State of antimicrobial protection of vaginal secrete in pregnant women during treatment of bacterial vaginosis
Tyutyunnik V.L., Mirzabekova D.D., Kan N.E., Khachatryan Z.V., Donnikov A.E.
Objective: A comprehensive analysis of the relationship between the antimicrobial activity of vaginal secretions and the clinical features of bacterial vaginosis in pregnant women during therapy, with consideration of vaginal pH dynamics and microbiological parameters.
Materials and methods: A total of 97 women participated in the study: 69 with bacterial vaginosis and 28 with normocenosis (normal vaginal flora). Patients with bacterial vaginosis were divided into a main group (n=37), who received combination therapy with an antibiotic and the immunomodulator "Superlymph", and a comparison group (n=32) on antibiotic monotherapy. After treatment, normocenosis was restored in all patients using the dosed cream "Acilact Duo". The antimicrobial activity of antimicrobial peptides was determined in the groups of pregnant women depending on the nature of the clinical symptoms of bacterial vaginosis against the background of its treatment.
Results: The results showed that the highest activity of antimicrobial peptides was observed in women with normocenosis. In bacterial vaginosis this activity decreased in proportion to the severity of the disease. In the main group, after combination therapy, this indicator increased almost twofold from the baseline. This regimen provided a significant improvement in clinical and laboratory parameters, a reduction in recurrences, prolonged remission, and restoration of the microbiota.
Conclusion: Including an assessment of immune status in the diagnosis of bacterial vaginosis in pregnant women, alongside a comprehensive evaluation of the vaginal microbiome, allows for personalized therapy. The results substantiate the advisability of implementing combination therapy into clinical practice.
Authors’ contributions: Tyutyunnik V.L., Mirzabekova D.D., Kan N.E., Khachatryan Z.V., Donnikov A.E. – developing of study design, obtaining data for analysis, reviewing publications on the topic of the article, statistical analysis of the obtained data, article writing.
Conflicts of interest: Authors declare lack of the possible conflicts of interest.
Funding: The study was conducted without sponsorship.
Ethical Approval: The study was approved by the ethics committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Tyutyunnik V.L., Mirzabekova D.D., Kan N.E., Khachatryan Z.V., Donnikov A.E. State of
antimicrobial protection of vaginal secrete in pregnant women during treatment of bacterial vaginosis.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (12): 53-62 (in Russian)
https://dx.doi.org/10.18565/aig.2025.338
Keywords
Bacterial vaginosis (BV) is one of the leading gynecological disorders in women of reproductive age. This condition is characterized by a critical reduction in the number of Lactobacilli (Lactobacillus spp.) and a compensatory increase in facultative and obligate anaerobes, as well as microaerophiles. Among the latter according to modern concepts, Gardnerella vaginalis and Atopobium vaginae play a key role in the etiopathogenesis of the disease, although the association also includes numerous representatives of anaerobic microflora (Peptostreptococcus, Prevotella, Bacteroides, etc.) [1–3]. Vaginal normocenosis is a complex ecosystem comprising over 40 species of microorganisms with the predominance of lactobacilli (90–95%). The remaining 5–10% are anaerobes, whose concentration in the vagina is much higher than that of aerobes [3, 4].
The stability of a healthy vaginal ecosystem is ensured by a multi-layered defense system. Lactobacilli produce lactic acid, by utilizing glycogen in the vaginal epithelium, the accumulation of which is stimulated by estrogen, maintaining an acidic environment (pH 4.0–4.5). They also produce hydrogen peroxide, which acts as a natural antiseptic. Lactobacilli also compete with pathogens for adhesion receptors on epithelial cells. Immune regulation is mediated by the activation of TLR receptors in the vaginal epithelium. Lactobacilli induce the production of proinflammatory cytokines in the number sufficient to control opportunistic microbiota [1, 5, 6].
The BV begins with a reduction in the lactobacillus population, which leads to a decrease in lactic acid production and a shift in pH toward the alkaline range. Spare ecological niche is then occupied by G. vaginalis, which acts synergistically with anaerobes. An important virulence factor for G. vaginalis is vagolysin, which has a cytotoxic effect on the epithelium and potentiates the activity of other gardnerella. In association with Lactobacterium iners, this microorganism is capable of producing cytolysin, which destroys epithelial cells [3, 5–7]. Intense cytolysis leads to the release of carbohydrates, which serve as a substrate for the synthesis of short-chain fatty acids (marker BV metabolites). A vicious cycle develops: glycogen and glucose deficiency exacerbates the destruction of vaginal epithelial cells, and the pH shifts toward alkalinity. These metabolites with their immunomodulatory effects suppress the inflammatory response [7–9].
The prevalence rate of bacterial vaginosis varies extensively from 12% to 80%, depending on the studied population. Among pregnant women BV is diagnosed in 36–40% of cases [1].
Only half of diagnosed women experience BV symptoms. The key symptoms include a grayish-white homogenous discharge, evenly covering the vaginal walls, and a specific “fishy” odor. Women may experience vulva discomfort, which intensifies after intercourse without the use of barrier contraception. Itching and burning are less common. However, certain signs of the vaginal and vulvar mucosa inflammation (hyperemia, swelling) are absent [1, 4, 9].
Diagnosis of BV is based on a comprehensive assessment of the clinical manifestation and laboratory test results [1–4, 10]. The main methods of laboratory diagnostics include: a microscopic method aimed at identifying “key” cells; determination of Gardnerella vaginalis, Atopobium vaginae, Lactobacillus spp. DNA and the total number of bacteria in vaginal discharge with the use of PCR-test (quantitative study); microbiological (cultural) examination of female genital discharge for aerobic and facultative anaerobic microorganisms [1, 10–13].
In general, two main diagnostic algorithms are used in clinical practice: Amsel’s criteria and the Nugent scoring system [14, 15]. The Amsel’s criteria are the most common method in routine practice due to their promptness and availability. To confirm the diagnosis any three of the four listed features should be present:
- vaginal discharge: thick, homogeneous, grayish-white, with an unpleasant odor;
- increased pH of vaginal secretions above 4.5;
- positive aminotest (a “fishy” odor when adding 10% potassium hydroxide solution to a discharge sample);
- detection of “key” cells (vaginal epithelial cells covered with a layer of bacteria) by microscopy [1, 14].
The Nugent's scale (Nugent's Diagnostic Criteria for Bacterial Vaginosis), proposed in 1991, is a more objective and sensitive method based on microscopy of a Gram-stained smear [15]. The study involves a quantitative assessment of the key microflora morphotypes:
- lactobacilli (Lactobacillus spp.): 0–4 points (inverse correlation – the fewer lactobacilli, the higher the score);
- opportunistic bacteria (Gardnerella, Bacteroides, Mobiluncus): 4–6 points;
- anaerobic microflora without lactobacilli: 7–10 points.
The total score is represented as follows: 0-3 points correspond to normocenosis, 4-6 points refer to borderline state, and 7–10 points confirm bacterial vaginosis [11, 14, 15].
BV is most common among women of reproductive age. Although this condition is not directly life-threatening, it is a significant risk factor for a number of serious complications [1, 16, 17]. The presence of bacterial vaginosis is associated with an increased risk of adverse gestational outcomes which often lead to low birth weight babies: spontaneous abortion, premature rupture of membranes, chorioamnionitis, preterm labor [16–20]. In addition to obstetric risks, women with BV are under threat of developing inflammatory processes in the postpartum period, in particular, endometritis, as well as sepsis after cesarean section [21, 22].
Therapy for BV during pregnancy is clinically justified, since it leads to a decrease in the number of gestational and perinatal complications [5, 17, 18]. A large number of studies dedicated to studying the effectiveness and features of the use of various therapeutic strategies for BV in pregnant women have been conducted [18, 20].
Treatment for BV includes antibacterial drugs from the nitroimidazole (metronidazole) and lincosamide (clindamycin) groups [1, 4, 23, 24]. The success of therapy is confirmed by the normalization of the clinical and microbiological presentation, associated with the elimination or a sharp reduction in the population of “key” cells during control microscopy [1–4, 12–14].
Despite the proven efficiency of oral antibiotic therapy, its use may be accompanied by adverse reactions, among which dyspeptic disorders predominate in pregnant women [17, 20, 24]. As opposed to the systemic approach, local drug administration has a number of advantages, such as targeted action at the site of infection, minimal systemic absorption, which allows for a reduction in dosage and the frequency of side effects [2, 9, 20–23]. In addition, a significant practical advantage of the intravaginal form of clindamycin is its convenient dosing regimen – just one administration of 100 mg at night for 6–7 days, which differs from the regimens for oral medications intake [17–23].
In addition to the protective function of normal flora, antimicrobial peptides (AMPs) are an integral component of the vaginal microecosystem. They are synthesized by mucosal barrier cells and neutrophils. These compounds play a key role in providing local immunity: they prevent pathogen adhesion, regulate the inflammatory response, and participate in the formation of acquired immunity by exhibiting direct antimicrobial activity. In scientific publications AMPs are often called “natural antibiotics”; however, the total diversity in the vaginal microbiome exceeds 500 species [27].
According to research data, AMP concentrations show an inverse correlation with the BV severity: the greatest decrease in their levels is identified in the most severe pathological forms. Reduced AMP activity weakens the vaginal epithelium ability to resist pathogen adhesion. This, in turn, provokes excessive growth of facultative microflora and exacerbates the pathological process, which clinically manifests with more severe symptoms [27, 28].
To date, two main subfamilies of AMPs have been identified: defensins and cathelicidins. Various AMPs, particularly α- and β-defensins, are synthesized by a wide range of cells, including lymphocytes, phagocytes, and epithelial cells of the respiratory, gastrointestinal, and urogenital tracts. Defensins play a key role in local immunity, serving as a first-line therapy against bacterial, fungal, and viral pathogens. Their local production by the vaginal and cervical epithelium ensures a rapid response to potential infection [28].
Restoration of AMP production by vaginal epithelium during BV therapy can become a prognostic marker reflecting the success of local antibacterial treatment.
Weakening of the immune system functions plays a leading role in the chronification of the pathology, associated with its prolonged course and the formation of therapy resistance [9, 29]. Excessive synthesis of proinflammatory cytokines results in the transition of the disease to a persistent form, and the accumulation of circulating immune complexes in tissues triggers the immunopathological processes. Therefore, to achieve stable BV remission, correction of the immunological status is necessary. A promising method to solve this problem is the inclusion of agents with immunomodulatory action into therapy [29]. “Superlymph” is one of such agents, which acts due to a complex of natural AMPs and cytokines that serve as stimulators of innate immunity. Its pharmacological activity is fulfilled through the activation of key components of the immune response. Furthermore, the use of Superlymph helps suppress inflammatory reactions, demonstrates an antioxidant effect, and stimulates tissue regeneration and epithelialization. The results of a series of independent clinical studies confirm its therapeutic efficacy [29, 30].
Thus, combination of etiotropic treatment with immunomodulatory therapy appears clinically sound. This comprehensive approach not only ensures accelerated symptom relief but also normalizes humoral immunity.
The second stage of treatment is aimed at restoring vaginal health using eubiotics. It is recommended to administer Acilact Duo intravaginally, one dose once a day (at night) for 10 days after finishing the basic course [31, 32].
Search for improved methods of BV diagnosis and therapy in pregnant women makes the study of the protective function of AMP and their response to infectious agents in this disease particular relevant.
Objective of the study: a comprehensive analysis of the relationship between the antimicrobial activity of vaginal secretions and the clinical features of BV in pregnant women during therapy, with consideration of vaginal pH dynamics and microbiological parameters.
Materials and methods
97 pregnant women were enrolled in a prospective randomized study. 69 of them were diagnosed with BV and 28 had normal vaginal microbiomes. Patients were selected based on their visits to specialized departments of the V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Inclusion criteria: singleton pregnancy at 22–39 weeks of gestation. Exclusion criterion for all patients was the presence of infection with obligate pathogens. Exclusion criterion for patients with BV covered delivery before completion of the full course of therapy. BV was detected based on three of the four Amsel criteria.
To assess the presence and severity of BV, we used standardized Nugent quantitative scale. Gram-stained smears were examined microscopically for lactobacilli, bacteroides, gram-positive cocci, and gardnerella. The quantitative characteristics of microorganisms were described using a 4-point system: 0 points corresponded to the absence of microflora, 1 point – single cells in the field of view, 2 points – a moderate amount, and 3 points – a significant abundance of cells.
At the first stage of the study, we analyzed the vaginal microbiome of the patients depending on the BV severity. Pregnant women with a confirmed diagnosis were classified into three clinical subgroups according to the severity of symptoms. To assess the condition, we developed a scoring scale, including the following clinical criteria: itching, burning, hyperemia, swelling, dysuria, dyspareunia, erosive lesions of the vulvovaginal mucosa, and signs of vaginal discharge. All patients underwent a comprehensive microbiological examination, which is mandatory determination of vaginal discharge pH and exclusion of infection by obligate pathogens.
To assess the antimicrobial activity of vaginal epithelium, we analyzed vaginal discharge samples. The antimicrobial characteristics were detected by incubating a 3-day test culture of Escherichia coli cells at 32°C in a mixture containing 40 μl of vaginal discharge and 10 μl of a bacterial suspension with a concentration of 10⁴ CFU/ml. Cultures were taken immediately after mixing the components and then again after a 2-hour incubation. The results were described as the percentage of bacterial cells killed during incubation. The antimicrobial activity of AMPs was identified in groups of pregnant women with varying severity of clinical symptoms during the treatment.
Fractionation of vaginal discharge proteins was performed by SDS-electrophoresis using 5–20% gradient polyacrylamide gel. Sample preparation was performed under native conditions: two parts of buffer were added to one part of the biological sample, after which 40 µl of the resulting mixture was applied to each well with the gel. Results were visualized using Coomassie R-250 dye. For molecular weight calibration we used a standard LMW kit (Amersham-Pharmacia).
At the second stage of the study, pregnant women with BV were randomly assigned to two groups. The main group (group 1, n=37) consisted of patients receiving combination therapy with an antibacterial drug and the immunomodulator Superlymph, while the comparison group (group 2, n=32) consisted of patients receiving antibiotic monotherapy.
Antibacterial therapy in both groups comprised clindamycin 2% cream, administered intravaginally once a day at bedtime for 7 days. This medication was chosen due to its high bioavailability, marked antibacterial activity, and favorable safety profile. Patients in the main group additionally received Superlymph at a dose of 10 U: 1 suppository rectally once a day for the first 10 days, followed by intravaginal administration over the next 10 days. Immunomodulatory therapy was initiated simultaneously with antibacterial therapy. After completion of the main course of treatment, all patients underwent vaginal normocenosis restoration using the eubiotic Acylact Duo according to the following regimen: 1 dose intravaginally once a day at night for 10 days.
To evaluate the effectiveness of treatment, we analyzed changes in the clinical symptoms 2 weeks after the start of therapy: the results of microbiological and microscopic examination of vaginal discharge, the pH of the vaginal environment and the level of antibacterial peptides.
Statistical analysis
Statistical analysis was performed using SPSS Statistics 22.0. The mean and standard deviation (M±SD) were calculated to describe the central tendency and dispersion of quantitative data in each study group. This method allows for a clear comparison of mean values between groups and an assessment of intragroup variability. It also describes microbiological parameters, vaginal pH, the percentage of antimicrobial activity in vaginal discharge, number of cells counted by microscopy, labor duration, blood loss volume, gestational age and birth weight, intercurrent period duration, and recurrence duration.
Statistical significance was assessed using parametric statistical analysis (Student's t-test). Fisher's exact test was used to compare proportions. These methods helped to investigate whether the observed differences between groups were statistically significant or could have originated by chance. Differences between samples were considered statistically significant at p-value<0.05. The odds ratio (OR) was presented with a 95% confidence interval (CI) to compare the risks of adverse perinatal and obstetric outcomes between treatment groups.
Results and discussion
After patient enrollment and a comprehensive examination, a qualitative characteristics of the microflora composition was conducted. The results of the comprehensive analysis are presented in Table 1.
According to the data in Table 1, the highest values of obligatory microflora were recorded in patients with normocenosis, while the lowest values were observed in patients with severe BV. The parameters of facultative microflora demonstrated the opposite trend: the lowest values were noted in the control group, and the peak values were observed in severe BV patients. A strong inverse correlation was found between the quantitative characteristics of obligatory and facultative microflora in all study groups. Moreover, the severity of BV clinical manifestations correlated positively with the level of facultative microflora and negatively – with the indicators of obligatory microflora.
Analysis of vaginal pH revealed a consistent value decrease in the group with a normal microbiome, which is explained by the physiological activity of Lactobacilli. Conversely, maximum pH values were recorded in severe forms of BV. The obtained data confirm a direct correlation between vaginal pH and the severity of the pathological process and the quantitative ratio of microbiological parameters in the studied biotope.
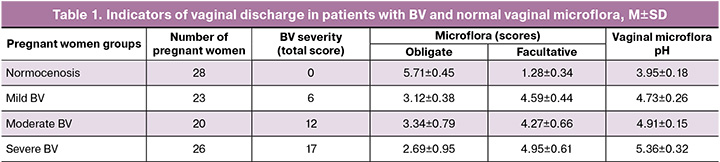
Then we assessed changes in the antimicrobial activity of vaginal secretions. The study results demonstrated maximum AMP activity in pregnant women with normal vaginal microbiota, reaching 77.9%. In patients with BV, a decrease in this indicator was noted, correlating with the severity of the disease: 42.8% in mild cases, 37.2% in moderate cases, and 35.3% in severe cases (Fig. 1).
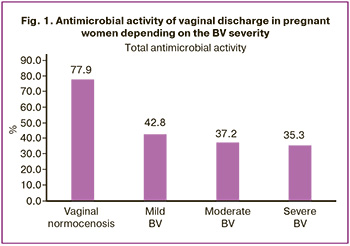
In the group of pregnant women with BV who received combined therapy with clindamycin and Superlymph, antimicrobial activity levels nearly doubled compared to the baseline. These values were 84.3% for mild cases, 75.5% for moderate cases, and 72.7% for severe cases. Meanwhile, in the group of healthy pregnant women, antimicrobial activity levels remained stable at 78.2% (Fig. 2).
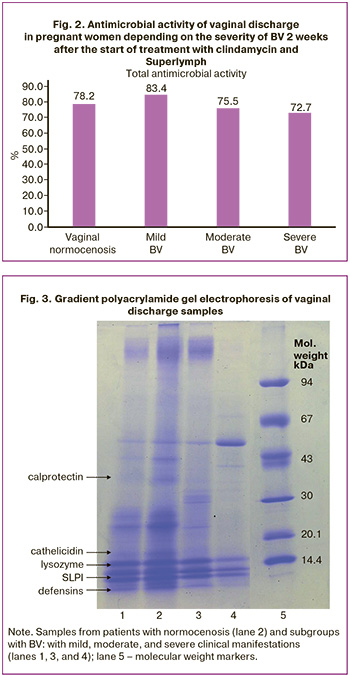
To identify the cause of the decrease in antimicrobial activity in BV, an electrophoretic assay of vaginal discharge proteins in a polyacrylamide gel gradient was performed (Fig. 3).
The obtained electrophoresis results demonstrate zones corresponding to AMP. In the sample from patients with normocenosis (lane No. 2) and maximum antimicrobial activity (100.0%), clear bands of polypeptides are visualized: calprotectin (37 kDa), cathelicidin hCAP18 (18 kDa), secretory leukocyte protease inhibitor (12 kDa), lysozyme (14.5 kDa), and defensins (<5 kDa).
In mild BV (lane 1, antimicrobial activity 66.7%) calprotectin is absent and the band intensity of other proteins is reduced. In moderate form of disease (lane 3, activity 21.6%) calprotectin is not detected and weak defensin expression is observed. The most pronounced changes were recorded in severe BV (lane No. 4): a complete absence of defensins, a sharp decrease in the expression of lysozyme and the absence of calprotectin with zero antimicrobial activity.
The results of the second stage of the study demonstrate that microscopic evaluation of vaginal microbiocenosis in both study groups revealed a morphological picture typical of BV: a significant number of clue cells, leukocytosis and an increased level of epithelial cells. Treatment results in both groups demonstrate positive dynamics, including a decrease in the number of epithelial cells and leukocytes, as well as a complete absence of key cells. It should be noted that in Group 1, which received combination therapy, the improvement of microscopic examination results was more pronounced, as shown in Table 2.
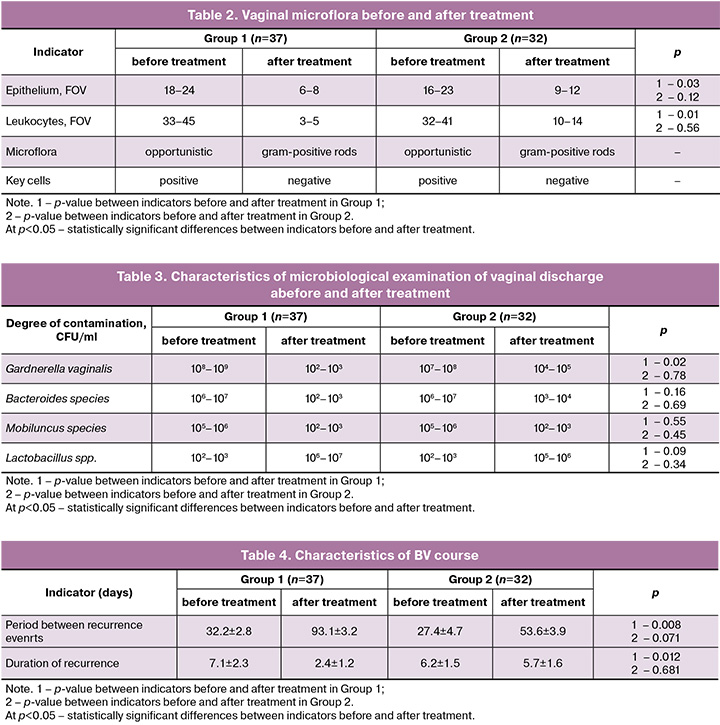
The nature of the microbiological presentation of vaginal discharge and the composition of the most frequent microorganisms are presented in Table 3. The results of the study demonstrated a decrease in the titer of microorganisms in both groups during the therapy. However, in group 2 which received monotherapy in 6/32 (18.8%) cases the average titer of Gardnerella vaginalis remained at >104 CFU/ml. The criterion for the effectiveness of treatment in both groups was an increase in the number of Lactobacillus spp., and in the combination therapy group the values were higher. According to the microbiological study, the vaginal microbiocenosis in pregnant women from the main group corresponded to the that of healthy pregnant women after termination of the course of treatment.
No treatment-related complaints were recorded in any case; patients in both groups demonstrated high compliance with the therapy. Group 1 patients did not complain of vaginal discharge, vaginal odor, discomfort, or itching in the perineal area at the follow-up visit. Meanwhile, six patients in Group 2 experienced persistent symptoms similar to those reported before treatment, although less marked. Vaginal pH levels in both groups normalized to <4.5 following treatment.
During the 3 months post-treatment follow-up period (including after delivery), we assessed the time between recurrence events and the duration of recurrence. In Group 1, the period between recurrence events at the first visit was 32.2±2.8 days, the duration of recurrence was 7.1±2.3 days, at the second visit, the period between recurrence events was 93.1±3.2 days, the duration of recurrence was 2.4±1.2 days. In Group 2, the period between recurrence events and their duration at the first visit were 27.4±4.7 and 6.2±1.5 days, respectively, at the second visit – 53.6±3.9 and 5.7±1.6 days, respectively. The data are presented in Table 4.
The data presented demonstrate treatment efficacy in both study groups. However, in Group 1 the post-treatment interval between recurrence events became significantly longer compared to Group 2, and the duration of recurrence was shorter.
In addition, we assessed obstetric and perinatal outcomes (Table 5).
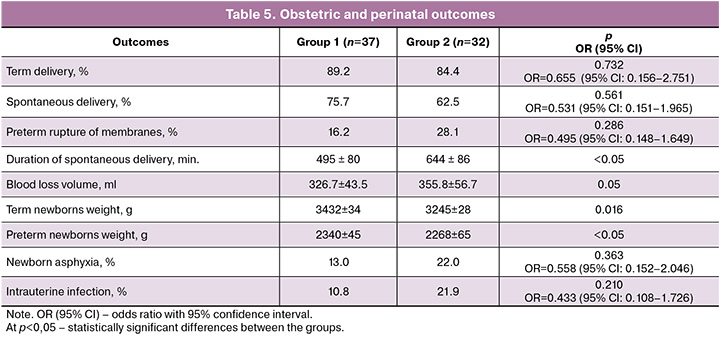
Term deliveries were observed in 33/37 of cases (89,2%) in Group 1 and in 27/32 of women (84,4%) in Group 2, preterm deliveries – in 4/37 of women (10,8%) in Group 1, and in 5/32 of patients (15,6%) in Group 2. Preterm rupture of membranes was diagnosed in 6/37 of patients (16,2%) in Group 1, and in 9/32 of patients (28,1%) in Group 2.
In Group 1 spontaneous deliveries happened in 28/37 of women (75,7%), mean duration of delivery was 8 hours 15 min. ± 1 hour 20 min., blood loss volume was 326,7±43,5 ml. In Group 2 vaginal delivery happened in 20/32 of women (62,5%), mean duration of delivery was 10 hours 44 min. ± 1 hour 26 min., blood loss volume was 355,8±56,7 ml.
The analysis of perinatal outcomes demonstrated that gestational age of newborns ranged 35–41 weeks. Apgar score valued 7–9 points in the main study group, and 5–9 points – in the comparison group. Number of preterm babies corresponded to preterm labor rate in the groups. Mean weight of term newborns in Group 1 was 3432±34 g, in Group 2 it comprised 3245±28 g, in Group 1 preterm newborns the mean weight was 2340±45 g, in Group 2 preterm newborns it was 2268±65 g. Newborn asphyxia was registered in 13% of cases (5/37) in the main group and in 26% (7/32) of cases in the comparison group; intrauterine infection symptoms were detected in: 10,8% (4/37) in Group 1 versus 21,8% (7/32) in Group 2.
Although no significant differences were found in the incidence rate of complicated pregnancy outcomes (preterm labor, premature rupture of membranes), combination therapy (Group 1) was associated with a more favorable labor course: labor duration was significantly shorter, and blood loss tended to decrease. Furthermore, neonatal asphyxia and signs of intrauterine infection were less common in the study group.
The results of the conducted analysis confirm active expression of innate immunity factors in reproductive tract mucosa. Vaginal microecosystem is regulated by dynamic exposure between local immune protective factors and opportunistic microflora population. Key components of this system are endogenic cationic AMPs, that participate mainly both the regulation of inflammatory reaction and in the formation of specific immune response [9, 28, 29]. Adequate synthesis of these peptides is critical to ensure effective antimicrobial protection. Along with a decrease in the functional activity of AMPs, a progressive increase in the number of facultative microflora is observed together with the reduction in the population of obligate microorganisms. In clinical practice it is manifested in more severe forms of BV.
Conclusion
Based on the results of the study, we can conclude that expanding the diagnostic algorithm for pregnant women with inflammatory and dysbiotic lesions in the lower reproductive tract which comprises incorporation of systemic and local immunity parameters, along with a comprehensive assessment of the vaginal microbiome, opens up opportunities for personalized therapy. Although BV does not pose an immediate threat to life, its treatment, especially during pregnancy, is often ineffective due to physiological immunosuppression and hormonal changes.
Furthermore, the obtained results demonstrate the advantages for the treatment of BV by combining antibacterial therapy with the Superlymph immunomodulator and the eubiotic Acylact Duo. This regimen reduced the recurrence rate and prolonged remission duration, proving the implementation of combination therapy into clinical practice for the management of pregnant women with BV. This approach not only alleviates acute symptoms of the disease but also restores normal vaginal microbiota, ensuring a long-lasting therapeutic effect.
References
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Бактериальный вагиноз. 2022. [Ministry of Health of the Russian Federation. Clinical guidelines. Bacterial vaginosis. 2022. (in Russian)].
- Coudray M.S., Madhivanan P. Bacterial vaginosis – a brief synopsis of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020; 245: 143-8. https://dx.doi.org/10.1016/j.ejogrb.2019.12.035
- Bhakta V., Aslam S., Aljaghwani A. Bacterial vaginosis in pregnancy: prevalence and outcomes in a tertiary care hospital. Afr. J. Reprod. Health. 2021; 25(1): 49-55. https://dx.doi.org/10.29063/ajrh2021/v25i1.6
- Дикке Г.Б., Баранов И.И., Байрамова Г.Р. Бактериальный вагиноз: парадокс XXI века. Акушерство и гинекология: новости, мнения, обучение. 2021; 9(4): 52-62. [Dikke G.B., Baranov I.I., Bairamova G.R. Bacterial vaginosis: paradox of the 21st century. Obstetrics and Gynecology: News, Opinions, Training. 2021; 9(4): 52-62 (in Russian)]. https://dx.doi.org/10.33029/2303-9698-2021-9-4-52-62.
- Chen X., Lu Y., Chen T., Li R. The female vaginal microbiome in health and bacterial vaginosis. Front. Cell. Infect. Microbiol. 2021; 11: 631972. https://dx.doi.org/10.3389/fcimb.2021.631972
- Chee W.J.Y., Chew S.Y., Than L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Fact. 2020; 19(1): 203. https://dx.doi.org/10.1186/s12934-020-01464-4
- Reiter S., Kellogg Spadt S. Bacterial vaginosis: a primer for clinicians. Postgrad. Med. 2019; 131(1): 8-18. https://dx.doi.org/10.1080/00325481.2019.1546534
- van den Tweel M., van den Munckhof E., van der Zanden M., Le Cessie S., van Lith J., Boers K. Testing on bacterial vaginosis in a subfertile population and time to pregnancy: a prospective cohort study. Arch. Gynecol. Obstet. 2024; 310(2): 1245-53. https://dx.doi.org/10.1007/s00404-024-07542-x
- Gilbert N.M., Ramirez Hernandez L.A., Berman D., Morrill S., Gagneux P., Lewis A.L. Social, microbial, and immune factors linking bacterial vaginosis and infectious diseases. J. Clin. Invest. 2025; 135(11): e184322. https://dx.doi.org/10.1172/JCI184322
- Чилова Р.А., Проклова Г.Ф., Гончаренко Н.В. Проблемы дифференциальной диагностики и лечения бактериального вагиноза. РМЖ. Мать и дитя. 2020; 3(1): 39-43. [Chilova R.A., Proklova G.F., Goncharenko N.V. Differential diagnosis and treatment for bacterial vaginosis. Russian Journal of Woman and Child Health. 2020; 3(1): 39-43 (in Russian)]. https://dx.doi.org/10.32364/2618- 8430-2020-3-1-39-43
- Redelinghuys M.J., Geldenhuys J., Jung H., Kock M.M. Bacterial vaginosis: current diagnostic avenues and future opportunities. Front. Cell. Infect. Microbiol. 2020; 10: 354. https://dx.doi.org/10.3389/fcimb.2020.00354
- Доброхотова Ю.Э., Шадрова П.А. Особенности диагностики бактериального вагиноза у беременных и его риски для репродукции. Медицинский оппонент. 2022; 2(18): 22-7. [Dobrokhotova Yu.E., Shadrova P.A. Features in diagnosis of bacterial vaginosis in pregnant women and its risks for reproduction. Medical Opponent. 2022; 2(18): 22-7 (in Russian)].
- Coronado O., Escamilla M.G., Vidal-Gutiérrez O., Garza-Rodríguez M.L. Hallmarks of bacterial vaginosis. Diagnostics (Basel). 2025; 15(9): 1090. https://dx.doi.org/10.3390/diagnostics15091090
- Colonna C., Steelman M. Amsel Criteria. 2023 Jul 4. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan.
- Theiler T., Schoeler S., Möllers M., Schuler F., Olaru I.D., Schaumburg F. Bacterial vaginosis in pregnant women: a comparison of the Nugent Score with a multiplex PCR. Diagn. Microbiol. Infect. Dis. 2024; 110(1): 116403. https://dx.doi.org/10.1016/j.diagmicrobio.2024.116403
- Sethi N., Narayanan V., Saaid R., Ahmad Adlan A.S., Ngoi S.T., Teh C.S.J. et al. Prevalence, risk factors, and adverse outcomes of bacterial vaginosis among pregnant women: a systematic review. BMC Pregnancy Childbirth. 2025; 25(1): 40. https://dx.doi.org/10.1186/s12884-025-07144-8
- Jayaram P.M., Mohan M.K., Konje J. Bacterial vaginosis in pregnancy - a storm in the cup of tea. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020; 253: 220-4. https://dx.doi.org/10.1016/j.ejogrb.2020.08.009
- Приступа Е.М., Баклыгина Е.А. Нарушение микробиоценоза влагалища как фактор риска развития истмико-цервикальной недостаточности. Современная наука: актуальные проблемы теории и практики. Серия: Естественные и технические науки. 2023; 9: 209-12. [Pristupa E.M., Baklygina E.A. Violation of vaginal microbiocenosis as a risk factor for the development of isthmic-cervical insufficiency. Modern Science: Current Issues in Theory and Practice. Series: Natural and technical sciences. 2023; 9: 209-212 (in Russian)]. https://dx.doi.org/10.37882/2223-2966.2023.09.26
- Gerede A., Nikolettos K., Vavoulidis E., Margioula-Siarkou C., Petousis S., Giourga M. et al. Vaginal microbiome and pregnancy complications: a review. J. Clin. Med. 2024; 13(13): 3875. https://dx.doi.org/10.3390/jcm13133875
- Blumenfeld Y.J., Marić I., Stevenson D.K., Gibbs R.S., Shaw G.M. Persistent bacterial vaginosis and risk for spontaneous preterm birth. Am. J. Perinatol. 2024; 41(S 01): e2081-8. https://dx.doi.org/10.1055/s-0043-1770703
- Jin J. Screening for bacterial vaginosis during pregnancy. JAMA. 2020; 323 (13): 1324. https://dx.doi.org/10.1001/jama.2020.3690
- Yefet E., Mirin D., Massalha M., Alter A., Nachum Z. Screening for and treatment of bacterial vaginosis reduced preterm delivery in high-risk pregnant women: a systematic review and meta-analysis. Gynecol. Obstet. Invest. 2025: 90(4): 353-62. https://dx.doi.org/10.1159/000543502
- Subtil D., Brabant G., Tilloy E., Devos P., Canis F., Fruchart A. et al. Early clindamycin for bacterial vaginosis in pregnancy (PREMEVA): a multicentre, double-blind, randomised controlled trial. Lancet. 2018; 392(10160): 2171-9. https://dx.doi.org/10.1016/S0140-6736(18)31617-9
- Wu J., Zhu X., Tang B., Wu J., Wei F., Wang X. et al. Effects of bacterial vaginosis treatment during pregnancy on maternal-fetal outcome: a systematic review and network meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2025; 307: 175-83. https://dx.doi.org/10.1016/j.ejogrb.2025.02.008
- Казанцева В.Д., Озолиня Л.А., Савченко Т.Н., Доброхотова Ю.Э. Современные стратегии лечения и профилактики бактериального вагиноза. РМЖ. Мать и дитя. 2025; 8(1): 27-31. [Kazantseva V.D., Ozolinya L.A., Savchenko T.N., Dobrokhotova Yu.E. Modern treatment tactics and prophylaxis of bacterial vaginosis. Russian Journal of Woman and Child Health. 2025; 8(1): 27-31 (in Russian)]. https://dx.doi.org/10.32364/2618-8430-2025-8-1-4
- Хашукоева А.З., Агаева М.И., Савченко Т.Н., Агаева З.А., Бурденко М.В., Лобачева Ю.И. Рациональное использование антимикробных препаратов в акушерской практике с учетом растущей антибиотикорезистентности. Лечащий врач. 2024; 6(27): 52-7. [Khashukoeva A.Z., Agaeva M.I., Savchenko T.N., Agaeva Z.A., Burdenko M.V., Lobacheva Yu.I. Rational use of antimicrobial drugs in obstetric practice, taking into account the growing antibiotic resistance. Lechashchiy Vrach. 2024; 6(27): 52-7 (in Russian)]. https://doi.org/10.51793/OS.2024.27.6.007
- Карапетян Т.Э., Арзуманян В.Г., Тютюнник В.Л., Кан Н.Е., Ломова Н.А., Канюкина А.А. Бактериальный вагиноз и антимикробная активность во время беременности. Акушерство и гинекология. 2018; 8: 85-90. [Karapetyan T.E., Arzumanyan V.G., Tyutyunnik V.L., Kan N.E., Lomova N.A., Kanyukina A.A. Bacterial vaginosis and antimicrobial activity during pregnancy. Obstetrics and Gynecology. 2018; (8): 85-90 (in Russian)]. https://dx.doi.org/10.18565/aig.2018.8.85-90
- Kumaresan V., Kamaraj Y., Subramaniyan S., Punamalai G. Understanding the dynamics of human defensin antimicrobial peptides: pathogen resistance and commensal induction. Appl. Biochem. Biotechnol. 2024; 196(10): 6993-7024. https://dx.doi.org/10.1007/s12010-024-04893-8
- Аполихина И.А., Саидова А.С., Тетерина Т.А. Эффективность применения локальной цитокинотерапии в комплексном лечении пациенток с хроническим циститом. Акушерство и гинекология. 2019; 12: 167-72. [Apolikhina I.A., Saidova A.S., Teterina T.A. Efficiency of local cytokine therapy used in the combination treatment of female patients with chronic cystitis. Obstetrics and Gynecology. 2019; (12): 167-72 (in Russian)]. https://dx.doi.org/10.18565/aig.2019.12
- Суханов А.А., Дикке Г.Б., Кукарская И.И., Шилова Н.В. Профилактика преждевременных родов у пациенток с бактериальным вагинозом с использованием комплекса природных антимикробных пептидов и цитокинов. Акушерство, гинекология и репродукция. 2024; 18(3): 300-15. [Sukhanov A.A., Dikke G.B., Kukarskaya I.I., Shilova N.V. Prevention of premature birth in patients with bacterial vaginosis using a complex of natural antimicrobial peptides and cytokines. Obstetrics, Gynecology and Reproduction. 2024; 18(3): 300-15 (in Russian)]. https://dx.doi.org/10.17749/2313-7347/ob.gyn.rep.2024.531
- Назарова Н.М., Девяткина А.Р., Межевитинова Е.А., Прилепская В.Н., Алиева Л.Э., Сычева Е.Г., Муравьева В.В. Молочная кислота как ключ к восстановлению микробиоты влагалища: физиологические и клинические аспекты. Акушерство и гинекология. 2025; 5: 40-8. [Nazarova N.M., Devyatkina A.R., Mezhevitinova E.A., Prilepskaya V.N., Alieva L.E., Sycheva E.G., Muravyeva V.V. Lactic acid as a key to restoring the vaginal microbiota: physiological and clinical aspects. Obstetrics and Gynecology. 2025; (5): 40-8 (in Russian)]. https://dx.doi.org/10.18565/aig.2025.125
- Wu L.Y., Yang T.H., Ou Y.C., Lin H. The role of probiotics in women's health: an update narrative review. Taiwan. J. Obstet. Gynecol. 2024; 63(1): 29-36. https://dx.doi.org/10.1016/j.tjog.2023.09.018
Received 20.11.2025
Accepted 15.12.2025
About the Authors
Victor L. Tyutyunnik, Professor, Dr. Med. Sci., Leading Researcher at the Research and Development Service, Academician V.I. Kulakov National Medical Research Centerfor Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, tioutiounnik@mail.ru, Researcher ID: B-2364-2015,
SPIN: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500, https://orcid.org/0000-0002-5830-5099
Dzhamilia D. Mirzabekova, PhD, obstetrician-gynecologist at the 1st Obstetric Physiological Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, Jamilya1705@yandex.ru,
https://orcid.org/0000-0002-2391-3334
Natalia E. Kan, Professor, Dr. Med. Sci., Honored Scientist of the Russian Federation, Deputy Director General for Research – Director of the Institute of Obstetrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow,
Ac. Oparina str., 4, kan-med@mail.ru, Researcher ID: B-2370-2015, SPIN: 5378-8437, Authors ID: 624900, Scopus Author ID: 57008835600,
https://orcid.org/0000-0001-5087-5946
Zarine V. Khachatryan, PhD, obstetrician-gynecologist of the Maternity Department, Perinatal Center of the European Medical Center, 125040, Russia, Moscow, Pravdy str., 15, build. 1, z.v.khachatryan@gmail.com, https://orcid.org/0009-0000-2652-6156
Andrey E. Donnikov, PhD, Head of the Laboratory of Molecular Genetic Methods, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, donnikov@dna-technology.ru, https://orcid.org/0000-0003-3504-2406



