Antiphosphatidylserine/prothrombin complex antibodies in patients with healthy pregnancies
Ischuk V.V., Kerchelaeva S.B., Aleksandrova E.N., Novikov A.A., Khashukoeva I.Z.
Antiphosphatidylserine/prothrombin complex (aPS/PT) antibodies are an additional set of biomarkers that can be used to detect thromboembolic and obstetric complications, in addition to the traditional panel of phospholipid antibodies.
Objective: To determine aPS/PT levels in patients with healthy pregnancies.
Materials and methods: A study of serum aPS/PT IgG and IgM levels was conducted in 50 pregnant women with normal pregnancies during the first, second, and third trimesters of gestation.
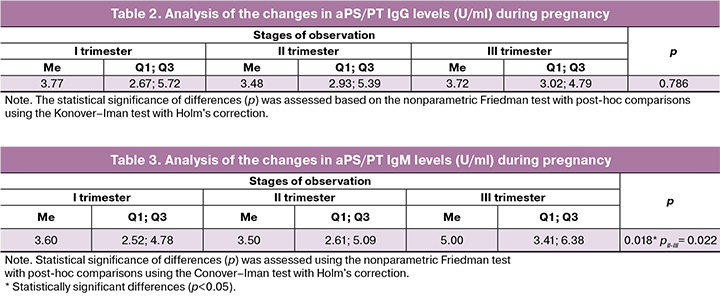
Results: The median level of aPS/PT IgG in pregnant women during the first trimester was 3.77
(2.67; 5.72) U/ml; in the second trimester, it was 3.48 (2.93; 5.39) U/ml; and in the third trimester, it was 3.72 (3.02; 4.79) U/ml. The median level of aPS/PT IgM in these patients was 3.6 (2.61; 5.09) U/ml in the first trimester; 4.1 (2.65; 5.4) U/ml in the second trimester; and 5.0 (3.41; 6.38) U/ml in the third trimester. Statistically significant changes were observed only in the third trimester (p=0.018).
Conclusion: The scientific community recognizes aPS/PT among non-criterial phospholipid antibodies as a promising diagnostic marker that may enhance the effectiveness of antiphospholipid syndrome diagnosis. Further studies involving patients with complicated pregnancies are needed to determine the significance of aPS/PT and confirm their potential use in the clinical practice of obstetricians and gynecologists.
Authors' contributions: Ischuk V.V. – patient examination, review of the relevant literature, drafting of the manuscript; Kerchelaeva S.B. – conception and design of the study, drafting and editing of the manuscript; Aleksandrova E.N. – collection and analysis of literature sources, drafting of the manuscript; Novikov A.A. – literature review, patient examination, drafting of the manuscript; Khashukoeva I.Z. – literature review, drafting of the manuscript. All of the authors contributed significantly to developing the concept, conducting the study, and drafting of the manuscript. They all read and approved the final version before submission.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical approval: The study was reviewed and approved by the Research Ethics Committee of the Pirogov RNRMU, Ministry of Health of Russia (Ref. No: 236 of 22.01.2024).
Patient Сonsent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Ischuk V.V., Kerchelaeva S.B., Aleksandrova E.N., Novikov A.A., Khashukoeva I.Z. Antiphosphatidylserine/prothrombin complex antibodies in patients with healthy pregnancies.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (11): 70-76 (in Russian)
https://dx.doi.org/10.18565/aig.2025.292
Keywords
Antiphosphatidylserine/prothrombin complex (aPS/PT) antibodies target a complex composed of phosphatidylserine, a negatively charged phospholipid found in cell membranes, and prothrombin, a blood plasma protein involved in hemostasis. The interaction between prothrombin and phosphatidylserine on the cell surface is crucial for activating the blood coagulation cascade [1–3]. Numerous obstetric studies on the pathogenesis of antiphospholipid syndrome (APS) have investigated the pathogenetic role of aPS/PT. Similar to the classic serological markers of APS, such as antibodies to cardiolipin (both IgG and IgM isotypes), antibodies to β2-glycoprotein I (both IgG and IgM isotypes) and lupus anticoagulant (aPS/PT) contribute to the activation of endothelial cells and platelets, as well as microcirculation disorders in the placenta. These mechanisms lead to clinical manifestations of APS, including arterial and venous thrombosis and pregnancy complications, such as miscarriage, preeclampsia, eclampsia, placental insufficiency, preterm birth, fetal growth restriction, and neonatal mortality [4–6]. Recent studies have indicated that aPS/PT can serve as an additional set of biomarkers for detecting thromboembolic and obstetric complications, complementing the traditional panel of phospholipid antibodies [7–9]. aPS/PT has garnered significant attention and has been incorporated into the Antiphospholipid score (APL-S) and the Global Antiphospholipid Syndrome Score (GАPSS), which are widely used systems for assessing the thrombotic risk of patients with APS [10, 11]. It is likely that in the near future, risk stratification for patients with APS, including pregnant women, will encompass other aPL or their cofactors, one of which is aPS/PT. Currently, the scientific community recognizes aPS/PT among non-criteria aPL as a promising diagnostic marker that may enhance the effectiveness of APS diagnosis [8, 9, 12]. In this context, we aimed to study the levels of aPS/PT in pregnant women.
This study aimed to determine aPS/PT levels in patients with healthy pregnancies.
Materials and methods
The study was conducted between 2023 and 2024 at the F.I. Inozemtsev City Clinical Hospital of the Moscow Healthcare Department. It involved 54 pregnant women who provided informed consent. All participants were registered at a maternity clinic and were monitored throughout their pregnancies, during childbirth and in the postpartum period.
The inclusion criteria were age 18–40 years and singleton pregnancies. Exclusion criteria were multiple pregnancies, hypertensive disorders, autoimmune diseases, thromboembolic complications, congenital heart defects, chronic kidney disease, adrenal gland disorders, and acute or exacerbated chronic infectious diseases.
Pregnant women were examined in accordance with Order No. 1130n of the Ministry of Health of the Russian Federation, dated October 20, 2020. All the patients underwent blood serum testing for aPS/PT IgG and IgM during pregnancy. aPS/PT IgG and IgM levels were determined using an enzyme-linked immunosorbent assay with a commercial AESKULISA SerinProthrombin-GM reagent kit (AESKU, Germany) on a MINDRAY MR-96A photometric enzyme-linked immunosorbent analyzer (PRC). The results were expressed in U/ml.
Statistical analysis
Statistical analysis was performed using Microsoft Excel 2010 (USA) and StatTech v. 4.8.3 (Russia). Continuous variables with a normal distribution are expressed as mean (M) and standard deviation (SD); otherwise, medians (Me) with interquartile ranges (Q3–Q1) are reported. Categorical data are presented as counts and percentages. For comparisons among three or more dependent populations with non-normal distributions, the nonparametric Friedman test was applied, followed by post-hoc comparisons using the Konover–Iman test with Holm's correction. Differences were considered statistically significant at p<0.05.
Results
The mean age of the patients was 30.1 (4.8) years. Analysis of somatic history revealed no history of familial thrombosis, autoimmune diseases, oncological diseases, hypertensive disorders, chronic kidney disease, diabetes mellitus, or diseases of the central nervous system. However, the following diseases were diagnosed in pregnant women: varicose veins of the lower extremities in 9/54 (16.7%), upper respiratory tract diseases, in particular bronchial asthma, in 2/54 (3.7%), and chronic bronchitis in 3/54 (5.6%). Among gastrointestinal tract diseases, gallstone disease was diagnosed in 1/54 (1.9%) cases, and gastroesophageal reflux disease combined with chronic gastritis was diagnosed in 3/54 (5.6%) cases. Bilateral post-traumatic coxarthrosis was detected in 1/54 (1.9%) patients.
Based on this study, we examined the gynecological history in detail and determined that small uterine fibroids were diagnosed in 2/54 (3.7%) patients, inflammatory diseases of the lower genital tract (vulvovaginal candidiasis, bacterial vaginosis, aerobic vaginitis) in 14/54 (25.9%), polycystic ovary syndrome in 2/54 (3.7%), cervical ectopia in 3/54 (5.6%), and endometriosis in 3/54 (5.6%). Among the examined pregnant women, 1/54 (1.9%) had a history of pregnancy complicated by ectopic localization, 1/54 (1.9%) underwent medical abortion without complications at the patient's request, and in 3/54 (5.5%) patients, the pregnancy ended with a missed miscarriage before 8 weeks of gestation. Parity of the women included in this study was examined during the analysis. The frequencies of primiparous and multiparous pregnancies were 26/54 (48.1%) and 28/54 (51.9%) respectively. The frequencies of primiparous and multiparous births were 31/54 (57.4%) and 23/54 (42.6%), respectively. Analysis of the maternal and perinatal outcomes of previous pregnancies in multiparous women revealed no serious complications during pregnancy, childbirth, or the postpartum period.
When studying the course of the current pregnancy, it was found that the mean body mass index at the time of registration for pregnancy was 21.8 (2.3) kg/m2. All the 54 patients underwent early prenatal screening. Biochemical screening revealed that 3/54 (5.6%) pregnant women were at high risk of preeclampsia, and 1/54 (1.9%) patients were at high risk of both preeclampsia and fetal growth restriction syndrome. Due to abnormal results, we excluded these patients from the study. Analysis of the first-trimester prenatal screening in the remaining 50 patients revealed no abnormalities. The analysis of complications during the current pregnancy is presented in Table 1.

Ultrasound screening in the second trimester of pregnancy revealed low placentation in 1/50 (2%) patients, whereas no pathological changes were found in the remaining 49/50 (98%) women. All women included in the study underwent ultrasound screening during the third trimester of pregnancy. As a result of this study, no abnormalities were found in 49/50 (98%) pregnant women. Placenta previa was recorded in 1/50 (2%) patients. According to antenatal calculation of the risk of venous thromboembolic complications, patients did not require low-molecular-weight heparin during pregnancy.
Serum levels of aPS/PT IgG and IgM were determined in all 50 pregnant women during the first, second, and third trimesters of gestation. The results of statistical processing using the nonparametric Friedman test with a posteriori comparisons using the Konover–Iman test with Holm's correction of aPS/ PT IgG levels during pregnancy show that aPS/PT IgG levels in pregnant women did not demonstrate statistically significant changes over time (Table 2). aPS/PT IgM levels were also evaluated in these patients. During pregnancy, statistically significant changes in the progression of gestation were noted only in the third trimester (Table 3).
During the study, we analyzed the course of labor in all pregnant women. Upon admission to the maternity hospital for delivery, all patients underwent complete clinical, laboratory, and instrumental examinations. Based on the analysis of laboratory test results obtained upon admission to the maternity hospital for delivery, the following mean values were calculated: platelets – 221.3 (34.2)×109/L, activated partial thromboplastin time – 27.8 (2.6) s, prothrombin time – 13.2 (1.9) s, international normalized ratio – 1.1 (0.2), fibrinogen – 4.9 (1.9) g/L.
All 50 patients delivered at full-term gestation, ranging from 38.6 to 40.5 weeks, with a median of 39.4 weeks. Vaginal delivery occurred in 43/50 (83.3%) patients. Complications during labor in the form of premature rupture of membranes occurred in 9/50 (18%) of women in labor, weakness of labor in 3/50 (6%), 1st degree perineal rupture in 9/50 (18%), 2nd degree perineal rupture in 4/50 (8%) of women in labor. Median blood loss during vaginal delivery was 286.5 (246.3; 318.8) ml. Of the 50 pregnant women, seven (14%) underwent planned cesarean section. Indications for operative delivery were deformation of the pelvic bones (bilateral posttraumatic coxarthrosis) in 1/50 (2%) cases, placenta previa in 1/50 (2%) cases, and breech presentation in combination with a large fetus in 2/50 (4%) cases. In 3/50 (6%) cases, the indication for operative delivery was failure of oxytocin induction. The median blood loss during operative delivery was 636.5 (546.3; 668.8) ml.
After calculating the risk of venous thromboembolic complications in the postpartum period, 11/50 (22%) patients required prophylactic low molecular weight heparin. In the postpartum period, antianemic therapy was required in 13/50 (26%) women due to mild anemia and in 4/50 (8%) women due to moderate anemia.
We analyzed the neonatal outcomes of the mothers included in the study. At delivery, the mean birth weight was 3468.9 (511.9) g, and the median height was 52.5 (51–54) cm. All newborns had Apgar scores of 8–10 in the first and fifth minute. After delivery, all 50 newborns were in a satisfactory condition, and the mother and baby were kept together. No neonatal complications were diagnosed in the maternity hospital for the newborns of the mothers included in the study. All mothers were discharged home with their newborns in satisfactory condition on days 3–4 under the supervision of an obstetrician-gynecologist and a local pediatrician, with recommendations.
One of the objectives of our study was to analyze placental morphology. Normal placental histology was obtained in 43/50 of the (86%) patients. Moderate compensatory changes in the morphological assessment of the placenta, characterized by chronic placental insufficiency, were detected in 7/50 (14%) women. Chronic compensated placental insufficiency included the following histological features: widespread involutional-dystrophic changes in the placenta and the presence of afunctional zones.
Discussion
In this study, we aimed to determine the serum aPS/PT levels in healthy pregnancies. Initially, the study cohort consisted of pregnant women without severe somatic or gynecological pathologies. The multiparous mothers had no history of pregnancy-related complications. Complications identified during current pregnancy, such as vomiting, gestational diabetes mellitus, placenta previa, and polyhydramnios, are common in the general population. Laboratory coagulation parameters did not exceed the reference values, and all pregnancies were concluded with timely delivery. The study results demonstrated a typical prevalence of complications during childbirth, including premature rupture of membranes, labor dystocia, and perineal tears. After delivery, no obstetric or perinatal complications were noted in either the mother or newborn. Consequently, no clinical manifestations indicative of APS were identified during pregnancy, delivery, or the postpartum period, and both the maternal and perinatal outcomes were favorable.
Recently, numerous studies have investigated aPS/PT in patients with rheumatic disease. Reshetnyak T.M. et al. [13] reported that the median levels of IgG and aPS/PT IgM in 100 healthy individuals were 10.9 (8.3; 15.0) U/ml and 2.4 (0.1; 4.4) U/ml, respectively. In our study, the median level of aPS/PT IgG in patients with a physiological course of pregnancy was slightly lower than that in the healthy cohort, whereas the levels of aPS/PT IgM were comparable. Classic laboratory markers, including antibodies to cardiolipin, antibodies to β2-glycoprotein I, and lupus anticoagulant, have been extensively studied in patients with healthy pregnancies and newborns [14]. Elbagir et al. [15] found a statistically significant difference in the levels of IgG and IgM isotypes of antibodies to cardiolipin and β2-glycoprotein I between non-pregnant and pregnant women. The authors suggested that this difference may be attributed to physiological changes that occur in the female body during pregnancy. This finding underscores the need to establish aPS/PT IgG/IgM levels in patients across the first, second, and third trimesters of a healthy pregnancy, an area that has not been previously explored.
The scientific community is increasingly interested in aPS/PT as a serological marker for detecting “seronegative” APS, characterized by clear clinical signs of APS despite persistently negative results according to classic laboratory criteria [16, 17]. The potential use of aPS/PT as an alternative test for lupus anticoagulant, particularly in patients receiving anticoagulant therapy, has also been examined [18]. Furthermore, a study indicated that “tetrapositivity,” which includes three classic aPL (antibodies to cardiolipin, antibodies to β2-glycoprotein I, and lupus anticoagulant) along with aPS/PT, increases the risk of developing APS-related complications compared to triple positivity [19]. It has also been established that circulation of aPS/PT is closely associated with thrombosis [20]. Several researchers have highlighted the clinical significance of aPS/PT in obstetric practice for diagnosing APS [6, 21, 22]. A review by Radin M. et al. [21] demonstrated a strong correlation between positive aPS/PT IgG and IgM test results and pregnancy pathology, with an odds ratio (OR) of 10.6 (95% confidence interval (CI): 3.54–35.38, p<0.05), particularly for aPS/PT IgG (OR 6.7; 95% CI: 3.04–21.6, p<0.05). Additionally, aPS/PT was significantly associated with pregnancy complications such as preterm birth (odds ratio [OR], 7.5; 95% CI: 1.4–40.8, p=0.008), preeclampsia, and fetal growth restriction. Moreover, aPS/PT IgG levels were inversely proportional to the birth weight of newborns, and histological examinations of the placenta in patients with fetal growth restriction and positive aPS/PT results revealed multiple markers of vascular damage [6, 22].
The existing literature on aPS/PT IgG and IgM in pregnant women and their impact on maternal and perinatal outcomes is less extensive than that on traditional aPL. Although aPS/PT levels have been proposed as promising diagnostic markers for APS, their association with complications during pregnancy remains debatable and requires further investigation.
Conclusion
In our study, we examined the aPS/PT IgG/IgM levels in healthy pregnancies. Based on our findings, we calculated the aPS/PT IgG/IgM levels across the first, second, and third trimesters of healthy pregnancies. Currently, the scientific community recognizes aPS/PT as a promising diagnostic marker among non-criterial aPLs, which may enhance the effectiveness of APS diagnosis.
Therefore, further research involving patients with complicated pregnancies is warranted to determine the significance of aPS/PT and confirm its potential application in the clinical practice of obstetricians and gynecologists.
References
- Bradacova P., Slavik L., Ulehlova J., Skoumalova A., Ullrychova J., Prochazkova J. et al. Current promising biomarkers and methods in the diagnostics of antiphospholipid synd rome: a review. Biomedicines. 2021; 9(2): 166. https://dx.doi.org/10.3390/biomedicines9020166
- Atsumi T., Ieko M., Bertolaccini M.L., Ichikawa K., Tsutsumi A., Matsuura E. et al. Association of autoantibodies against the phosphatidylserine-prothrombin complex with manifestations of the antiphospholipid syndrome and with the presence of lupus anticoagulant. Arthritis Rheum. 2000; 43(9): 1982-93. https://dx.doi.org/10.1002/1529-0131(200009) 43:93.0.CO;2-2
- Александрова Е.Н., Новиков А.А., Лукина Г.В. Новые аспекты лабораторной диагностики антифосфолипидного синдрома. Лабораторная медицина. 2024; 15(1-2): 25-30. [Aleksandrova E.N., Novikov A.A., Lukina G.V. New aspects of laboratory diagnostics of antiphospholipid syndrome. Laboratory medicine. 2024; 15(1-2): 25-30 (in Russian)]. https://dx.doi.org/10.58953/15621790_2024_15_1-2_25
- Alijotas-Reig J., Esteve-Valverde E., Anunciación-Llunell A., Marques-Soares J., Pardos-Gea J., Miró-Mur F. Pathogenesis, diagnosis and management of obstetric antiphospholipid syndrome: a comprehensive review. J. Clin. Med. 2022; 11(3): 675. https://dx.doi.org/10.3390/jcm11030675
- Liu L, Sun D. Pregnancy outcomes in patients with primary antiphospholipid syndrome: a systematic review and meta-analysis. Medicine (Baltimore). 2019; 98(20): e15733. https://dx.doi.org/10.1097/MD.0000000000015733
- D’Ippolito S., Barbaro G., Paciullo C., Tersigni C., Scambia G., Di Simone N. Antiphospholipid syndrome in pregnancy: new and old pathogenetic mechanisms. Int. J. Mol. Sci. 2023; 24(4): 3195. https://dx.doi.org/10.3390/ijms24043195
- Canti V., Del Rosso S., Tonello M., Lucianò R., Hoxha A., Coletto L.A. et al. Antiphosphatidylserine / prothrombin antibodies in antiphospholipid syndrome with intrauterine growth restriction and preeclampsia. J. Rheumatol. 2018; 45(9): 1263-72. https://dx.doi.org/10.3899/jrheum.170751
- Moyle K.A., Branch D.W., Peterson L.K., Guerra M.M., Allshouse A.A., Benson A.E. et al. Association between novel antiphospholipid antibodies and adverse pregnancy outcomes. Obstet. Gynecol. 2025; 145(1): 55-64. https://dx.doi.org/10.1097/AOG.0000000000005729
- Xiang J., Pan Y., Bao R., Cai Z. Correlation of anti-phosphatidylserine / prothrombin and anti-phosphatidylserine antibodies with pregnancy outcomes. Am. J. Reprod. Immunol. 2024; 92(1): e13890. https://dx.doi.org/10.1111/aji.13890
- Otomo K., Atsumi T., Amengual O., Fujieda Y., Kato M., Oku K. et al. Efficacy of the antiphospholipid score for the diagnosis of antiphospholipid syndrome and its predictive value for thrombotic events. Arthritis Rheum. 2012; 64(2): 504-12. https://dx.doi.org/10.1002/art.33340
- Sciascia S., Sanna G., Murru V., Roccatello D., Khamashta M.A., Bertolaccini M.L. GAPSS: the global anti-phospholipid syndrome score. Rheumatology (Oxford). 2013; 52(8): 1397-403. https://dx.doi.org/10.1093/rheumatology/kes388
- Менжинская И.В., Ванько Л.В., Кречетова Л.В., Тетруашвили Н.К., Сухих Г.Т. Спектр, иммунохимические свойства и диагностическое значение антифосфолипидных антител у женщин с ранним привычным выкидышем. Акушерство и гинекология. 2020; 8: 39-46. [Menzhinskaya I.V., Vanko L.V., Krechetova L.V., Tetruashvili N.K., Sukhov G.T. Spectrum, immunochemical properties and diagnostic value of antiphospholipid antibodies in women with early habitual miscarriage. Obstetrics and Gynecology. 2020; (8): 39-46 (in Russian)]. https://dx.doi.org/10.18565/aig.2020.8.39-46
- Решетняк Т.М., Чельдиева Ф.А., Черкасова М.В., Лила А.М., Насонов Е.Л. Антитела к комплексу фосфатидилсерин/протромбин у пациентов с антифосфолипидным синдромом. Терапевтический архив. 2022; 94(5): 628-34. [Reshetnyak T.M., Cheldieva F.A., Cherkasova M.V., Lila A.M., Nasonov E.L. Antibodies to the phosphatidylserine / prothrombin complex in the diagnosis of antiphospholipid syndrome. Ter. Arkh. 2022; 94(5): 628-34 (in Russian)]. https://dx.doi.org/10.26442/00403660.2022.05.201488
- Озолиня Л.А. Керчелаева С.Б., Лапина И.А., Макаров О.В. Венозные тромбоэмболические осложнения в акушерстве и гинекологии: учебник. 2-е изд., испр. и доп. М.: ГЭОТАР-Медиа; 2020. 288 с. [Ozolinja L.A. Kerchelaeva S.B., Lapina I.A., Makarov O.V. Venous thromboembolic complications in obstetrics and gynecology: textbook. 2nd ed., rev. and exp. Moscow: GEOTAR-Media; 2020. 288 p. (in Russian)].
- Elbagir S., Mohammed N.A., Kaihola H., Svenungsson E., Gunnarsson I., Manivel V.A. et al. Elevated IgA antiphospholipid antibodies in healthy pregnant women in Sudan but not Sweden, without corresponding increase in IgA anti-β2 glycoprotein I domain 1 antibodies. Lupus. 2020; 29(5): 463-73. https://dx.doi.org/10.1177/0961203320908949
- Ding Z., Pan H., Yang Z., Yang C., Shi H. Beyond the classics: the emerging value of anti-phosphatidylserine / prothrombin antibodies in antiphospholipid syndrome. Clin. Immunol. 2023; 256: 109804. https://dx.doi.org/10.1016/j.clim.2023.109804
- Truglia S., Riitano G., Mancuso S., Recalchi S., Rapino L., Garufi C. et al. Antibody profiles in the mosaic of 'seronegative' APS syndrome. Clin. Exp. Immunol. 2024; 218(3): 275-82. https://dx.doi.org/10.1093/cei/uxae079
- Saadalla A., Nandakumar V. Anti-phosphatidyl-serine / prothrombin (aPS / PT) antibodies are superior predictors of LAC presence and APS diagnoses: a single center study. Clin. Chim. Acta. 2024; 554: 117761. https://dx.doi.org/10.1016/j.cca.2024.117761
- Cattini M.G., Bison E., Pontara E., Cheng C., Denas G., Pengo V. Tetra positive thrombotic antiphospholipid syndrome: major contribution of anti-phosphatidyl-serine / prothrombin antibodies to lupus anticoagulant activity. J. Thromb. Haemost. 2020; 18(5): 1124-32. https://dx.doi.org/10.1111/jth.14765
- Knight J.S., Branch D.W., Ortel T.L. Antiphospholipid syndrome: advances in diagnosis, pathogenesis, and management. BMJ. 2023; 380: e069717. https://dx.doi.org/10.1136/bmj-2021-069717
- Radin M., Foddai S.G., Cecchi I., Rubini E., Schreiber K., Roccatello D. et al. Antiphosphatidylserine / prothrombin antibodies: an update on their association with clinical manifestations of antiphospholipid syndrome. Thromb. Haemost. 2020; 120(4): 592-8. https://dx.doi.org/10.1055/s-0040-1705115
- Žigon P., Perdan Pirkmajer K., Tomšič M., Kveder T., Božič B., Sodin Šemrl S. et al. Anti-phosphatidylserine / prothrombin antibodies are associated with adverse pregnancy outcomes. J. Immunol. Res. 2015; 2015: 975704. https://dx.doi.org/10.1155/2015/975704
Received 17.10.2025
Accepted 11.11.2025
About the Authors
Valeriya V. Ischuk, PhD student at the Departments of Obstetrics and Gynecology, Institute of Surgery, N.I. Pirogov Russian National Research Medical University,Ministry of Health of Russia, 117513, Russia, Moscow, Ostrovityanova str., 1. lerchonokmark@mail.ru, https://orcid.org/0000-0003-4328-2370
Svetlana B. Kerchelaeva, Dr. Med. Sci., Professor at the Departments of Obstetrics and Gynecology, Institute of Surgery, N.I. Pirogov Russian National Research Medical University, Ministry of Health of Russia, 117513, Russia, Moscow, Ostrovityanova str., 1, ksb65@mail.ru, https://orcid.org/0000-0002-4411-4478
Elena N. Aleksandrova, Dr. Med. Sci., Head of the Laboratory of Clinical Immunology, A.S. Loginov Moscow Clinical Scientific Center, Moscow Healthcare Department, 111123, Russia, Moscow, Novogireevskaya str., 1, aleksandrovaen2015@yandex.ru, https://orcid.org/0000-0003-4074-5907
Alexander A. Novikov, Dr. Bio. Sci., Leading Researcher at the Laboratory of Clinical Immunology, A.S. Loginov Moscow Clinical Scientific Center, Moscow Healthcare Department, 111123, Russia, Moscow, Novogireevskaya str., 1, irramnlab@yandex.ru, https://orcid.org/0000-0002-2738-2956
Ilona Z. Khashukoeva, student, H.M. Berbekov Kabardino-Balkarian State University, 360004, Russia, Nalchik, Chernyshevsky str., 173, ilona_1017@mail.ru,
https://orcid.org/0009-0000-4378-910X
Corresponding author: Valeriya V. Ischuk, lerchonokmark@mail.ru



