs. 18/120 (15.5%); CAH – 2/116 (1.7%) vs. 3/120 (2.6%); GAH – 4/116 (3.3%) in Group 1, 6/120 (5.2%) in Group 2; low birth weight – 1/116 (0.8%) vs. 4/120 (3.4%); tendency to deliver a large fetus –16/116 (13.3%) in Group 1, 11/120 (9.5%) in Group 2; large fetus – 16/116 (13.3%) vs. 9/120 (7.8%); low birth weight by gestational age – 2/116 (1.7%) vs. 1/120 (0.9%); intrahepatic cholestasis of pregnancy – 2/116 (1.7%) vs. 0/120 (0%) (p>0.05).
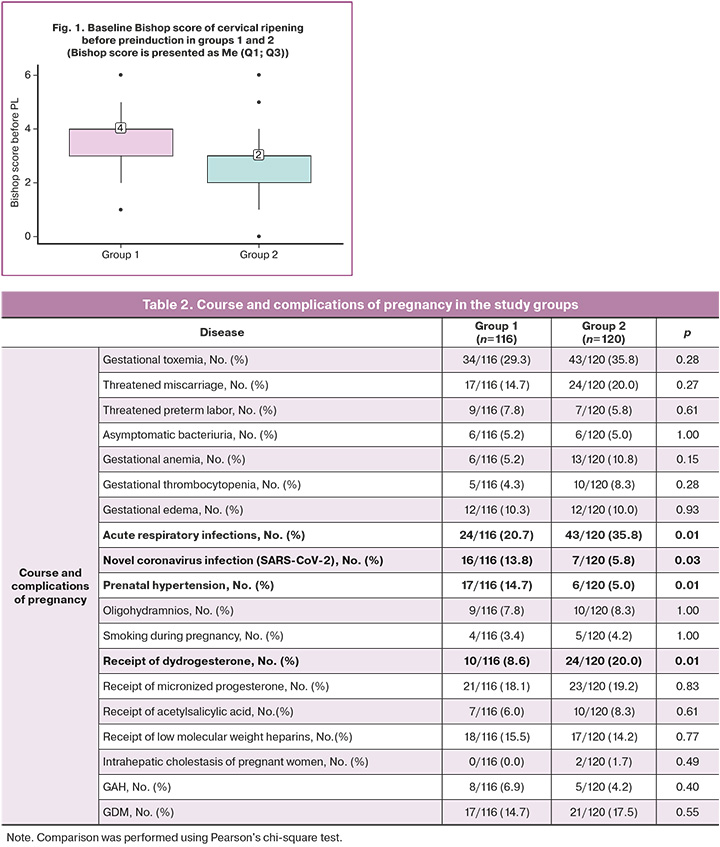
The timing of PL initiation in both groups was comparable, with a median of 280 days (275; 284), corresponding to 40 weeks (39.2; 40.4). The median PL completion time was 281 days (276; 285) to 40 weeks 1 day (39.3; 40.5) for group 1, and 282 days (277; 286) to 40 weeks 2 days (39.4; 40.6) for Group 2. The Bishop score before preinduction was 4 (3; 4) in Group 1 and 3 (2; 3) in Group 2, which was statistically significantly different (p<0.001) (Fig. 1).
Significant differences were found in the number of days from PL onset to PL completion: the median number of days in Group 1 that responded to 1 tablet of mifepristone was 2 (2; 2), while in Group 2 it was 3 (2; 3), p<0.001.
The data obtained on the method of delivery showed that the rate of vaginal delivery was 103/116 (88.8%) in Group 1 and 99/120 (82.5%) in Group 2, which was not statistically significantly different (p=0.169). At the same time, there were no differences in the duration of labor between the two groups: the median in Group 1 was 6 h 25 min (5.25; 7.43) versus 6 h 55 min (5.78; 8.30) in Group 2 (p=0.158). Both groups were comparable in terms of labor complications. The bleeding rates in both groups were 1/116 (0.9%) and 1/120 (0.8%), respectively (p=1.00). There were significant differences between the groups in terms of episiotomy performed due to threatened perineal laceration: in Group 1, 20/116 (17.2%) in Group 2, 43/120 (35.8%), p=0.001). There were no differences in postpartum blood loss in the study groups: 250 (170; 300) ml and 250 (200; 312) ml, p=0.30.
The cesarean delivery rates did not differ between the groups, amounting to 13/116 (11.2%) in Group 1 and 21/120 (17.5%) in Group 2. Indications for cesarean section were comparable in most cases, except for cases of a clinically narrow pelvis, which were 3/116 (2.6%) and 13 (10.8%), respectively (p=0.01).
The 'vacuum delivery rates also showed no significant difference between the two study groups, 4/116 (3.4%) vs. 4/120 (3.3%), respectively, p=1.00.
In all cases, live preterm infants were born with a median Apgar score of 8 (8; 8) at 1 minute (p=0.87) and 9 (9; 9) at 5 minutes (p=0.11) in both groups.
Birth weight differed and was 3490 (422) (95% CI 3412–3567) g in Group 1 and 3596 (375) (95% CI 3528–3663) g in Group 2 (p=0.04). The body length and head circumference of newborns in both groups were not significantly different. There were also no differences in neonate sex.
A prognostic model based on binary logistic regression was developed to determine the probability of successful PL depending on variables "Parity", "Polyhydramnios" and "Bishop score before preinduction".
The regression analysis included the traits of pregnant women who responded to PL/IL as the dependent variable (response variable). Significant variables included as independent (predictor) variables were "Parity" (values 0, 1, 2), "Polyhydramnios" (values 0, 1), "Bishop score before preinduction" (values 1, 2, 3, 4).
Univariate analysis (logistic regression), was performed 3 times for each of the variables. The glm function in the R programming language was used. The parameter "Parity" showed a significance p<0.03. The parameter "Polyhydramnios " was significant at p=0.017. The parameter "Bishop scale score before preinduction" showed high significance at p=0.001 level.
The independent variables were tested for collinearity (Table 3). The correlation coefficients of the independent variables did not exceed 0.17, and they were included in the regression model.
The process of variable selection according to significance (contribution to the model) was carried out by stepwise inclusion.
The total number of observations was 236. The observed dependence, the use of which is possible in order to predict a successful response to PL with mifepristone, is described by the equation:
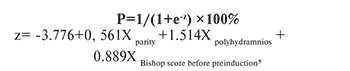
where P is the probability of PL success, Xparity is the N of given births by count, Xpolyhydramnios is polyhydramnios (0 is normal water count, 1 is polyhydramnios), and the XBishop scale score before preinduction is the Bishop scale score before PL.
The regression model obtained was statistically significant (p<0.001).
Multiple regression analysis used the four independent variables described above. The regression coefficients and p-values for each variable are summarized in Table 4.
The wald.test function of the aod library (R language) was used to test for the possibility of zero coefficient values according to the Wald test. For the variables "Parity"+"polyhydramnios" Chi-squared test: χ2=6.6, df=2, P(>χ2)=0.036. For variables "Bishop scale score before preinduction" Chi-squared test: χ2=33.9, df=2, P(>χ2) =0.004 Since the p is less than 0.05, the null hypothesis of Wald test can be rejected.
When the Parity score increased by 1, the odds of preinduction success increased by 1.57 times. When the Polyhydramnios Score was assessed, the odds of successful preinduction increased by a factor of 4.22. When the preinduction Bishop score was increased by 1, the odds of successful preinduction increased 2.16-fold (Table 4).
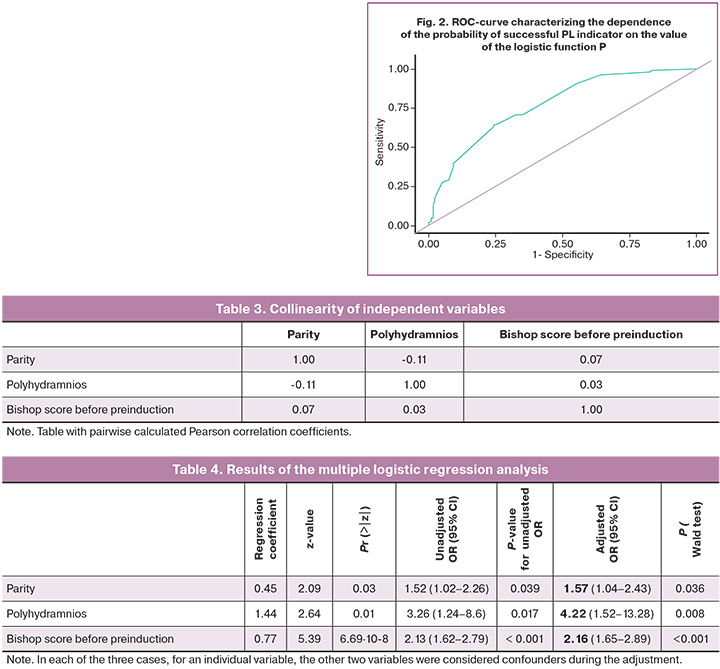
When the dependence of the probability of successful PL on the value of the logistic function, P, was evaluated using ROC analysis, the curve shown in Figure 2 was obtained.
The area under the ROC curve was 0.771±0.032 (95% CI 0.708–0.833). The resulting model was statistically significant (p<0.001).
The threshold value of the logistic function P at the cutoff point, which corresponded to the highest value of the Youden index, was 0.561. The response to one mifepristone tablet (Group 1) was predicted when the value of the logistic function P was greater than or equal to this value. The sensitivity and specificity of this model were 64.2% and 75.8%, respectively.
The positive predictive value was 72.6, the negative predictive value was 67.9.
Discussion
In this study, we investigated possible predictors contributing to successful preinduction of labor, as well as shortening the time interval to prepare for delivery. We took one of two starting points as the basis for preinduction of labor success in our study: achievement of cervical ripening according to the Bishop ≥8, or development of regular labor contractions within 24 hours of a single 200 mg dose of oral mifepristone.
Cervical maturation is a known predictor of induction of labor success/failure [38, 39]. However, we found no studies in the literature available to us at present that have determined the value of the Bishop score at which a rapid response to preinduction with mifepristone is expected. Our study showed that this value was four points.
We found a correlation between parity and successful preinduction, which was significantly higher in multiparous mothers. These findings have been confirmed in several studies [40, 41].
A difficult-to-explain finding of our study is the association between a history of induced abortion and the success of preinduction. However, no association was found between the efficacy of preinduction and missed miscarriage and ectopic pregnancy.
The correlation between acute respiratory infections during pregnancy and novel coronavirus infection and the response to preinduction is also controversial. Given the lack of detailed data on the causative agents, duration, and severity of the course of acute respiratory viral infections in the pregnant women included in the study, it is not possible to provide a convincing explanation of the correlation between acute respiratory viral infections and longer preinduction of labor, as well as more frequent use of additional methods of cervical ripening.
The results showed that a new coronavirus infection during pregnancy was combined with a rapid response to preinduction after a single use of mifepristone. It can be speculated that SARS-CoV-2, by initiating an inflammatory cascade in the maternal body, promotes the production of cytokines, leading to activation of the myometrium on the one hand and a decrease in progesterone levels on the other, thereby initiating labor [42, 43].
We found that there was no correlation between the use of micronized natural progesterone early in pregnancy to prevent and treat threatened miscarriage and a more complex and longer preinduction of labor regimen, whereas women who received dydrogesterone at the same gestational age showed the opposite. In other words, the preinduction of labor efficacy was lower in women treated with dydrogesterone early in pregnancy. However, this requires further study.
We found a marked difference between groups 1 and 2 with regard to preinduction of labor depending on the presence of polyuria. Thus, the development of regular labor or achievement of cervical ripening within 24 h from the start of preinduction was more frequent in women with polyhydramnios. This mechanism is most likely related to mechanical stretching of the myometrium, which stimulates the onset of labor through the production of monocyte chemotactic protein-1 (MCP-1) [44, 45].
In our study, we found statistically significant differences in the need for epidural analgesia for labor pain between the two groups: patients in Group 2 (with longer and more multicomponent preinduction of labor) required analgesia significantly more often. This fact can be explained by the initial lower readiness of the organism as a whole for the onset of labor activity, as well as by the longer stay of the pregnant woman in the hospital and several stages of preinduction of labor, which affects the maternal psycho-emotional state. These findings were consistent with those reported by Voskresensky et al. [46].
The higher frequency of episiotomy due to threatened perineal lacerations in Group 2 was correlated with a lower Bishop score in this group. This probably confirms the lower degree of readiness of the organism for labor, which is expressed in the immaturity of both cervical and other tissues of the birth canal.
We found differences in birth weight between the groups. There was an association between higher birth weight and less successful preinduction of labor. Similar findings were reported by Kamlungkuea et al. (2022), who showed that successful vaginal delivery after preinduction was associated with fetal weight <3500 g [47]. However, fetal weight was a less significant factor than parity, Bishop score, and polyhydramnios.
Our study had several limitations, primarily due to its retrospective design. Data obtained from outpatient cards and delivery case records do not always fully reflect the entire pregnancy course and medication intake. More accurate data can be obtained in a prospective study that includes more accurate information about a woman’s pregnancy history and course.
Conclusion
Based on the above, predictors of more successful and faster preinduction of labor are primarily the initial Bishop score, parity, and polyhydramnios. The issue of prior viral disease, particularly novel coronavirus infection, remains controversial and requires further study. The association between progesterone use during pregnancy also needs to be clarified. Our data showed that women who initially had a higher Bishop score were less likely to require epidural analgesia during labor and episiotomy because of the risk of perineal laceration, which correlates with a higher degree of body readiness for labor.
References
- Министерство здравоохранения Российской Федерации. Клинические рекомендации "Неудачная попытка стимуляции родов (подготовка шейки матки к родам и родовозбуждение)". 2021. [Ministry of Health of the Russian Federation. Clinical guidelines "Failed attempt at labor stimulation (cervical preparation and labor induction)". 2021 (in Russian)].
- Saucedo A.M., Cahill A.G. Evidence-based approaches to labor induction.. Obstet. Gynecol. Surv. 2023; 78(3):171-83. https://dx.doi.org/10.1097/ogx.0000000000001110.
- Marconi A.M. Recent advances in the induction of labor. F1000Research. 2019; (8): F1000 Faculty Rev-1829. https://dx.doi.org/10.12688/f1000research.17587.1.
- Simon C.E., Grobman W.A. When has an induction failed? Obstet. Gynecol. 2005;105(4): 705-9. https://dx.doi.org/10.1097/01.aog.0000157437.10998.e7.
- Caughey A.B., Sundaram V., Kaimal A.J., Gienger A., Cheng Y.W., McDonald K.M. et al. Systematic review: elective induction of labor versus expectant management of pregnancy. Ann. Intern. Med. 2009; 151(4): 252-63, W53-63. https://dx.doi.org/10.7326/0003-4819-151-4-200908180-00007.
- Lin M.G., Rouse D.J. What is a failed labor induction? Clin. Obstet. Gynecol. 2006; 49(3): 585-93. https://dx.doi.org/10.1097/00003081-200609000-00018
- Walker K.F., Bugg G.J., Macpherson M., McCormick C., Grace N., Wildsmith C. et al. Randomized trial of labor induction in women 35 years of age or older. N. Engl. J. Med. 2016; 374(9): 813-22. https://dx.doi.org/10.1056/nejmoa1509117.
- Knight H.E., Cromwell D.A., Gurol-Urganci I., Harron K., van der Meulen J.H., Smith G.C.S. Perinatal mortality associated with induction of labour versus expectant management in nulliparous women aged 35 years or over: аn English national cohort study. PLoS Med. 2017;14(11):e1002425. https://dx.doi.org/10.1371/journal.pmed.1002425
- Spong C.Y., Berghella V., Wenstrom K.D., Mercer B.M., Saade G.R. Preventing the first cesarean delivery: summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists Workshop. Obstet. Gynecol. 2012; 120(5): 1181-93. https://dx.doi.org/10.1097/aog.0b013e3182704880.
- Middleton P., Shepherd E., Crowther C.A. Induction of labour for improving birth outcomes for women at or beyond term. Cochrane Database Syst. Rev. 2018; 5(5):CD004945. https://dx.doi.org/10.1002/14651858.cd004945.pub4.
- Batinelli L., Serafini A., Nante N., Petraglia F., Severi F.M., Messina G. Induction of labour: clinical predictive factors for success and failure. J. Obstet. Gynaecol. 2018;38(3):352-8. https://dx.doi.org/10.1080/01443615.2017.1361388.
- Ludmir J., Sehdev H.M. Anatomy and physiology of the uterine cervix. Clin. Obstet. Gynecol. 2000; 43(3):433-9. https://dx.doi.org/10.1097/00003081-200009000-00003.
- Feghali M., Timofeev J., Huang C.C., Driggers R., Miodovnik M., Landy H.J. et al. Preterm induction of labor: predictors of vaginal delivery and labor curves. Am. J. Obstet. Gynecol. 2015; 212(1): 91.e1-91.e7. https://dx.doi.org/10.1016/j.ajog.2014.07.035.
- Gibson K.S., Waters T.P. Measures of success: Prediction of successful labor induction. Semin. Perinatol. 2015; 39(6):475-2. https://dx.doi.org/10.1053/j.semperi.2015.07.012.
- Subramaniam A., Jauk V.C., Goss A.R., Alvarez M.D., Reese C., Edwards R.K. Mode of delivery in women with class III obesity: planned cesarean compared with induction of labor. Am. J. Obstet. Gynecol. 2014; 211(6): 700.e1-700.e9. https://dx.doi.org/10.1016/j.ajog.2014.06.045.
- Pandis G.K., Papageorghiou A.T., Ramanathan V.G., Thompson M.O., Nicolaides K.H. Preinduction sonographic measurement of cervical length in the prediction of successful induction of labor. Ultrasound Obstet. Gynecol. 2001; 18(6):623-8. https://dx.doi.org/10.1046/j.0960-7692.2001.00580.x.
- Rane S.M., Guirgis R.R., Higgins B., Nicolaides K.H. The value of ultrasound in the prediction of successful induction of labor. Ultrasound Obstet. Gynecol. 2004; 24(5): 538-9. https://dx.doi.org/10.1002/uog.1100.
- Gómez Laencina A.M, Sánchez F.G., Gimenez J.H., Martínez M.S., Valverde Martínez J.A., Vizcaíno V.M. Comparison of ultrasonographic cervical length and the Bishop score in predicting successful labor induction. Acta Obstet. Gynecol. Scand. 2007; 86(7): 799-804. https://dx.doi.org/10.1080/00016340701409858
- Park K.H., Kim S.N., Lee S.Y., Jeong E.H., Jung H.J., Oh K.J. Comparison between sonographic cervical length and Bishop score in preinduction cervical assessment: a randomized trial. Ultrasound Obstet. Gynecol. 2011; 38(2):198-204. https://dx.doi.org/10.1002/uog.9020.
- Khalifa M.A., Abbas A.M., Gaber M.A., Salah M. Bishop score versus transvaginal ultrasonographic measurement of cervical length in predicting successful labor induction in post-term pregnancy: prospective cohort study. Int. J. Reprod. Contracept. Obstet. Gynecol. 2018; 7(11): 4646-51. https://dx.doi.org/10.18203/2320-1770.ijrcog20184523.
- Бабич Д.А., Баев О.Р., Федоткина Е.П., Гус А.И. Диагностические возможности эхоэластографии в акушерстве и гинекологии. Акушерство и гинекология. 2019; 7: 5-12. [Babich D.A., Baev O.R., Fedotkina E.P., Gus A.I. Diagnostic opportunities of echo elastography in obstetrics and gynecology. Obstetrics and Gynecology. 2019; (7): 5-12. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.7.5-12.
- Yang Q., Zhou C.C., Chen Y., Pei J.D, Hua X.L, Yao L.P. Prediction model for successful induction of labor by cervical strain elastography diagnosed at late-term pregnancy in nulliparous women: a prospective cohort study. BMC Pregnancy Childbirth. 2023; 23(1): 114. https://dx.doi.org/10.1186/s12884-023-05426-7.
- Lockwood C.J., Senyei A.E., Dische M.R., Casal D., Shah K.D., Thung S.N. et al. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. N. Engl. J. Med. 1991; 325(10): 669-74. https://dx.doi.org/10.1056/nejm199109053251001.
- Garite T.J., Casal D., Garcia-Alonso A., Kreaden U., Jimenez G., Ayala J.A. et al. Fetal fibronectin: a new tool for the prediction of successful induction of labor. Am. J. Obstet. Gynecol. 1996;175(6):1516-21. https://dx.doi.org/10.1016/s0002-9378(96)70100-x.
- Crane J.M. Factors predicting labor induction success: a critical analysis. Clin. Obstet. Gynecol. 2006; 49(3):573-84. https://dx.doi.org/10.1097/00003081-200609000-00017.
- Kiss H., Ahner R., Hohlagschwandtner M., Leitich H., Husslein P. Fetal fibronectin as a predictor of term labor: a literature review. Acta. Obstet. Gynecol. Scand. 2000; 79(1):3-7. https://dx.doi.org/10.1080/j.1600-0412.2000.079001003.x.
- Adedeji M.O., Olumodeji A.M., Fabamwo A.O., Oyedele O.Y. Quantitative cervicovaginal fetal fibronectin as a predictor of cervical ripening and induced labour duration in late-term pregnancy. J. Obstet. Gynaecol. 2023; 43(1):2204975. https://dx.doi.org/10.1080/01443615.2023.2204975.
- Sciscione A., Hoffman M.K., DeLuca S., O’Shea A., Benson J., Pollock M. Vakili B. Fetal fibronectin as a predictor of vaginal birth in nulliparas undergoing preinduction cervical ripening. Obstet. Gynecol. 2005; 106 (5 Pt 1): 980-5. https://dx.doi.org/10.1097/01.aog.0000185288.75896.98.
- Droulez A., Girard R., Dumas A.M., Mathian B., Berland M. Prediction of successful induction of labor: a comparison between fetal fibronectin assay and the Bishop score. J. Gynecol. Obstet. Biol. Reprod. (Paris). 2008; 37(7): 691-6. https://dx.doi.org/10.1016/j.jgyn.2008.05.009.
- Ojutiku D., Jones G., Bewley S. Quantitative foetal fibronectin as a predictor of successful induction of labour in post-date pregnancies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002; 101(2):143-6. https://dx.doi.org/10.1016/s0301-2115(01)00544-9.
- Roman A.S., Pessel C., Fox N., Klauser C.K., Saltzman D., Rebarber A. Vaginal fetal fibronectin as a predictor of spontaneous preterm delivery in triplet gestations. J. Matern. Fetal. Neonatal. Med. 2012; 25(10):1921-3. https://dx.doi.org/10.3109/14767058.2012.677964.
- Lau S.L., Kwan A., Tse W.T., Poon L.C. The use of ultrasound, fibronectin and other parameters to predict the success of labour induction. Best Pract. Res. Clinin. Obstet. Gynaecol. 2022; 79: 27-41. https://dx.doi.org/10.1016/j.bpobgyn.2021.10.002.
- Riboni F., Garofalo G., Pascoli I., Vitulo A., Dell’avanzo M., Battagliarin G. et al. Labour induction at term: clinical, biophysical and molecular predictive factors. Arch. Gynecol. Obstet. 2012; 286(5):1123-9. https://dx.doi.org/10.1007/s00404-012-2432-1.
- Rathore A., Sharma R., Kar R., Tandon A., Suneja A., Guleria K. Role of cervical phosphorylated insulin-like growth factor-binding protein 1 (phIGFBP1) for prediction of successful induction among primigravida with prolonged pregnancy. J. Obstet. Gynaecol. India. 2021; 71(1):38-44. https://dx.doi.org/10.1007/s13224-020-01372-y.
- Schneider-Kolsky M., D’Antona D., Evans L.W., Taylor N., O’Connor A., Groome N.P. Maternal serum total activin A and follistatin in pregnancy and parturition. BJOG. 2000; 107(8):995-1000. https://dx.doi.org/10.1111/j.1471-0528.2000.tb10402.x.
- Funghi L., Torricelli M., Novembri R., Vannuccini S., Cevenini G., Di Tommaso M. et al. Placental and maternal serum activin A in spontaneous and induced labor in late-term pregnancy. J. Endocrinol. Invest. 2018;41(2):171-7. https://dx.doi.org/10.1007/s40618-017-0640-z.
- Özdemir B.G., Özdemir H., Atalay C.R. The importance of fetal adrenal gland volume measurement in successful labor induction with oxytocin. J. Obstet. Gynaecol. Res. 2022; 48(10):2514-21. https://dx.doi.org/10.1111/jog.15361.
- Isono W., Nagamatsu T., Uemura Y., Fujii T., Hyodo H., Yamashita T. et al. Prediction model for the incidence of emergent cesarean section during induction of labor specialized in nulliparous low-risk women. J. Obstet. Gynaecol. Res. 2011;37(12):1784-91. https://dx.doi.org/10.1111/j.1447-0756.2011.01607.x.
- Teixeira C., Lunet N., Rodrigues T., Barros H. The Bishop score as a determinant of labour induction success: a systematic review and meta-analysis. Arch. Gynecol. Obstet. 2012; 286(3):739-53. https://dx.doi.org/10.1007/s00404-012-2341-3.
- Keepanasseril A., Suri V., Bagga R., Aggarwal N. A new objective scoring system for the prediction of successful induction of labour. J. Obstet. Gynaecol. 2012; 32(2):145-7. https://dx.doi.org/10.3109/01443615.2011.637142.
- Mohammed M., Oumer R., Mohammed F., Walle F., Mosa H., Ahmed R. et al. Prevalence and factors associated with failed induction of labor in Worabe Comprehensive Specialized Hospital, Southern Ethiopia. PloS One. 2022; 17(1):e0263371. https://dx.doi.org/10.1371/journal.pone.0263371.
- Shah S.B. COVID-19 and progesterone: Part 1. SARS-CoV-2, progesterone and its potential clinical use. Endocr. Metab. Sci. 2021; 5:100109. https://dx.doi.org/10.1016/j.endmts.2021.100109.
- Fell D.B., Dimanlig-Cruz S., Regan A.K., Håberg S.E., Gravel C.A., Oakley L. et al. Risk of preterm birth, small for gestational age at birth, and stillbirth after covid-19 vaccination during pregnancy: population based retrospective cohort study. BMJ. 2022; 378: e071416. https://dx.doi.org/10.1136/bmj-2022-071416.
- Shynlova O., Tsui P., Dorogin A., Lye S.J. Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J. Immunol. 2008; 181(2):1470-9. https://dx.doi.org/10.4049/jimmunol.181.2.1470.
- Гайдарова А.Р. Гусар В.А., Баев О.Р. Инициация родовой деятельности как многофакторный механизм коммуникации компартментов матери и плода. Акушерство и гинекология. 2022; 2: 20-6. [Gaidarova A.R., Gusar V.A., Baev O.R. Initiation of labor activity as a multifactor mechanism of communication between maternal and fetal compartments. Obstetrics and Gynecology. 2022; (2): 20-6 (in Russian)]. https://dx.doi.org/10.18565/aig.2022.2.20-26
- Воскресенский C.Л., Тесакова М.Л., Небышинец Л.М., Мельник Е.В., Шилкина Е.В., Малолеткина О.Л. Характеристика различных методов индукции родов. Охрана материнства и детства. 2012;1(19):29-35. [Voskresensky S.L., Tesakova M.L., Nebyishinec L.M., Melnik E.V., Shilkina E.V., Maloletkina O.L.Description of various methods of labour induction. Maternal and child care. 2012; 1(19):29-35. (in Russian)].
- Kamlungkuea T., Manonai J., Suriyawongpaisal P., Hansahiranwadee W. Factors predicting successful vaginal delivery following induction of labor in term pregnancy. Int. J. Womens Health. 2022; 14: 245-55. https://dx.doi.org/10.2147/IJWH.S347878.
Received 30.05.2023
Accepted 24.10.2023
About the Authors
Asiyat R. Gaidarova, PhD Student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, a_gadzhieva@oparina4.ru, https://orcid.org/0000-0003-1415-3318, 117997, Russia, Moscow, Ac. Oparina str., 4.Oleg R. Baev, Dr. Med. Sci., Professor, Head of the 1st Maternity Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, 117997, Russia, Moscow, Ac. Oparina str., 4; Professor at the Department of Obstetrics, Gynecology, Perinatology and Reproductology, I.M. Sechenov First Moscow State Medical University, Ministry of Health of the Russian Federation (Sechenov University),
119991, Russia, Moscow, Trubetskaya str., 8-2, o_baev@oparina4.ru, https://orcid.org/0000-0001-8572-1971
Vladislava A. Gusar, PhD, Senior Researcher at the Laboratory of Applied Transcriptomics of the Department of Systems Biology in Reproduction, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, v_gusar@oparina4.ru,
https://orcid.org/0000-0003-3990-6224, 117997, Russia, Moscow, Ac.Oparina str., 4.
Irina V. Edilberg, PhD Student, I.M. Sechenov First Moscow State Medical University, Ministry of Health of the Russian Federation (Sechenov University),
i_edilberg@oparina4.ru, https://orcid.org/0000-0003-4194-8730, 119991, Russia, Moscow, Bolshaya Pirogovskaya str., 2-4.
Oleg V. Tysyachnyi, PhD, Researcher at the 1st Maternity Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, o_tysyachny@oparina4.ru, https://orcid.org/0000-0001-9282-9817, 117997, Russia, Moscow, Ac. Oparina str., 4.
Aleksey A. Alekseev, Junior Researcher at the Laboratory of Cytology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, a_alekseev@oparina4.ru, https://orcid.org/0000-0002-5347-6884, 117997, Russia, Moscow, Ac. Oparina str., 4.
Corresponding author: Asiyat R. Gaidarova, a_gadzhieva@oparina4.ru



