Clinical and morphological variants of insufficient uterine scar after cesarean section
Nesterov V.F., Malgina G.B., Dyakova M.M., Grishkina A.A.
Objective: To explore the morphological features and clinical manifestations of insufficient uterine scar after cesarean section.
Materials and methods: The study included 109 patients with uterine scar after cesarean section. They were divided into four groups depending on the clinical and morphological features of insufficient uterine scar: group I – “vascular”type (n=23), group II – “connective tissue” type (n=39); III – “inflammatory” type (n=27), and Group IV – the comparison group (n=20). Histological and histomorphological evaluation of excised scar tissue, clinical assessment of anamnestic indicators, and statistical data analysis were performed.
Results: Three main morphological variants of insufficient uterine scar were identified – "inflammatory," "vascular," and "connective tissue." The clinical and morphological parallels of insufficient uterine scar were assessed taking into account the histological variant. The patients with the "vascular" type of insufficient uterine scar had a history of irregular menstrual cycles (r=0.71; 95% CI 0.81;0.89 p<0.001). The main indication for delivery was the risk of uterine rupture along the scar (r=0.67; 95% CI 0.78;0.95 p<0.001). The patients with the "connective tissue" type of insufficient uterine scar underwent the first cesarean section due to abnormal labor (r=0.81; 95% CI 0.65;0.74 p<0.001). Uterine scar insufficiency – thinning of the uterine scar (< 2 mm) was detected by ultrasound. At the same time, the clinical picture showed no risk of uterine rupture along the scar (r=0.88; 95% CI 0.76;0.85 p<0.001). The "inflammatory type" of insufficient uterine scar correlated with recurrent miscarriage in history (r=0.65;
95% CI 0.58;0.83 p<0.001) and fetal distress in current pregnancy (r=0.63; 95% CI 0.61;0.81 p<0.001).
Conclusion: The obtained data indicate that there is a need for an in-depth study of the pathogenetic mechanisms of uterine scar formation and the development of a prediction model.
Authors' contributions: Nesterov V.F., Malgina G.B. – the concept and design of the study; Nesterov V.F., Dyakova M.M., Grishkina A.A. – data collection, analysis and interpretation of the results, the draft manuscript writing; Malgina G.B., Grishkina A.A. – the critical revision of the draft manuscript and formation of the final version. All authors approved the final version of the article before publication and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved.
Conflicts of interest: The authors confirm that they have no conflicts of interest to declare.
Funding: The study was conducted without any sponsorship.
Ethical Approval: The study was approved by the Ethics Committee of the Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia
Patient Consent for Publication: The patients have signed informed consent for publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Nesterov V.F., Malgina G.B., Dyakova M.M., Grishkina A.A. Clinical and
morphological variants of insufficient uterine scar after cesarean section.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (10): 62-72 (in Russian)
https://dx.doi.org/10.18565/aig.2025.197
Keywords
Currently, global cesarean section (CS) rates are steadily increasing. There is increasing risk of placenta previa and placenta accreta, hemorrhage, and hysterectomy with each subsequent CS, that in turn, can lead to near misses and maternal mortality [1, 2]. This can be avoided by appropriate timing of elective abdominal delivery in women suspected of having insufficient uterine scars. Morphological uterine scar insufficiency leads to the clinical inadequacy associated with rough cicatricial changes in the scar tissue lacking the elasticity during pregnancy and optimal contractile activity during labor. Uterine scarring poses a threat to women's health and future childbirth. It is known that after CS several possible scenarios to restore uterine wall include complete regeneration of myometrium, complete or incomplete formation of the scar (myometrial defect), and placental invasion into the scar tissue. Insufficient uterine scar can cause uterine rupture not only in subsequent delivery, but also in pregnancy [3, 4].
To date, the criteria for prenatal assessment of insufficient uterine scar and the choice of safe delivery method is not unanimous. In most cases, the presence of uterine scar is the main indication for repeat operative delivery, although vaginal delivery is possible in 25–70% of parturient women with uterine scar after CS [5]. However, the absence of clear criteria for assessment of uterine scar condition does not allow to achieve reduction in the frequency of repeat abdominal delivery.
According to the WHO, insufficient uterine scar is a CS scar of <2,5–3 mm thickness and is characterized by lack of elasticity, inadequate contractility, the presence of niche, thickening and indentation at the site of the scar [6]. However, according to Nozhnitseva O.A. et al. (2019) [7], it is more appropriate to use the term “local thinning of uterine scar”, since uterine scar condition can be assessed only during labor and birth.
According to Belyaeva E.K. (2024), morphological examination of insufficient uterine scar showed dense connective tissue between the bundles of muscle fiber with the presence of numerous blood vessels of different size – predominantly venules, capillaries, and small arteries. Dystrophic muscle fibers were surrounded by thin layers of connective tissue. Hypertrophy was often observed at the sites, where muscle fibers were surrounded by dense connective tissue [8]. However, Ewies A. et al (2022) showed that the identified hypertrophy of muscle fibers is not a significant morphological marker of insufficient uterine scar formation [9].
Thus, there is no unanimity of opinions about the pathogenic variants of uterine scar insufficiency after CS. This complicates exploration of pathogenetic mechanisms of its formation, as well as identification of clinical determinants of different morphological variants of scar insufficiency, that serves as a base for planning optimal and safe delivery.
The objective of the study was to explore the morphological features and clinical manifestations of insufficient uterine scar after cesarean section.
Materials and methods
The single-center prospective cohort study included 109 patients with uterine scar after one CS. Cesarean deliveries were at 37–40 weeks of gestation. The material was collected using continuous data collection technique. Uterine scar tissue was intraoperatively excised within the unchanged tissue and sent for histological examination, including light microscopic evaluation of the excised scar tissue obtained during cesarean section. For histological examination the samples of the excised scar tissue were fixed in 10% neutral buffered formalin, embedded in paraffin, cut into sections of 5 µm thickness, and stained with hematoxylin and eosin. Microscopic evaluation was performed using Leica DM 2500 microscope (Germany). Histological verification of uterine scar was based on assessment of sections of 5 µm thickness stained using hematoxylin and eosin (most frequently used technique), and using van Gieson's picrofuchsin. Based on the results, 4 groups of patients were verified: group I (n=23) – the patients with “vascular type” of uterine scar insufficiency, group II (n=39) the patients with “connective tissue type”, group III (n=27) the patients with “inflammatory type”, and the comparison group IV (n=20) – the patients with “normal” uterine scar. The patients with insufficient uterine scar were identified based on clinical and morphological manifestations of uterine scar insufficiency. The reasoning for division into groups was the prevalence of certain parameters (over 60%). Comparative analysis of anamnestic, ultrasound-based and clinical factors between the three groups of patients with uterine scar insufficiency and the comparison group was performed.
The inclusion criteria in the study groups were the following: the presence of one uterine scar after cesarean delivery, weeks of gestation in term pregnancy, patients’ informed consent for uterine scar tissue sample collection and histological examination. Non-inclusion criteria were the patients with two or more cesarean scars, and uterine scars after gynecological surgeries, patient’s informed consent to participate in the study. The study was approved by the Ethics Committee (protocol No. 12 of October 19, 2022). The main limitation of the second stage of this single-center study was selection bias.
Statistical analysis
Statistical data analysis was performed using software package Microsoft Excel (2010), SPSS Statistics V22.0 (IBM Microsoft, USA).
Hypothesis testing for normal sample distribution was done using the Kholmogorov–Smirnov and Shapiro–Wilk tests with data validation tools in IBM SPSS Statistics V22.0.
The F-test in SPSS Statistics V22.0 was used to determine the equality of variances and was a part of procedure of hypothesis testing for the difference in the means. The data are presented as arithmetic mean (M) and standard deviation (SD). The qualitative categorical variables are presented as the number of subjects (n) and percentage (%). Comparison of the frequency of the qualitative characteristics was carried using χ2 test for fourfold tables.
Relationship between variance of the resulting feature and variance of feature-factor was assessed using Spearman's rank correlation coefficient (׀r׀≤0.25 – weak correlation; 0.25<׀r׀<0.75 – moderate correlation; ׀r׀ ≥0.75 – strong correlation).
The differences between the groups were considered to be statistically significant at the level of significance of 5% (р<0,05). The Bonferroni correction was used for comparisons of more than two groups, critical value at р<0.05/3 was 0.017.
Results
From 2021 to 2024, there were 1343 deliveries in women with uterine scars at the Ural Research Institute of Maternity and Child Care of the Ministry of Health of Russia. It should be noted that intraoperatively and histologically confirmed uterine scar insufficiency was in 43.5% of women (584/1343).
The main macroscopic signs of insufficient uterine scars registered by the operating surgeons in surgical protocols were the following: scar spreading, local thinning of the scar, scar retraction and thinning on the periphery of the scar, hemorrhage and extensive vascular network in the scar area.
The age of pregnant women in all groups was 20 – 42 years. The mean age was 30.9 (4,6) years; 30.5 (4.2) years in group I, 31.4 (5.3) years in group II, and 30.2 (5.6) years in group III. The mean age in the comparison group was 30.9 (6.8) years.
There were no statistically significant differences between the study groups in the mean age and social status (p>0.05).
Figure 1 shows macroscopic evaluation of uterine scars and the signs of uterine scar insufficiency registered by the operating surgeons in surgical protocols.
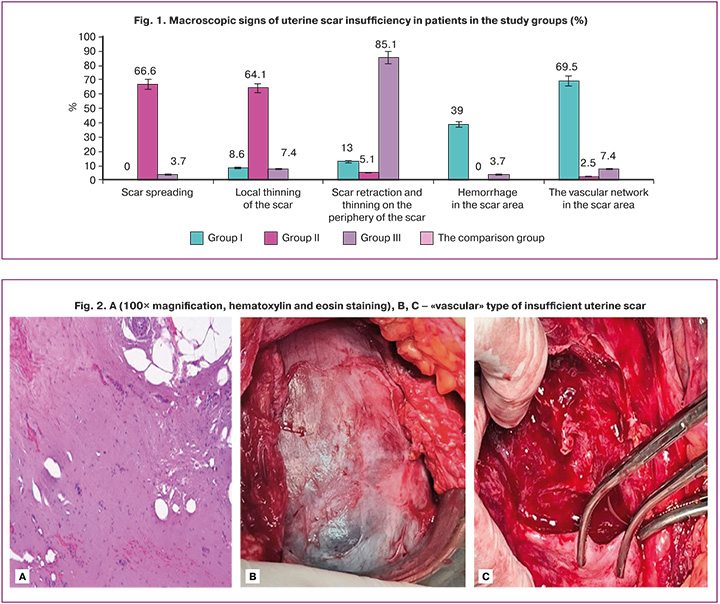
In group I, vascular signs of uterine scar insufficiency prevailed – 9/23 (39.1%) patients. Extensive vascular network in the scar area was in 16/23 (69.5%) patients. Other signs are described by surgeons as single cases. For example, scar spreading occurred in none of patients.
In contrast, in women in group II, uterine scar insufficiency included scar spreading (26/39, 66.6%) and local thinning of the scar (2/39, 56,4%). Vascular signs were described as single cases.
In patients in group III, the main macroscopic sign of uterine scar insufficiency was scar retraction and thinning on the periphery of the scar (23/27, 85.2%). In single cases other defects were observed – local thinning of the scar (7.4%) and extensive vascular network (7,4%). Scar spreading was in 1 out of 27 (3.7%) patients, and this patient was included in group III due to the absence of other signs.
Thus, according to the microscopic signs, the groups of patients with uterine scar insufficiency are heterogeneous. However, the macroscopic description of the uterine scar contains the elements of subjectivity on the part of the operating surgeon. Therefore, microscopic signs of scar defects in patients in groups I – III are of particular interest (Table 1.
Analysis of the data presented in Table 1 showed the following: the prevalence of connective tissue (>50%) with randomly arranged muscle fibers and edema of intermuscular stroma was typical for of all patients with uterine scar insufficiency. The signs of disorganized connective tissue were also found in 15% of patients with a “normal” uterine scar, but in women with uterine scar insufficiency, microscopic examination showed its predominant manifestation. However, in each of the three groups distinctive microscopic changes were identified and classified.
The most characteristic feature of uterine scar insufficiency in patients in group I was hemorrhage (22/23, 95.6%). Extensive vascular network ranked second –19/23 (82.6%). These morphological signs were observed in patients in other groups significantly less often.
In patients in group II, the signs of connective tissue dysplasia were found significantly more often, that is also reported by many authors [10–12]. Along with the prevalence of connective tissue (>50%), in 69.2% of cases (27/39) large lesions of dense fibrous connective tissue were found. Extensive vascular network was found significantly less often.
In patients in group III, the prevalence of lymphohistiocytic infiltration was statistically significant, that was a sign of chronic inflammation.
Therefore, we can conditionally divide the patients with insufficient uterine scar into groups I, II, III, that are described by the above data and illustrated by macro- and microphographs, as shown below. In group I (n=23), the scars were characterized predominantly with connective tissue interspersed with the bundles of small smooth muscle tissue, extensive vascular network, small hemorrhage, and hemosiderin deposition (Fig. 2A).
Phenotypically, “local thinning of the scar” was rarely observed. There was no clear boundary with the intact myometrium. Large vessels were visualized. Excision of this type of scar was accompanied by bleeding at the edges of the wound (Fig. 2B, C).
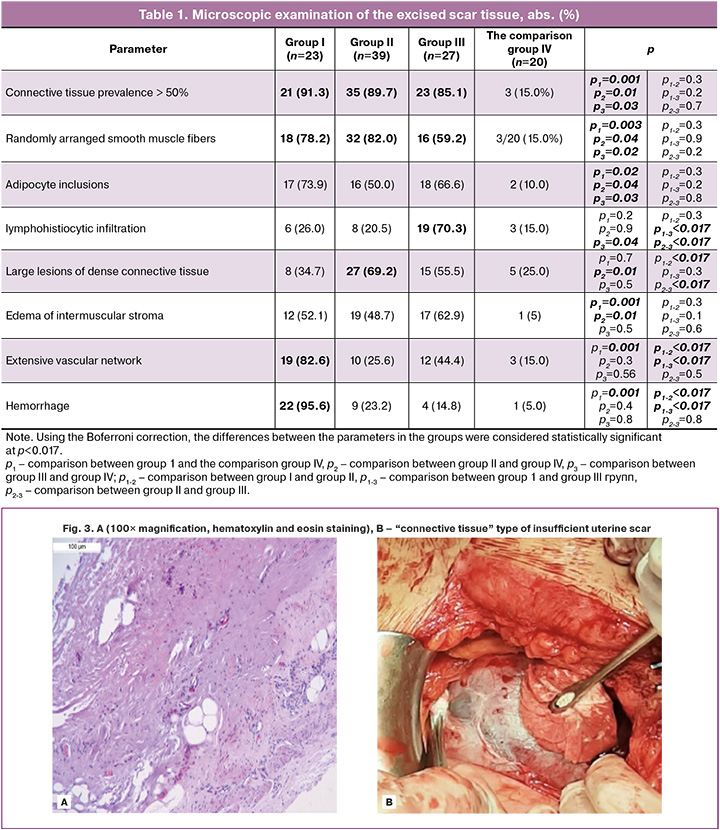
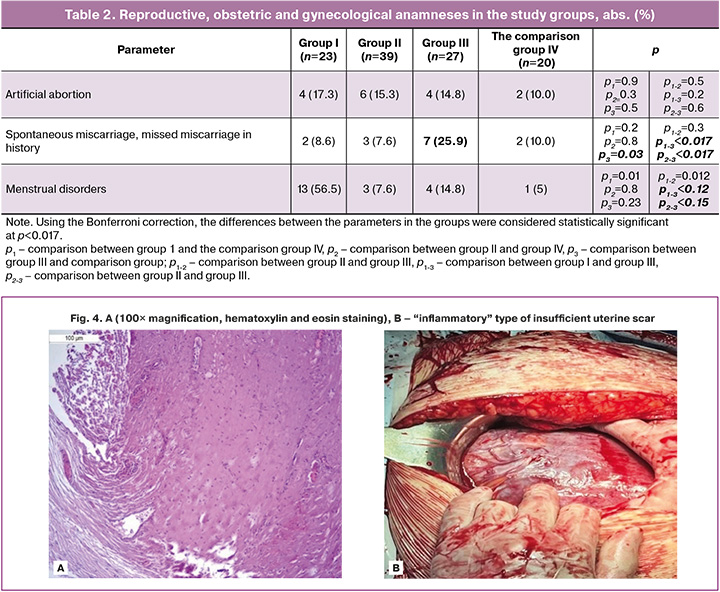
“Connective tissue” type of uterine scar insufficiency was in group II (n=39). This variant was characterized by small amounts of interspersed muscle fibers divided by the bundles of disorganized connective tissue, adipocyte inclusions, lymphohistiocytic infiltration, and poor vascular network (Fig. 3A). Phenotypically (intraoperatively), such scars showed “local thinning” or “spreading”, and formation of the clear boundary with unchanged myometrium (Fig. 3B). At the same time, there was no extensive vascular component in the scar tissue.
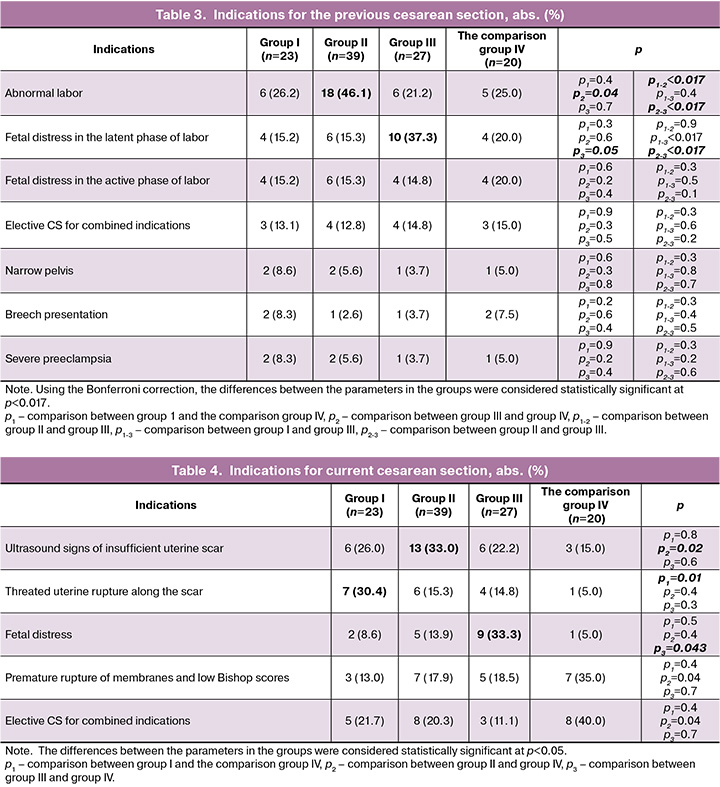
“Inflammatory” type of uterine scar insufficiency was in Group III (n=27). Microscopic evaluation showed that this type of scar insufficiency was characterized by the presence of muscle fibers with the bands of dense fibrous connective tissue and increased leukocyte infiltration with the signs of hyalinosis (Fig. 4A). Phenotypically, these scars showed white area, retractions and often thinning around the edge of the boundary with unchanged myometrium, and uneven thickness (Fig. 4B).
The second stage of the study was a search for anamnestic determinants of the three identified variants of scar insufficiency.
The analysis of somatic anamnesis found that there was approximately equal frequency of gastrointestinal diseases, disorders of the urinary system, and cardiovascular diseases in patients in the compared groups. In group II, myopia was significantly more often (14/39; 31.6%, р<0.017).
In the structure of gynecological anamnesis, the number of women with menstrual disorders was significantly higher in group I (13/23; 56,5%). They had prolonged and painful menstrual periods, that was reported after the primary CS.
Analysis of reproductive anamnesis (Table 2) in the study groups showed significant difference in the number of spontaneous miscarriages and missed miscarriages in history in women in group III (7/27; 27.9%, р<0.017). There were no statistically significant differences found between the groups in other parity indicators. Higher rates of spontaneous miscarriage and missed miscarriage in patients in group III can be associated with chronic inflammation of the uterine cavity. Due to this, lymphohisteocytic infiltration occurred in the scar tissue as a sign of chronic inflammation.
Analysis of indications for the previous cesarean section in the cohort of patients in group II showed that primary CS was performed significantly more often due to abnormal labor (18/39, 46.1%, р<0.05). In group III, the most common indication for CS in group III was fetal distress in the latent phase of labor (10/27, 37.3%, p<0.05). The frequency of indications for CS, such as breech presentation, narrow pelvis, severe preeclampsia and combined indications for CS was similar in other groups (Table 3).
The mean gestational age for delivery in current pregnancy in groups I, II, III was 38.1 (37.4; 38.5) weeks, and it was statistically significantly higher in the comparison group – 39.2 (38.5; 39.6) weeks (р<0.005).
Analysis of indications for CS in current pregnancy (Table 4) showed that in group 1 threatened miscarriage was most often. It was characterized by clinical manifestations, such as pain, hypertonic pelvic floor, that were detected even with no signs of labor in 7/23 (30.4%) patients (р<0.017). At the same time, according to ultrasound examination, no local thinning was found.
In group II, ultrasound examination showed the following statistically significant manifestations of scar insufficiency in 13/30 (33.0%) women: thinning in the scar area, uneven residual myometrial thickness, bulging “hernia” in the scar area (р<0.017).
In group III, the main indication for CS was fetal distress in labor (9/27; 33.3%). There were no ultrasound signs or clinical markers of insufficient uterine scar, as well as markers of inflammation were detected.
Assessment of ultrasound parameters (Table 5) before delivery found significant thinning of pelvic floor with average thickness of 2.1 mm (1.6; 2.6) (p<0.05) in group II, whereas in other groups this parameter was similar. Scar retractions and hyperechoic lesions were found significantly more often in patients in group III – 8/29 (6%) and 7/25 (9%), respectively (р<0.05). Hypervascularization in pelvic floor was found significantly more often in patients in group I – 9/39 (1%) (р<0.05).
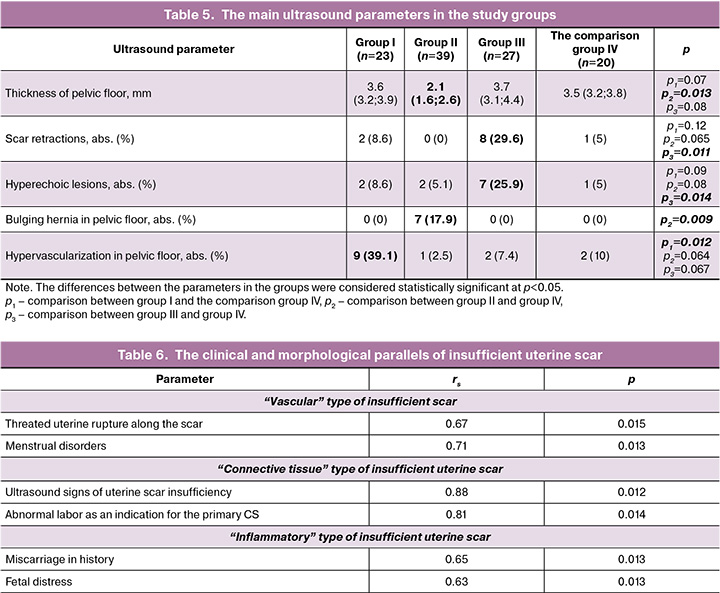
Analysis of the obtained data made it possible to determine the clinical and motphological parallels of insufficient uterine scar taking into account its histological variant and calculating correlation coefficient (Table 6).
Based on the results of correlation analysis, the most significant positive correlation between morphological variants and clinical and anamnestic manifestations of scar insufficiency for each variant was identified.
In patients with "vascular" type of scar insufficiency, strong positive association was found with menstrual disorders (rs=0,71), and indication for delivery in these patients was threatened uterine rupture along the scar, that was manifested by hypertonicity of the anterior wall of the uterus and local pain. In patients with “connective tissue” type of scar insufficiency, the primary CS was performed due to abnormal labor (rs=0,81). Manu authors explored the relationship between undifferentiated connective tissue dysplasia (CTD) and abnormal labor [13, 14]. Strong positive correlation with ultrasound signs of scar insufficiency inferiority was also found. “Inflammatory type” of insufficient scar had no specific ultrasound and macroscopic signs, however correlated with recurrent miscarriage in history (rs=0.65), and fetal distress in current pregnancy (rs=0.63). The signs of chronic inflammation were confirmed only by miscroscopic examination.
Discussion
According to Kan N.E. et al. (2025), the morphological criteria of uterine scar insufficiency include lesions of abnormal connective tissue structure characterized by fibrinoid swelling and fibrinoid necrosis, leiomyocyte necrosis, intramural hematomas and multiple petechial hemorrhages, inflammatory reaction (lymphohistiocytic infiltration), and neoangiogenesis [15].
Exploration of risk factors for insufficient uterine scar formation is of special interest. CTD is a risk factor for insufficient uterine scar formation. This pathological condition is genetically determined and has different clinical forms due impairment of connective tissue formation and reduction of certain types of collagen, that leads to reduction in tensile strength of connective tissue, and can lead to impairment in the repair processes. In patients with CTD, insufficient uterine scar is characterized by reduced levels of angiogenesis, that can lead to dystrophy of fibers and cells caused by local ischemia [16–18].
A number of domestic and foreign authors reported that adenomyosis or endometriosis of postoperative uterine scar can be a factor influencing insufficient uterine scar formation [19, 20]. According to Tskhai B.V. et al. (2016), histological evaluation of the excised post-cesarean scar tissue showed scar fibrosis and scar endometriosis in 21.4% of cases [21, 22].
In the etiology of insufficient uterine scar of great importance is a subtle form of postpartum endometritis, which is manifested by short duration of the acute phase, subclinical symptoms, subinvolution of the uterus, and is characterized by an intermittent disease course, late manifestation, predominance of infiltrative and necrotic forms of inflammation and the absence of basic laboratory indicators of acute inflammatory response [2, 23].
Based on the mentioned published data it can be suggested that the group of patients with insufficient uterine scar is heterogeneous, and the pathogenic variants of insufficient scar formation in these patients depend on risk factors and their clinical manifestations. In some patients, the cause of insufficient scar formation is undifferentiated connective tissue dysplasia. In other patients, chronic inflammation in the scars and around the scars prevails. In some women, scar endometriosis and excessive neoangiogenesis come to the forefront.
The data obtained by us confirm these suggestions. The clinical and anamnestic and morphological determinants make it possible to determine the types of insufficient scar. Formation of “vascular type” is most likely due to scar endometriosis with specific clinical manifestations: threatened uterine rupture along the scar in pregnancy, and menstrual disorders in non-pregnant women. Ultrasound examination shows hypervascularization in pelvic floor.
«Connective tissue” type is the most explored type of the scar. It is characterized by ultrasound signs of uterine scar insufficiency (thinning and bulging hernia in the scar area). Specific anamnestic feature is performance of primary CS due to abnormal labor. Ultrasound examination shows thinning of pelvic floor and bulging hernia in the scar area.
The third type of insufficient uterine scar is “inflammatory” type. The rate of miscarriage is statistically significant in this category of patients, that can indirectly indicate subclinical infection and inflammation during uterine scar formation [2], which influenced the subsequent pregnancy. Fetal distress in current pregnancy is possibly associated with chronic inflammatory processes in the mother-placenta-fetus system. Ultrasound examination shows multiple hyperechoic lesions and scar retractions.
Conclusion
The pathogenic variants described by us require further in-depth study, using new research instrumentation technologies, the development of prediction models of uterine scar insufficiency at the stage of planning labor and birth.
Based on the analysis of macro- and microscopic examination of excised scar tissues, three main morphological variants of insufficient uterine scar were identified – “vascular”, “connective tissue” and “inflammatory”, that provides the prospects for further study of the pathogenetic mechanisms of their formation, prediction of complications and optimization of childbirth.
References
- Савина Л.В., Ящук А.Г., Масленников А.В., Савин А.М., Шаяхметов А.М. Факторы риска формирования несостоятельности рубца на матке после операции кесарева сечения. Международный научно-исследовательский журнал. 2022; 6(120): 107-12. [Savina L.V., Yashchuk A.G., Maslennikov A.V., Savin A.M., Shayahmetov A.M. Risk factors of uterus scar insolvency after a c-section operation. International Research Journal. 2022; 6(120): 107-12.(in Russian)]. https://dx.doi.org/10.23670/IRJ.2022.120.6.050
- Глухов Е.Ю., Дикке Г.Б., Нефф Е.И., Глухова В.Е., Свяжина А.В. Хронический эндометрит и несостоятельный рубец на матке после кесарева сечения: отдаленные результаты метропластики. Акушерство и гинекология. 2019; 2: 126-34. [Glukhov E.Yu., Dikke G.B., Neff E.I., Glukhova V.E., Svyazhin A.V. Chronic endometritis and an incompetent uterine scar after cesarean section: long-term outcomes of metroplasty. Obstetrics and Gynecology. 2019; (2): 126-34 (in Russian)]. https://dx.doi.org/10.18565/aig.2019.2.126-134
- Савельева Г.М., Бреслав И.Ю. Разрыв оперированной матки во время беременности и родов. Вопросы гинекологии, акушерства и перинатологии 2015; 14(3): 22-7. [Savel'eva G.M., Breslav I.Yu. Rupture of the operated uterus during pregnancy and labour. Gynecology, Obstetrics and Perinatology. 2015; 14(3): 22-7 (in Russian)].
- Щукина Н.А., Буянова С.Н., Чечнева М.А., Земскова Н.Ю. Основные причины формирования несостоятельного рубца на матке после кесарева сечения. Российский вестник акушера-гинеколога. 2018; 4: 57-61. [Shchukina N.A., Buyanova S.N., Chechneva M.A., Zemskova N.Yu. Main reasons for the formation of an incompetent uterine scar after cesarean section. Russian Bulletin of Obstetrician-Gynecologist. 2018; 4: 57-61 (in Russian)]. https://dx.doi.org/10.17116/rosakush201818457
- Мудров В.А., Мочалова М.Н., Мудров А.А. Особенности родоразрешения беременных с рубцом на матке через естественные родовые пути на современном этапе. Журнал акушерства и женских болезней. 2018; 67(1): 26-37. [Mudrov V.A., Mochalova M.N., Mudrov A.A. Features of women’s vaginal delivery with uterine scar at present stage. Journal of Obstetrics and Women's Diseases. 2018; 67(1): 26-37 (in Russian)]. https://dx.doi.org/10.17816/JOWD67126-37
- Торобаева М.Т., Буянова С.Н., Пучкова Н.В. Несостоятельный рубец на матке после кесарева сечения как отдельная нозология. Российский вестник акушера-гинеколога. 2023; 23(3): 19-28. [Torobaeva M.T., Buyanova S.N., Puchkova N.V. Incompetent uterine scar after caesarean section as a separate nosology. Russian Bulletin of Obstetrician-Gynecologist. 2023; 23(3): 19-28 (in Russian)]. https://dx.doi.org/10.17116/rosakush20232303119
- Ножницева О.Н., Семенов И.А., Беженарь В.Ф. Рубец на матке после операции кесарева сечения и оптимальный алгоритм диагностики его состояния. Лучевая диагностика и терапия. 2019; 2: 85-90. [Nozhnitseva O.N., Semenov I.A., Bezhenar V.F. The scar on the uterus after cesarean section and the optimal algorithm for diagnostics. Diagnostic radiology and radiotherapy. 2019; 2: 85-90 (in Russian)]. https://dx.doi.org/10.22328/2079-5343-2019-10-2-85-90
- Беляева Е.К., Старцева Н.М., Маркарян Н.М., Хамошина М.Б., Логинова Е.В., Михалева Л.М., Бирюков А.Е. Клинико-морфологические параллели состоятельности рубца на матке после кесарева сечения. Акушерство и гинекология: новости, мнения, обучение. 2024; 12 Спецвыпуск: 13-9. [Belyaeva E.K., Startseva N.M., Markaryan N.M., Khamoshina M.B., Loginova E.V., Mikhaleva L.M., Biryukov A.E. Clinical and morphological parallels of the consistency of the uterine scar after cesarean section. Obstetrics and Gynecology: News, Opinions, Training. 2024; 12 Supplement: 13-9 (in Russian)]. https://dx.doi.org/10.33029/2303-9698-2024-12-suppl-13-19
- Ewies A.A.A., Qadri S., Awasthi R., Zanetto U. Caesarean section operation is not associated with myometrial hypertrophy - a prospective cohort study. J. Obstet. Gynaecol. 2022; 42(6): 2474-79. https://doi.org/10.1080/01443615.2022.2074787
- Ofili-Yebovi D., Ben-Nagi J., Sawyer E., Yazbek J., Lee C., Gonzalez J. et al. Deficient lower-segment cesarean section scars: prevalence and risk factors. Ultrasound Obstet. Gynecol. 2008; 31(1): 72-7. https://dx.doi.org/10.1002/uog.5200
- Vink J., Feltovich H. Cervical etiology of spontaneous preterm birth. Semin. Fetal Neonatal Med. 2016; 21(2): 106-12. https://dx.doi.org/10.1016/j.siny.2015.12.009
- Игитова М.Б., Дмитриенко К.В., Боровков В.А., Нестеров Ю.Н. Клинико-морфологическое сопоставление течения гестации и состояния рубца на матке после однократного кесарева сечения. Фундаментальная и клиническая медицина. 2023; 8(3): 37-43. [Igitova M.B., Dmitrienko K.V., Borovkov V.A., Nesterov Yu.N. Clinicopathological features of gestation course associated with uterine scar dehiscence in women with a past medical history of a single caesarean section. Fundamental and Clinical Medicine. 2023; 8(3): 37-43 (in Russian)]. https://dx.doi.org/10.23946/2500-0764-2023-8-3-37-43
- Ильина И.Ю., Чикишева А.А. Особенности течения беременности у пациенток с дисплазией соединительной ткани. РМЖ. Мать и дитя. 2020; 3(3): 182-8. [Il’ina I.Yu., Chikisheva A.A. Course of the pregnancy in women with connective tissue disorders. RMJ. Mother and child. 2020; 3(3): 182-8 (in Russian)]. https://dx.doi.org/10.32364/2618-8430-2020-3-3-182-188
- Manuck T.A. The genomics of prematurity in an era of more precise clinical phenotyping: a review. Semin. Fetal Neonatal Med. 2016; 21(2): 89-93. https://dx.doi.org/10.1016/j.siny.2016.01.001
- Кан Н.Е., Тютюнник В.Л., Демура Т.А., Кесова М.И. Особенности формирования рубца на матке после кесарева сечения при недифференцированной дисплазии соединительной ткани. Акушерство и гинекология. 2015; 1: 93-7. [Kan N.E., Tyutyunnik V.L., Demura T.A., Kesova M.I. Specific features of uterine scar formation after cesarean section in undifferentiated connective tissue dysplasia. Obstetrics and Gynecology. 2015; (1): 93-7 (in Russian)].
- Гарифуллова Ю.В., Журавлева В.И. Пластика несостоятельного рубца на матке влагалищным доступом при сопутствующей дисплазии соединительной ткани. Практическая медицина. 2019; 17(4): 85-7. [Garifullova Yu.V., Zhuravleva V.I. Vaginal access repair of uterine scar dehiscence with concomitant connective tissue dysplasia. Practical medicine. 2019; 17(4): 85-7 (in Russian)]. https://doi.org/10.32000/2072-1757-2019-4-85-87
- Окулова Е.О., Михельсон А.А., Мелкозерова О.А., Телякова М.И., Чистякова Г.Н., Лазукина М.В. Эндометриоз несостоятельного рубца на матке после операции кесарева сечения: воспаление или дисплазия? Проблемы репродукции. 2022; 28(4): 145-50. [Okulova E.O., Mikhelson A.A., Melkozerova O.A., Telyakova M.I., Chistyakova G.N., Lazukina M.V. Endometriosis of a post-cesarean incompetent of uterine scar: inflammatory or connective tissue dysplasia. Russian Journal of Human Reproduction. 2022; 28(4): 145-50 (in Russian)]. https://dx.doi.org/10.17116/repro202228041145
- Краснопольский В.И., Буянова С.Н., Щукина Н.А., Логутова Л.С. Несостоятельность шва (рубца) на матке после кесарева сечения: проблемы и решения (редакционная статья). Российский вестник акушера-гинеколога. 2015; 15(3): 4-8. [Krasnopol'skiĭ V.I., Buianova S.N., Shchukina N.A., Logutova L.S. Uterine suture (scar) incompetence after cesarean section: problems and solutions (an editorial). Russian Bulletin of Obstetrician-Gynecologist. 2015; 15(3): 4-8. (in Russian)]. https://dx.doi.org/10.17116/rosakush20151534-8
- Sholapurkar S.L. Etiology of cesarean uterine scar defect (niche): detailed critical analysis of hypotheses and prevention strategies and peritoneal closure debate. J. Clin. Med. Res. 2018; 10(3): 166-73. https://dx.doi.org/10.14740/jocmr3271w
- Pirtea L., Balint O., Secoşan C., Grigoraş D., Pirtea P. Case report: laparoscopic isthmocele repair on an 8 weeks pregnant uterus. Front. Med. (Lausanne). 2022; 9: 831588. https://dx.doi.org/10.3389/fmed.2022.831588
- Цхай В.Б., Леванович Е.В., Кельберг В.Г. Эндометриоз несостоятельного рубца на матке после операции кесарева сечения. Акушерство и гинекология. 2016; 8: 119-23. [Tshai V.B., Levanovich E.V., Kelberg V.G. Endometriosis in an incompetent uterine scar after cesarean section. Obstetrics and Gynecology. 2016; (8): 119-23 (in Russian)]. https://dx.doi.org/10.18565/aig.2016.8.119-123
- Телякова М.И., Михельсон А.А., Окулова Е.О., Мелкозерова О.А., Погорелко Д.В. Современные представления об эндометриозе несостоятельного рубца на матке после операции кесарево сечение. Лечение и профилактика. 2021; 11(1): 46-53. [Telyakova M.I., Mikhelson A.A., Okulova E.O., Melkozerova O.A., Pogorelko D.V. Inconsistency of the uterine scar after cesarean section in combination with endometriosis of the uterine scar. Treatment and prevention. 2021; 11(1): 46-53 (in Russian)].
- Gurbuz A.S., Gode F., Ozcimen N. Non-invasive isthmocele treatment: a new therapeutic option during assisted reproductive technology cycles? J. Clin. Med. Res. 2020; 12(5): 307-14. https://dx.doi.org/10.14740/jocmr4140
Received 18.07.2025
Accepted 30.09.2025
About the Authors
Vitaly F. Nesterov, PhD, Senior Researcher, Head of the Obstetric Department, Ural Scientific Research Institute for Maternal and Child Care, Ministry of Health of Russia, 620028, Russia, Yekaterinburg, Repin str., 1, +7(965)521-34-94, dr.nesterov2014@yandex.ru, https://orcid.org/0000-0002-5532-6587Galina B. Malgina, Dr. Med. Sci., Professor, Scientific Secretary, Leading Researcher, Ural Scientific Research Institute for Maternal and Child Care, Ministry of Health
of Russia, 620028, Yekaterinburg, Repin str., 1, +7(343)371-08-78, galinamalgina@mail.ru, https://orcid.org/0000-0002-5500-6296
Maria M. Dyakova, PhD, Junior Researcher, Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia,
620028, Russia, Ekaterinburg, Repin str., 1, +7(343)37-189-11, +7(950)550-06-52, mariadakova40@mail.ru, https://orcid.org/0000-0001-7911-6783
Anastasia A. Grishkina, PhD, Pathologist, Head of the Department of Pediatric Pathology, Sverdlovsk Regional Bureau of Anatomical Pathology,
620028, Russia, Yekaterinburg, Volgogradskaya str., 185a, xumukyc.ru@mail.ru, https://orcid.org/0000-0001-7433-2217
Corresponding author: Vitaly F. Nesterov, dr.nesterov2014@yandex.ru



