Perinatal outcomes and characteristics of placental disorders in term pregnancies among convalescents after novel coronavirus infection (COVID-19) experienced at different gestational ages
Kazachkova E.A., Voropaeva E.E., Ishchenko L.S., Kazachkov E.L., Kholopova A.Yu., Zatvornitskaya A.V., Shamaeva T.N.
Objective: To evaluate perinatal outcomes and the characteristics of placental disorders in term pregnancies among convalescents after COVID-19, experienced at different gestational ages.
Materials and methods: This study included 120 patients. The main group consisted of 80 women who gave birth at a gestational age of 370–416 weeks during the recovery period after COVID-19 and experienced various trimesters of pregnancy. The control group included 30 women without COVID-19 during their current pregnancy, who also gave birth at term. A retrospective analysis was conducted to assess obstetric and gynecological history, somatic pathology, course of pregnancy and childbirth, perinatal outcomes, and placental disorders.
Results: Convalescents after COVID-19, regardless of gestational age, who received low molecular weight heparin in prophylactic doses and vitamins C, E, and D in an extended regimen, exhibited favorable perinatal outcomes in term pregnancies. The most pronounced histological changes indicative of placental dysfunction were observed in cases in which COVID-19 was experienced during the second and third trimesters. In the placentas of convalescents at term, there was a statistically significant increase in the expression of the hypoxia markers HIF-1α and EPO compared to the control group. Additionally, a statistically significant increase in the apoptosis marker Caspase-3 was noted in the placentas of convalescents who experienced COVID-19 during the first and second trimesters.
Conclusion: Favorable perinatal outcomes were observed in convalescents with term pregnancies, regardless of the trimester in which the disease first manifested. However, pronounced histological and immunohistochemical changes in the placenta suggest the presence of placental insufficiency. Therefore, it is advisable to closely monitor this cohort of newborns from infancy to implement timely measures aimed at reducing the risk of potential long-term consequences.
Authors' contributions: Voropaeva E.E., Ishchenko L.S., Kazachkova E.A. – conception and design of the study; Voropaeva E.E., Ishchenko L.S. – data collection and processing; Ishchenko L.S., Kholopova A.Yu., Zatvornitskaya A.V., Shamaeva T.N. – data analysis; Kazachkova E.A., Ishchenko L.S. – drafting of the manuscript; Kazachkova E.A., Kazachkov E.L. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the South Ural State Medical University, Ministry of Health of the Russian Federation, Russia (Ref. No: 8 of 20.09.2021).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Kazachkova E.A., Voropaeva E.E., Ishchenko L.S., Kazachkov E.L., Kholopova A.Yu., Zatvornitskaya A.V., Shamaeva T.N. Perinatal outcomes and characteristics
of placental disorders in term pregnancies among convalescents
after novel coronavirus infection (COVID-19) experienced at different gestational ages.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (8): 100-108 (in Russian)
https://dx.doi.org/10.18565/aig.2025.168
Keywords
The placenta is a provisional organ that is essential for fetal life and offers protection against adverse effects. Effective placental function is crucial for successful pregnancy [1].
Placental insufficiency (PI), characterized by intrauterine hypoxia and fetal growth restriction (FGR), is a leading cause of perinatal morbidity and mortality. Additionally, PI is linked to impaired adaptation processes in newborns and negatively affects their future health [2].
PI arises when compensatory adaptive processes at the molecular, cellular, and tissue levels are disrupted owing to pathological changes in the uteroplacental and fetoplacental complexes. The causes of PI are numerous and diverse, often related to chronic maternal extragenital pathology, pregnancy complications, placental abnormalities, and fetal issues [3]. Infection is one of the factors that contribute to PI [2]. Therefore, it is important to investigate the role of the novel coronavirus disease (COVID-19) in the development of PI.
COVID-19 is associated with a complex array of adverse effects, including hypoxic, inflammatory, and thrombotic factors, that affect the entire body of a pregnant woman [4]. The placenta, an independent and newly formed organ, is not exempt from these effects. Numerous studies have documented the development of PI in the context of both acute COVID-19 [5–9] and in convalescents [10–12], regardless of the severity and timing of the infection. Histological examination of placentas at various gestational ages has revealed features indicative of PI, such as maternal and/or fetal malperfusion, massive fibrinoid deposition, histiocytic intervillitis, trophoblast necrosis, intervillous hemorrhages and thromboses, deciduitis, and villitis. However, the severity and nature of these placental disorders can vary among studies.
Gutikova et al. established statistically significant differences (p<0.05) in the frequency of FGR in convalescents after COVID-19 – 15/69 (21.7%) cases (gestational age at the time of delivery 265 (262–270) days) compared to pregnant women without COVID-19 – 0/25 (0.0%) cases (gestational age at the time of delivery 273 (270–275) days). In addition, the study noted a statistically significant (p<0.05) predominance of FGR frequency in convalescents with COVID-19 manifestation in the I–II trimesters (13/36 (36.1%) cases) compared to the manifestation of infection in the III trimester (2/33 (6.1%) cases). During the pathomorphological study of placentas, a statistically significantly lower mass (p<0.05), size (p<0.05), and thickness (p<0.05) of the placenta were determined in the group of convalescents after COVID-19 than in the group without COVID-19 [10]. Borovaya S.Yu. et al. revealed a statistically significant increase in the frequency of FGR (p=0.02) and fetal distress (p=0.002) in convalescents after COVID-19 with manifestation up to 34 weeks of gestation compared to pregnant women without COVID-19. In the early neonatal period, meconium aspiration was observed significantly more often in newborns from convalescents with COVID-19 manifestation up to 16 weeks of pregnancy (p=0.001), pneumonia in newborns from convalescents with COVID-19 manifestation up to 16 weeks and from 17 to 34 weeks of pregnancy (p<0.001), which required hospitalization in the intensive care unit for newborns [11]. Dagelic A. et al. did not reveal statistically significant differences in the birth weight (p=0.780) and body length (p=0.874) of newborns from patients after COVID-19 in any trimester [12].
Given this ambiguous information, it is advisable to further investigate perinatal outcomes and the characteristics of placental disorders in patients with COVID-19, particularly those experienced at different gestational ages.
This study aimed to evaluate perinatal outcomes and the characteristics of placental disorders in term pregnancies among convalescents after COVID-19, experienced at different gestational ages.
Materials and methods
A retrospective study was conducted involving 80 patients who gave birth at term during the convalescence period after COVID-19. The study group was divided into three subgroups: the first subgroup consisted of 20 women with COVID-19 in the first trimester, the second subgroup included 30 patients with COVID-19 in the second trimester, and the third subgroup comprised 30 women with COVID-19 in the third trimester of gestation. All patients received treatment for acute COVID-19 at the COVID hospital in Chelyabinsk from April 2020 to December 2021, covering the first four waves of the pandemic.
The control group consisted of 30 women who delivered in a timely manner at the obstetric hospital of Regional Clinical Hospital No. 3 in Chelyabinsk between July 2020 and February 2021.
To evaluate the expression levels of immunohistochemical (IHC) markers of hypoxia (hypoxia-inducible factor 1α [HIF-1α] and erythropoietin [EPO]) and apoptosis (Caspase-3), 60 placentas were randomly selected: 15 placentas from each subgroup and a control group.
The inclusion criteria were as follows: delivery at 370–416 weeks of gestation, reproductive age of the woman, availability of medical records, informed consent to participate in the study, and agreement to the publication of results in the public domain.
Additional criteria for the study group included a convalescence period of more than 30 days after confirmed COVID-19 (U07.1) during the current pregnancy, complex treatment for COVID-19 during hospitalization based on infection severity in accordance with current guidelines, and continuation of low-molecular-weight heparin prophylaxis for up to 6 weeks after discharge, along with vitamin and mineral complexes for pregnant women containing vitamins C, E, and D throughout the remainder of the pregnancy.
For the control group, the criteria included the absence of COVID-19 or respiratory viral infections during the current pregnancy, and a negative result for SARS-CoV-2 based on nasopharyngeal material at the time of delivery.
Exclusion criteria included multiple pregnancies, decompensated somatic diseases, HIV infection, malignant or mental illnesses, and perinatal death or congenital malformations of the fetus.
Morphological examination of placentas was conducted at the Chelyabinsk Regional Pathological Anatomy Bureau, which is affiliated with the Department of Anatomical Pathology and Forensic Medicine at the South Ural State Medical University, Ministry of Health of Russia. Histological examination of the placentas was performed using standard methods to assess placental disorders according to the Amsterdam Placenta Workshop Group classification. IHC studies were performed manually using the N-Histofine Simple Stain MAX PO (MULTI) visualization system with DAB chromogen and ready-to-use rabbit mAbs. Hypoxia in the placenta was assessed using monoclonal antibodies against HIF-1α (clone EP118, manufacturer Epitomics) and EPO (Cat. No. GTX112834, manufacturer GeneTex Inc.). Apoptosis was studied using monoclonal antibodies against Caspase-3 (clone ET 1602-39, manufacturer Hangzhou Huaan Biotechnology Co., Ltd). The area of brown staining in the cytoplasm of trophoblast cells was calculated to determine the expression levels of the IHC markers. Histo scans of histological micropreparations were obtained using a Hamamatsu digital microscope (NanoZoomer S210, Japan) and digitally archived. Automated counting of IHC-positive structures was performed using QuPath digital bioimage analysis software.
The study was approved by the Research Ethics Committee of the South Ural State Medical University, Ministry of Health of the Russian Federation (Ref. No: 8 of 20.09.2021). Informed consent was obtained from all the participants.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 19. Quantitative variables are presented as Me (Q1; Q3), where Me is the median, and Q1 and Q3 are the first and third quartiles, respectively, due to deviations from the normal distribution identified by the Shapiro–Wilk test. The statistical significance of differences in these variables was assessed using Kruskal–Wallis and Mann–Whitney tests. Qualitative parameters are presented as counts (n) and percentages (%). For analyzing contingency tables, the Pearson χ² test or Fisher's exact test was used when the conditions for the Pearson χ² test were not met. Differences were considered statistically significant at p<0.05. For multiple pairwise comparisons, the Bonferroni correction was applied, resulting in a corrected significance level of p≤0.008.
Results and discussion
The patients in the study groups were of a similar age: 32 (29.3; 35.8), 31.5 (27.8; 35.0), 31.0 (26.0; 35.0), and 29.5 (24.0; 34.0) years, respectively in the 1st-3rd subgroups and the control group (p=0.432).
In the 1st study subgroup, no patients had severe COVID-19, which led to statistical differences in this parameter. Mild infection was observed in 12/20 (60.0%), 10 (33.3%), 10 (33.3%) women, moderate – in 8/20 (40.0%), 10 (33.3%), 14 (46.7%) patients, severe – in 0 (0.0%), 10 (33.3%), 6 (20.0%) women in the 1st-3rd subgroups, respectively (p=0.040, p1,2=0.013, p1,3=0.042, p2,3=0.435).
Analysis of obstetric and gynecological history showed the overall homogeneity of the study groups by the main parameters. A statistically significant difference was found only in cesarean section (CS) in the history of the patients in the study groups (Table 1).
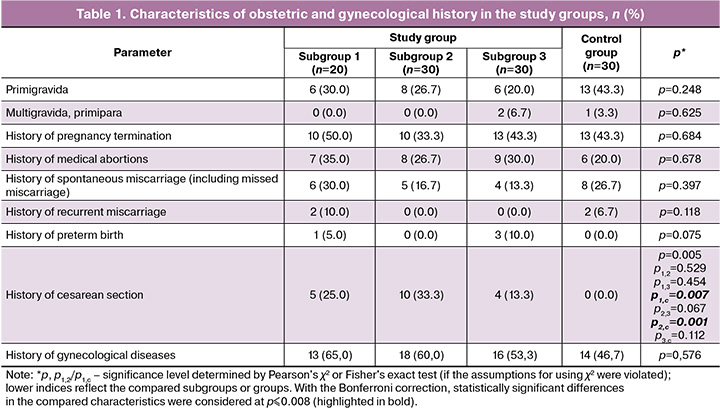
No statistically significant differences were found in the pregnancy characteristics during convalescence between the study and control groups (Table 2). Fetal distress during pregnancy and premature placental abruption were not observed.
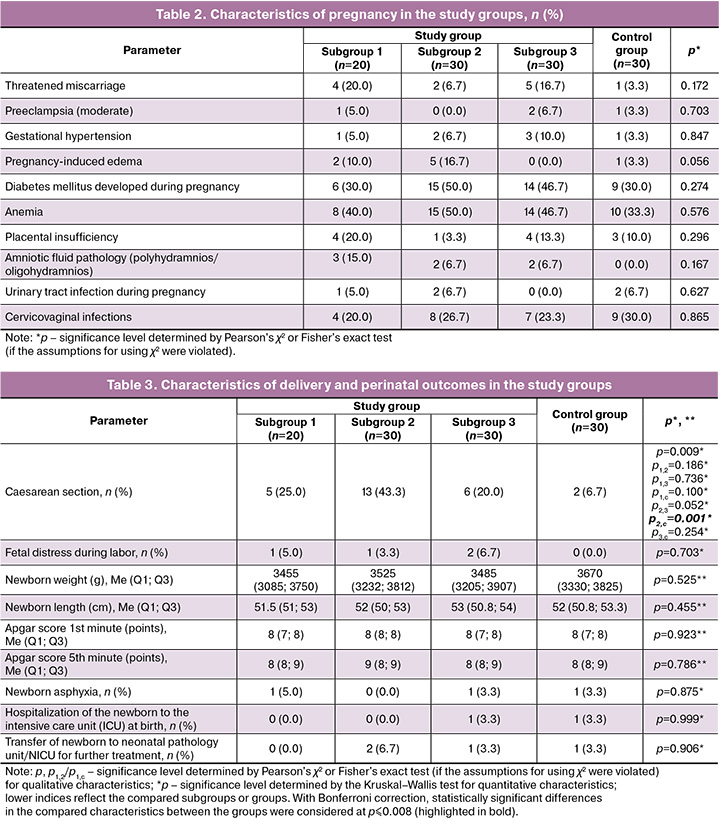
Convalescents after COVID-19 manifestation in the II trimester statistically significantly more often underwent cesarean section compared to women in the control group (p2,с=0.001) and 1.7 and 2.2 times more often compared to convalescents after COVID-19 manifestation in the I and III trimesters, respectively, without statistically significant difference (p1,2=0.186; p2,3=0.052). No statistically significant differences were noted in the perinatal outcomes between the study groups. Anthropometric data and Apgar scores of newborns in convalescents, regardless of the COVID-19 manifestation trimester, and in the control group did not differ significantly (Table 3).
Despite favorable perinatal outcomes in the study groups, we found a statistically higher frequency of placental disorders of varying severity in the placentas of convalescents after COVID-19 in different trimesters of gestation (Table 4). Thus, in convalescents, regardless of the COVID-19 manifestation trimester, compared to placentas of women without COVID-19, chronic inflammation in the form of lymphoplasmacytic deciduitis was statistically significantly more frequently registered (p1,с=0.001; p2,с=0.007; p3,с<0.001). These data are consistent with other studies showing a significantly higher frequency of deciduitis and villitis in placentas of convalescents after COVID-19 [10, 11, 16]. Pronounced signs of maternal and fetal malperfusion were observed. Villous vessel thrombosis was significantly more often detected in convalescents in the 2nd and 3rd subgroups than in the control group (p2,с<0.001; p3,с=0.005), which may indicate the persistence of thrombotic disorders in placentas by full-term pregnancy with COVID-19 manifestation in the II and III trimesters. Avascular villous groups were observed significantly more often in the 2nd subgroup relative than in the control group (p2,с=0.005). Decidual arteriopathy was significantly more often detected in the placentas of the 2nd and 3rd subgroups than in the control group (p2,с=0.001; p3,с=0.005). Villous agglutination was observed with statistically significant predominance in the study group placentas (p1,с=0.007; p2,с<0.001; p3,с=0.004). Massive perivillous fibrinoid deposition was significantly more predominant in the 2nd subgroup more often that in the control group (р2,с=0.004). The obtained results are consistent with studies demonstrating a high frequency of maternal [10, 11] and fetal [10, 11, 17] malperfusion in the placentas of convalescents after COVID-19.
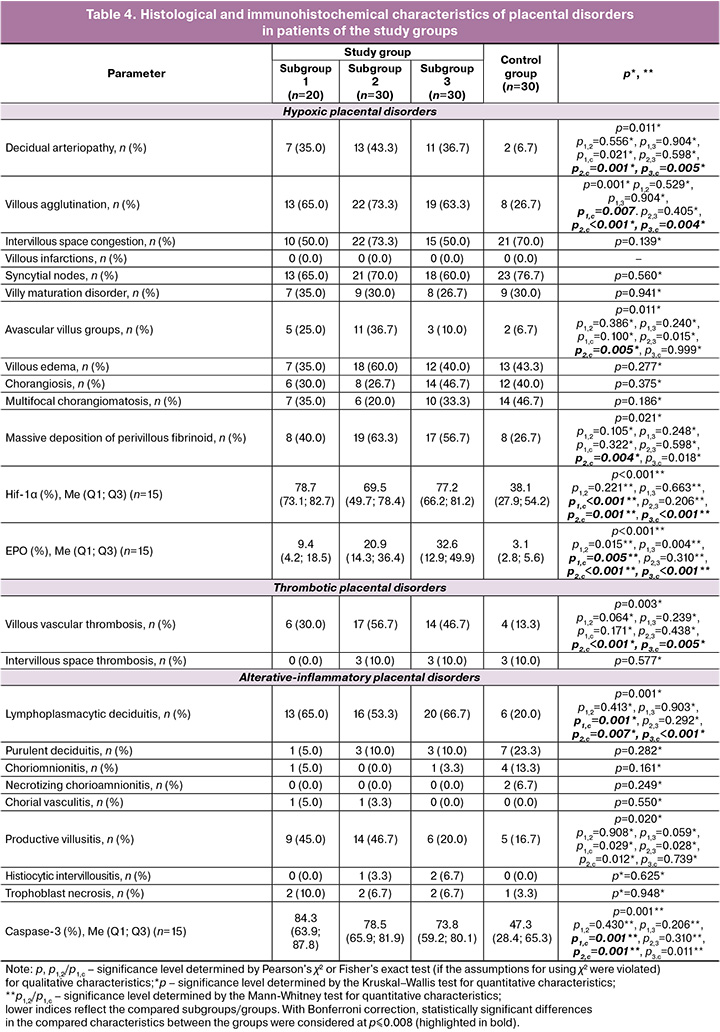
We registered a statistically significantly higher expression level of HIF-1α (p1,с<0.001; p2,с=0.001; p3,с<0.001) and EPO (p1,с=0.005; p2,с<0.001; p3,с<0.001) in placentas of convalescents after COVID-19, regardless of the infection manifestation trimester, relative to placentas of pregnant women without COVID-19 (Table 4). The Caspasе-3 expression level statistically significantly predominated in placentas of convalescents in the 1st (p1,с=0.001) and 2nd (p2,с=0.001) subgroups relative to the control group (Table 4).
Several studies have established a statistically significant increase in HIF-1α expression in the placentas of women with PI [18, 19]. Albers R.E. et al. reported significant changes in the branching and remodeling processes of spiral arteries in response to prolonged high levels of HIF-1α expression [20]. Few studies have assessed the severity of hypoxia and apoptosis in the placentas of individuals recovering from COVID-19. For instance, Voropaeva E.E. et al. demonstrated a statistically significant increase in HIF-1α expression (p<0.001) and a decrease in the expression of apoptosis-inducing factor (AIF) (p<0.001) in the placentas of convalescents compared to those in acute COVID-19 with critical lung damage during preterm labor [21]. Our findings of elevated HIF-1α and EPO expression suggest enhanced anti-hypoxic activity in the placentas of convalescents, regardless of the trimester in which COVID-19 occurred. Additionally, a higher level of apoptosis was observed in the placentas of convalescents compared to those of women without COVID-19, with statistically significant differences noted after COVID-19 in the first and second trimesters (p1,c=0.001 and p2,c=0.001, respectively). The simultaneous presence of high EPO and Caspase-3 expression, along with inflammatory changes in the placentas of convalescents, indicates a disruption in the balance between pro-apoptotic and anti-apoptotic mechanisms, particularly after COVID-19 before 28 weeks of gestation. Pronounced apoptosis may contribute to PI development by disrupting the physiological processes of placental angiogenesis. Prophylactic administration of low-molecular-weight heparin, along with vitamins C, E, and D throughout pregnancy, can help compensate for functional disorders in the placenta, leading to favorable perinatal outcomes [12, 22].
Currently, PI of various origins is considered a model for fetal programming that may lead to structural pathologies and functional disorders in organs and systems later in life [3, 23–25]. This necessitates the careful monitoring of newborns to implement timely preventive measures to mitigate the risks of potential long-term consequences.
Conclusion
In conclusion, convalescents at term pregnancy, regardless of the trimester in which COVID-19 first manifested., tend to experience favorable perinatal outcomes. However, significant histological and immunohistochemical changes in the placenta suggest the presence of PI, likely compensated by the prolonged use of low-molecular-weight heparin in prophylactic doses and vitamins C, E, and D throughout pregnancy. In light of fetal programming theory, the existence of placental disorders poses a risk for the development of various chronic pathologies in children. Therefore, it is advisable to closely monitor this cohort of newborns from infancy to ensure timely prevention aimed at reducing the risk of potential long-term consequences.
References
- Kreis N.N., Ritter A., Louwen F., Yuan J. A message from the human placenta: structural and immunomodulatory defense against SARS-CoV-2. Cells. 2020; 9(8): 1777. https://dx.doi.org/10.3390/cells9081777
- Пестрикова Т.Ю., Юрасова Е.А., Ткаченко В.А. Плацентарная недостаточность как базовая патология осложнений и исходов гестационного периода. Российский вестник акушера-гинеколога. 2020; 20(1): 5-15. [Pestrikova T.Iu., Iurasova E.A., Tkachenko V.A. Placental insufficiency as the underlying condition of the complications and outcomes of the gestation period. Russian Bulletin of Obstetrician-Gynecologist. 2020; 20(1): 5-15 (in Russian)]. https://dx.doi.org/10.17116/rosakush2020200115
- Тапильская Н.И., Мельников К.Н., Кузнецова И.А., Глушаков Р.И. Плацентарная недостаточность и синдром задержки роста плода: этиология, профилактика, лечение. Медицинский алфавит. 2020; 4: 6-10. [Tapilskaya N.I., Mel’nikov K.N., Kuznetsova I.A., Glushakov R.I. Placental insufficiency and fetal growth restriction: etiology, prevention, and treatment. Medical Alphabet. 2020; 4: 6-10 (in Russian)]. https://dx.doi.org/10.33667/2078-5631-2020-4-6-10
- Министерство здравоохранения Российской Федерации. Методические рекомендации. Организация оказания медицинской помощи беременным, роженицам, родильницам и новорожденным при новой коронавирусной инфекции COVID-19. Версия 5 (28.12.2021). [Ministry of Health of the Russian Federation. Methodological recommendations. Organization of medical care for pregnant women, women in labor, women in labor, and newborns with the new coronavirus infection COVID-19. Version 5 (28.12.2021) (in Russian)]. https://static-0.minzdrav.gov.ru/system/attachments/attaches/000/059/052/original/BMP_preg_5.pdf
- Щеголев А.И., Туманова У.Н., Серов В.Н. Поражения плаценты у беременных с SARS-CoV-2-инфекцией. Акушерство и гинекология. 2020; 12: 44-52. [Shchegolev A.I., Tumanova U.N., Serov V.N. Placental lesions in pregnant women with SARS-CoV-2 infection. Obstetrics and Gynecology. 2020; (12): 44-52 (in Russian)]. https://dx.doi.org/10.18565/aig.2020.12.44-52
- Воропаева Е.Е., Хайдукова Ю.В., Казачкова Э.А., Казачков Е.Л., Шамаева Т.Н., Алиева А.А., Ищенко Л.С., Холопова А.Ю., Сычугов Г.В. Перинатальные исходы и результаты морфологического исследования плацент у беременных с критическим поражением легких при новой коронавирусной инфекции COVID-19. Уральский медицинский журнал. 2023; 22(2): 109-21. [Voropaeva E.E., Khaidukova Yu.V., Kazachkova E.A., Kazachkov E.L., Shamaeva T.N., Aliyeva A.A., Ishchenko L.S.,
- Holopova A.Yu., Sychugov G.V. Perinatal outcomes and morphological examination of placentas in pregnant women with critical lung lesions in new COVID-19 coronavirus infection. Ural Medical Journal. 2023; 22(2): 109-21 (in Russian)]. https://dx.doi.org/10.52420/2071-5943-2023-22-2-109-121
- Theiler R.N., Warring S.K., Young M.C., Santos J., Branda M.E., Quinton R.A. et al. Association of SARS-CoV-2 infection during pregnancy with placental weight and histopathologic lesions. Placenta. 2025; 159: 180-6. https://dx.doi.org/10.1016/j.placenta.2024.12.017
- Ищенко Л.С., Воропаева Е.Е., Казачков Е.Л., Казачкова Э.А., Холопова А.Ю., Шамаева Т.Н. Структура перинатальных потерь и особенности плацентарных повреждений у пациенток с острым COVID-19 и преждевременными родами. Вестник новых медицинских технологий. Электронное издание. 2025; 3. [Ishchenko L.S., Voropaeva E.E., Kazachkov E.L., Kazachkova E.A., Kholopova A.Yu., Shamaeva T.N. Structure of perinatal losses and features of placental damage in patients with acute COVID-19 and preterm labor. Journal of New Medical Technologies. eEdition. 2025; 3 (in Russian)]. https://dx.doi.org/10.24412/2075-4094-2025-3-1-3
- Воропаева Е.Е., Ищенко Л.С., Михайлова С.А., Александров Д.И., Хайдукова Ю.В., Казачкова Э.А., Казачков Е.Л. Благоприятный исход крайне тяжелого течения новой коронавирусной инфекции COVID-19 при беременности с тотальным поражением легких, острым миокардитом и инфарктом миокарда. Акушерство и гинекология. 2021; 10: 179-86. [Voropaeva E.E., Ishchenko L.S., Mikhailova S.A., Aleksandrov D.I., Khaidukova Yu.V., Kazachkova E.A., Kazachkov E.L. Favorable outcome of the extremely severe course of the new coronavirus infection COVID-19 during pregnancy in the presence of overall lung damage, acute myocarditis, and myocardial infarction. Obstetrics and Gynecology. 2021; (10): 179-86 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.10.179-186
- Гутикова Л.В., Ганчар Е.П., Зверко В.Л., Лучко Е.В., Лупачик Е.И., Пашенко Е.Н. Патогистология плаценты, плацентарного ложа у женщин, перенесших COVID-19 во время беременности. Охрана материнства и детства. 2023; (2): 55-65. [Gutikova L.V., Ganchar E.P., Zverko V.L., Luchko E.V., Lupachik E.I., Pashenko E.N. Pathohistology of the placenta, placental bed in women who had COVID-19 during pregnancy. Maternity and Child Welfare. 2023; (2): 55-65 (in Russian)].
- Боровая С.Ю., Якимова А.В., Агеева Т.А., Мудров В.А. Оценка влияния COVID-19, перенесенной в различные сроки гестации, на перинатальные исходы и структурные изменения плаценты. Журнал акушерства и женских болезней. 2024; 73(1): 17-28. [Borovaya S.Yu., Yakimova A.V., Ageeva T.A., Mudrov V.A. Assessment of the impact of COVID-19 experienced at different stages of gestation on perinatal outcomes and structural changes in the placenta. Journal of Obstetrics and Women's Diseases. 2024; 73(1): 17-28 (in Russian)]. https://dx.doi.org/10.17816/JOWD624435
- Dagelic A., Stefanovic V., Karara J.R., Prusac I.K., Roje D., Kosovic I. et al. Does COVID-19 infection acquired in different pregnancy trimester influence placental pathology? J. Perinat. Med. 2023; 51(5): 607-13. https://dx.doi.org/10.1515/jpm-2022-0452
- Allen D.C., Cameron R.I., eds. Histopathology specimens: Clinical, pathological and laboratory aspects. London; New York: Springer; 2004. 519 p.
- Щеголев А.И. Современная морфологическая классификация повреждений плаценты. Акушерство и гинекология. 2016; 4: 16-23. [Shchegolev A.I. Modern morphological classification of placental damage. Obstetrics and Gynecology. 2016; (4): 16-23 (in Russian)]. https://dx.doi.org/10.18565/aig.2016.4.16-23
- Pell R., Oien K., Robinson M., Pitman H., Rajpoot N., Rittscher J. et al. The use of digital pathology and image analysis in clinical trials. J. Pathol. Clin. Res. 2019; 5(2): 81-90. https://dx.doi.org/10.1002/cjp2.127
- Wong Y.P., Tan G.C., Omar S.Z., Mustangin M., Singh Y., Salker M.S. et al. SARS-CoV-2 infection in pregnancy: placental histomorphological patterns, disease severity and perinatal outcomes. Int. J. Environ. Res. Public Health. 2022; 19(15): 9517. https://dx.doi.org/10.3390/ijerph19159517
- Boyraz B., James K., Hornick J.L., Roberts D.J. Placental pathology from COVID-19-recovered (nonacute) patients. Hum. Pathol. 2022; 125: 18-22. https://dx.doi.org/10.1016/j.humpath.2022.04.005
- Беженарь В.Ф., Павлова Н.Г., Большакова М.В., Пастушенков В.Л., Карев В.Е. Экспрессия гипоксия-индуцируемого фактора (HIF-1-α) в плацентах при хронической плацентарной недостаточности в конце беременности. Уральский медицинский журнал. 2020; 188(5): 141-5. [Bezhenar V.F., Pavlova N.G., Bolshakova M.V., Pastushenkov V.L., Karev V.E. Expression of hypoxia-induced factor (HIF-1-α) in placentas with chronic placental insufficiency at the end of pregnancy. Ural Medical Journal. 2020; 188(5): 141-5 (in Russian)]. https://dx.doi.org/10.25694/URMJ.2020.05.29
- Михайлин Е.С., Толибова Г.Х., Траль Т.Г. Морфофункциональные особенности последов у несовершеннолетних женщин. Журнал акушерства и женских болезней. 2016; 65(5): 41-8. [Mikhaylin E.S., Tolibova G.K., Tral T.G. Morfological and functional features of placentas in minor women. Journal of Obstetrics and Women's Diseases. 2016; 65(5): 41-8 (in Russian)]. https://dx.doi.org/10.17816/JOWD65541-48
- Albers R.E., Kaufman M.R., Natale B.V., Keoni C., Kulkarni-Datar K., Min S. et al. Trophoblast-specific expression of Hif-1α results in preeclampsia-like symptoms and fetal growth restriction. Sci. Rep. 2019; 9(1): 2742. https://dx.doi.org/10.1038/s41598-019-39426-5
- Воропаева Е.Е., Хайдукова Ю.В., Холопова А.Ю., Алиева А.А., Казачкова Э.А., Казачков Е.Л. Процессы апоптоза и гипоксии в плацентах у беременных женщин при COVID-19 и критическом поражении легких. Непрерывное медицинское образование и наука. 2024; 19(1): 28-33. [Voropaeva E.E., Hajdukova Yu.V., Holopova A.Yu., Alieva A.A., Kazachkova E.A., Kazachkov E.L. Apoptosis and hypoxia processes in placentas of pregnant women with COVID-19 and critical lung injury. Continuing medical education and science. 2024; 19(1): 28-33 (in Russian)].
- Heras N.D.L., Giménez V.M.M., Ferder L., Manucha W., Lahera V. Implications of oxidative stress and potential role of mitochondrial dysfunction in COVID-19: therapeutic effects of vitamin D. Antioxidants (Basel). 2020; 9(9): 897. https://dx.doi.org/10.3390/antiox9090897
- Barker D.J., Osmond C., Simmonds S.J., Wield G.A. The relation of small head circumference and thinness at birth to death from cardiovascular disease in adult life. BMJ. 1993; 306(6875): 422-6. https://dx.doi.org/10.1136/bmj.306.6875.422
- Calkins K., Devaskar S.U. Fetal origins of adult disease. Curr. Probl. Pediatr. Adolesc. Health Care. 2011; 41(6): 158-76. https://doi.org/10.1016/j.cppeds.2011.01.001
- Гуменюк Е.Г., Ившин А.А., Светова К.С. Задержка роста плода как предиктор здоровья на протяжении будущей жизни. Акушерство и гинекология. 2024; 3: 5-12. [Gumeniuk E.G., Ivshin A.A., Svetova K.S. Fetal growth retardation as a predictor of health during the future life. Obstetrics and Gynecology. 2024; (3): 5-12 (in Russian)]. https://dx.doi.org/10.18565/aig.2023.277
Received 24.06.2025
Accepted 01.07.2025
About the Authors
Ella A. Kazachkova, Dr. Med. Sci., Professor, Professor at the Department of Obstetrics and Gynecology, South Ural State Medical University, Ministry of Health of Russia,64 Vorovsky str., Chelyabinsk, 454000, Russia, +7(912)478-62-90, https://orcid.org/0000-0002-1672-7058
Ekaterina E. Voropaeva, Dr. Med. Sci., Professor, Professor at the Department of Pathological Anatomy and Forensic Medicine, South Ural State Medical University,
Ministry of Health of Russia, 64 Vorovsky str., Chelyabinsk, 454000, Russia; Chief Physician, Regional Perinatal Center, Chelyabinsk, Russia, +7(912)896-61-36,
https://orcid.org/0000-0002-9405-0134
Lyudmila S. Ishchenko, PhD, Associate Professor, Associate Professor at the Department of Obstetrics and Gynecology, South Ural State Medical University,
Ministry of Health of Russia, 64 Vorovsky str., Chelyabinsk, 454000, Russia; Obstetrician-Gynecologist, Regional Clinical Hospital No. 2, Chelyabinsk, Russia,
+7(919)352-91-14, lyudalyn@mail.ru, https://orcid.org/0000-0002-9405-0134
Evgeny L. Kazachkov, Dr. Med. Sci., Professor, Head of the Department of Pathological Anatomy and Forensic Medicine, South Ural State Medical University,
Ministry of Health of Russia, 64 Vorovsky str., Chelyabinsk, 454000, Russia, +7(912)323-39-74, https://orcid.org/0000-0002-4512-3421
Anna Yu. Kholopova, PhD student at the Department of Pathological Anatomy and Forensic Medicine, South Ural State Medical University, Ministry of Health of Russia,
64 Vorovsky str., Chelyabinsk, 454000, Russia, +7(922)231-63-66, https://orcid.org/0000-0001-5559-0069
Alexandra V. Zatvornitskaya, PhD, Teaching Assistant at the Department of Pathological Anatomy and Forensic Medicine, South Ural State Medical University,
Ministry of Health of Russia, 64 Vorovsky str., Chelyabinsk, 454000, Russia; Chief Physician, Regional Perinatal Center, Chelyabinsk, Russia, +7(919)400-75-35,
https://orcid.org/0000-0002-9245-3749
Tatiana N. Shamaeva, PhD, Associate Professor at the Department of Mathematics, Medical Informatics, Informatics and Statistics, South Ural State Medical University, Ministry of Health of Russia, 64 Vorovsky str., Chelyabinsk, 454000, Russia, +7(912)473-16-10, https://orcid.org/0000-0001-6327-2685
Corresponding author: Lyudmila S. Ishchenko, lyudalyn@mail.ru



