Relationship between the severity of endothelial dysfunction and metabolic disorders and the severity of climacteric syndrome
Aim. Investigation of the character and extent of changes in endothelial functional activity and metabolic disorders (visceral obesity and insulin resistance, lipid imbalance) in climacteric women, depending on severity of climacteric syndrome (CS). Materials and methods. The study included 168 women in perimenopause and 216 women in early perimenopause with CS. Modified Kupperman–Uvarova menopausal index was assessed. The parameters of lipid metabolism, fasting blood glucose, the level of immunoreactive insulin (IRI) and the HOMA-IR index were assessed. Waist circumference was measured and Waist-Hip Ratio (WHR) was calculated. Flow-dependent vasodilation (FDV) of brachial artery was assessed using the reactive hyperemia test. Enzyme immunoassay was used to assess the levels of endothelial dysfunction (ED) markers – endothelin-1 (ET-1, nitric oxide metabolites, von Willebrand factor antigen (VWF:Ag) and asymmetric dimethylarginine (ADMA) in blood plasma. Results. Significant disturbance of endothelial function was found in women with moderate and severe symptoms of CS in comparison to the women with mild disease: the change in brachial artery diameter (10.1±5.2% versus 12.2±6.5% (р<0.01)) and lower level of NO2 metabolites (12.1μml/l (10,3;13,8) versus 13.8 ) μml/l (12.2;15.3, respectively (р=0,042). Along with more severe abdominal obesity and IRI, the progressive intensity of vasomotor symptoms of CS was noted. In women with severe CS compared to a mild disease, obesity and dyslipidemia were more frequent (in 60.2% versus 40% of patients, p<0.001), as well as the metabolic syndrome (in 58.7% versus 41.3% , respectively (p<0.01)). Conclusion. The severity of CS was associated with significant metabolic changes and endothelial dysfunction. The severity of the vasomotor symnptoms of CS may reflect the severity of ED in menopausal women.Tolstov S.N., Salov I.A., Rebrov A.P.
Keywords
Many studies have been devoted to the problem of cardiovascular diseases (CVDs) in women of menopausal age due to the high morbidity and mortality rate [1, 2]. However, hormonal changes in women start quite early, in a few years before the decline in ovarian function and the onset of menopause, which leads to a wide range of metabolic and vascular changes that significantly increase the risk of cardiovascular diseases [3].
Despite significant variability of signs of menopausal syndrome (MS), most women in the menopausal period note the appearance of vasomotor symptoms; only a quarter of them seek medical help [4, 5], however this period is the best time for taking preventive measures to reduce cardiovascular risks [3, 6].
Vasomotor symptoms are most typical for MS and are associated not only with the deterioration of quality of life, but also have other consequences. Recent studies indicate an increased cardiovascular risk in women with severe vasomotor symptoms [7–9].
In some studies, a relationship between the severity of vasomotor symptoms of MS and an increased risk of metabolic syndrome was noted [10].
Other studies did not reveal the relationship between vasomotor symptoms and increased cardiovascular risk [11, 12].
The few and inconsistent findings [13, 14] regarding the relationship between the severity of MS and the risk of CVD was one of the reasons to conduct this study.
The aim of the study was to determine the nature and severity of changes in the functional activity of the endothelium, and metabolic disorders (the severity of visceral obesity, insulin resistance, and lipid disorders) in women in menopause, depending on the severity of MS.
Materials and methods
We performed a cross-sectional open non-randomized comparative study, which included 384 women aged 51.1 (48.0; 56.0) with clinical manifestations of MS. The patients included in the study were divided into two groups based on the universal criteria of the aging of the female reproductive system STRAW+10: group 1 included 168 women with the late menopause transition onset, group 2 included 216 women with the early postmenopause onset [15].
The exclusion criteria were: atherosclerosis with current or past acute cardiovascular symptoms, chronic forms of coronary artery disease, diabetes mellitus, arterial hypertension during reproductive period, premature or early menopause, sterilization, thyroid diseases, and other severe somatic disorders.
The study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of the V.I. Razumovsky Saratov State Medical University of the Ministry of Health of Russian Federation (protocol No. 2 dated October 04, 2016). All women included in the study had signed informed consent to participate in the study.
All women were tested for basic biochemical blood parameters, including the lipid metabolism parameters.
The modified Kupperman–Uvarova menopausal index (MMI) was used to assess the severity of vasomotor, psychoemotional, endocrine and metabolic symptoms of MS.
For neurovegetative disorders, the sum of points less than 10 was considered as the absence of vasomotor symptoms of the MS; a weak severity of neurovegetative symptoms was considered at 10–20 points; a medium severity – at 21 to 30 points, and severe manifestation – at the sum of points over 30. The metabolic, endocrine, and psychoemotional disorders were assessed in the same way: absence of symptoms at 0 points, a weak severity was considered at 1–7 points, a medium severity – at a total of 8–14 points, and a severe form of MS – at a sum of points more than 14 [16].
The following anthropometric indicators were assessed: body mass index (BMI), waist circumference, hip circumference, and waist–hip ratio.
All women were tested for fasting blood glucose level, and plasma immunoreactive insulin (IRI) level by enzyme-linked immunosorbent assay. The HOMA-IR index (Homeostasis Model Assessment of Insulin Resistance) was calculated using the formula: HOMA-IR = fasting IRI (µU/ml) × fasting glucose (mmol/l)/22.5. Based on the results, the presence of insulin resistance syndrome (IR) was assessed, which was confirmed at IRI values ≥12 μU/ml, and HOMA index >2.77 c.u.
The functional state of the vascular endothelium was studied noninvasively by a duplex ultrasound examination with assessment of flow-dependent vasodilation (FDV) of the brachial artery during reactive hyperemia test using ultrasound system Medison EKO 7. The absence of endothelial dysfunction was confirmed by an increase in the diameter of the brachial artery by 10% or more from the initial level.
Biochemical markers of endothelial dysfunction (ED) were assessed in blood samples taken during mornimg fasting with the use of enzyme-linked immunosorbent assay using ELISA kits (USA) according to the instructions. The content of endothelin-1 (ET-1), nitric oxide metabolites (nitrite NO2 and nitrate NO3), and quantitative level of total stable nitric oxide metabolites (NOx). The level of von Willebrand factor (vWF:Ag) antigen, and the level of asymmetric dimethylarginine (ADMA) were determined.
Statistical analysis
The statistical software package Statistica 10.0 (StatSoft Inc., USA) was used for statistical processing of data.
The normality of the distribution was assessed using the Shapiro–Wilk test. In case of normal distribution of characteristic variations, data was described by arithmetic mean and standard deviation M (SD), in case of non-normal distribution of characteristic variations – by median (Me) and interquartile range of Me (Q1;Q3). Qualitative indicators were presented as absolute and relative values (% of the total number of cases).
To compare the mean values of indicators in two independent samples in the case of normal distribution, the Student's t-test was used for each of the samples. In the case of distribution other than normal, the nonparametric Mann–Whitney U-test was used for two independent samples. The Kruskal–Wallis test with the Bonferroni correction was used for cross-sectional comparison of three independent groups. The analysis of differences by qualitative characteristics was performed using the Pearson's χ2 test.
The Spearman's rank correlation coefficient (r) was used to identify and assess the strength of relationship between the studied characteristics. Differences were considered statistically significant at p <0.05.
Results
Table 1 demonstrates the main clinical and demographic characteristics of the women included in the study.
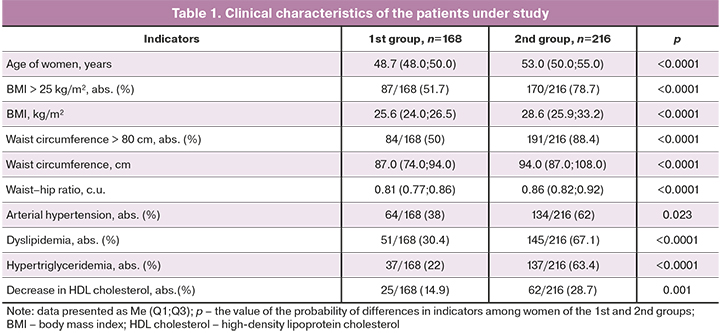
An increase in the incidence and severity of cardiovascular risk factors and lipid metabolism disorders was found in women of group 2 in comparison with women of group 1. The most typical changes in women in menopause were rapid weight gain with visceral adipose tissue distribution, which was more pronounced in women in postmenopause.
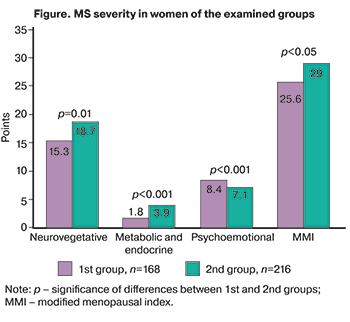 MS was more severe in postmenopausal women than in women during the menopause transition (Figure).
MS was more severe in postmenopausal women than in women during the menopause transition (Figure).
Psycho-emotional disorders were more common among premenopausal women while neurovegetative vasomotor and neuroendocrine symptoms predominated in postmenopausal women.
The severity of ED was assessed in relationship to the severity of menopausal disorders, in the two subgroups of women, either with moderate or severe MS (group 1a, n=115) and mild MS (group 1b, n=269).
The modified Kupperman–Uvarova menopausal index in women of the studied groups was 38.0 (6.5) and 24.4 (4.6) points, respectively (p <0.0001).
Women in group 1 had significantly more pronounced disorders of the vasoregulatory function of the endothelium (flow-dependent vasodilation of brachial artery was 10.1 (5.2)%, and 12.2 (6.5)% for group 1a and group 1b, respectively, p<0.01) and lower levels of nitric oxide metabolites compared to women of group 1b (Table 2). The levels of other ED markers (ET-1, vWF:Ag, and ADMA) did not differ significantly.
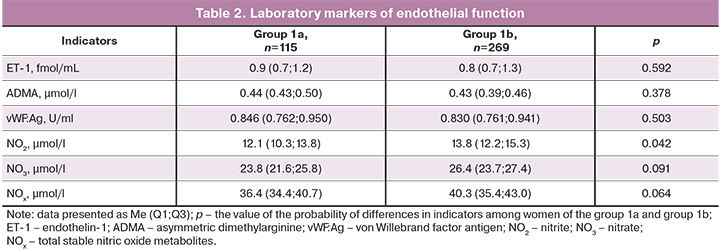
The study revealed the relationship between the severity of vasomotor manifestations of MS and changes in the vasoregulatory function of the brachial artery (r=-0.25; p=0.03).
To assess the relationship of the severity of menopausal disorders and lipid metabolism disturbance, the patients were divided into three groups: women with normal body weight, women with excess body weight, and women with obesity (Table 3).
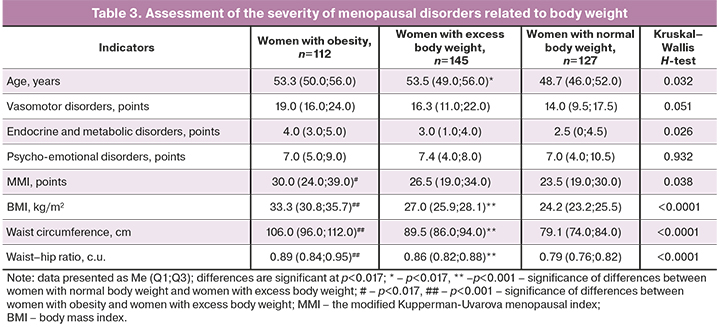
The severity and nature of obesity in women of the studied groups differed significantly.
Normal body weight was observed in 78/168 (46.4%) of women during the menopausal transition. Excess body weight (114/216 (52.8%) of women) and obesity (100/216 (46.3%) of women) were more common in postmenopausal women.
The pairwise comparison in women of the studied groups did not reveal any significant differences in the severity of menopausal disorders (the Bonferroni correction, significant level of differences at p<0.017), however there was a clear tendency to an increase in the severity of vasomotor, endocrine and metabolic disorders with increasing body weight. Most women with normal body weight had mild MS (99/127 (78%) of women); no severe MS were found in this group of women.
Mild MS was noted in 98/145 (67.6%) of women with excess body weight (less often than in women with normal body weight, χ2=3.63, p=0.056), moderate MS was diagnosed in 31/145 (21.4%) of women in this group, and severe MS was registered in 16/145 (11%) of women (χ2=14.8, p<0.001).
The most severe MS were observed in the group of women with obesity. Mild course of MS was registered in 52/112 (46.4%) of women in this group, and moderate or severe MS occurred more often in this group – in 40/112 (35.7%) and 20/112 (17.9%) of women, respectively, i.e. more commonly than in women with excess body weight (χ2=2.46, p=0.081).
Associations between BMI and vasomotor disorders (r=0.41, p<0.0001), endocrine and metabolic disorders (r=0.42, p<0.0001), and MS severity (r=0.38, p<0.0001) were found. Similar associations were noted between the severity of abdominal obesity, vasomotor (r=0.32, p=0.006) and endocrine and metabolic manifestations of MS (r=0.38, p<0.0001), and MS severity (r=0.31, p=0.008).
The relationship between the severity of MS and the severity of IR were studied in two subgroups: group 2a included women without IR, group 2b – women with IR.
IR was revealed in 60/168 (35.7%) of women during the menopausal transition and in 147/216 (68%) of women in the early postmenopausal period (χ2=39.7, p<0.0001).
The age of women in groups 2a and 2b differed significantly: 49 (46; 55) years and 52 (49; 56) years, respectively (p=0.02). Abdominal obesity was significantly more common in women with IR. While BMI in the two subgroups was similar (26.0 (24.2; 30.8) kg/m2 in group 2a and 28.1 (25.7; 31.2) kg/m2 in group 2b, p=0.392), women in group 2b had larger waist circumference than women of group 2b: 92 (85; 104) cm versus 85 (82; 95) cm (p=0.054), and waist–hip ratio: 92 (85; 104) cm versus 85 (82; 95) cm, respectively (p=0.009).
The severity of MS (in the presence of IR) is presented in Table 4.
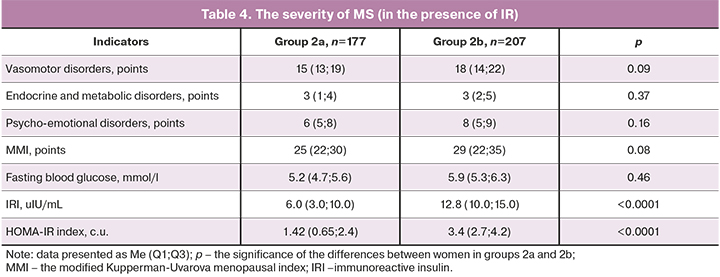
Despite the fact that the character and severity of menopausal disorders were comparable, a clear tendency to an increase in the severity of vasomotor symptoms and menopausal disorders was noted in women with IR.
Dyslipidemia was more common in patients with moderate and severe MS (69/115 (60%) women), compared to patients with mild MS (127/269 (47.2%) women), χ2=5.27, p=0.021. Significant differences were also found in the incidence of metabolic syndrome: this disorder was found in 64/269 (23.8%) and 91/115 (79.1%) of women with mild, moderate, and severe MS, respectively (χ2=100.1, p<0.001).
Discussion
Previous studies did not show clear relationship between the severity of vasomotor symptoms of MS and an increased risk of CVD. These conditions were considered to occur independently of each other [13, 14, 17].
Some researchers reported a decrease in the risk of overall and cardiovascular mortality in women with nighttime hot flashes, regardless of the existing cardiovascular risk factors [11].
In recent years, more evidence was found demonstrating that the severity of MS may be associated with an increase in cardiovascular risk, and the severity of vasomotor syndromes may reflect the severity of metabolic and vascular disorders; however, this association require further study [7, 8, 9, 18].
The results of a recent pooled analysis of six prospective studies which included 23,365 women of menopausal age with varying degrees of MS severity are of particular interest. The relationship between the MS symptoms frequency and severity, and menopause onset with an increased risk of a CVD was assessed.
Increased intensity of hot flashes or night sweats was associated with a higher risk of CVD, regardless the time of symptoms onset (during the menopausal transition or in postmenopause). The CVD risk in women with pronounced hot flashes, night sweats, and any vasomotor symptoms was 1.83 (95% CI 1.22–2.73), 1.59 (95% CI 1.07–2.37), and 2.11 (95% CI 1.62–2.76), respectively [19].
Some researchers state that neurohumoral imbalance occurring in women with MS is one of the factors responsible for the association between the severity of MS and an increased risk of CVD.
Vasomotor symptoms are associated with increased activity of the sympathetic nervous system, which, in turn, is associated with metabolic syndrome – a known risk factor for CVD [10].
Our study revealed that the severity of vasomotor symptoms of MS increased with the progression of obesity and increase of IR. We also found the association between the severity and character of obesity and the severity of vasomotor symptoms of MS.
In our study, dyslipidemia was significantly more common in women with more severe MS. Some researchers noted an increase in blood triglyceride levels in women with severe vasomotor symptoms [20].
These findings are consistent with the results of other studies which also noted the relationship between the severity of vasomotor symptoms of MS and the risk of metabolic syndrome [10]. In our study, metabolic syndrome was also significantly more common in women with more severe MS.
Another possible cause of this relationship is endothelial dysfunction in women with severe MS [21].
In our study, the severity of MS in women was associated with changes in the functional activity of the vascular endothelium. A significant decrease in vasoregulatory function of the brachial artery, and levels of nitric oxide metabolites was found in patients with moderate and severe MS compared to women with mild MS. Regression analysis showed the relationship between the severity of vasomotor symptoms and the severity of MS with the level of NO2.
In our opinion, the severity of vasomotor symptoms of MS may provide additional information about the severity of endothelial dysfunction (in addition to CVD risk factors) in women of menopausal age.
Conclusion
The severity of MS was associated with more pronounced metabolic changes (increased body weight, severity of abdominal obesity, increased IR, and lipid disorders), and the severity of ED. The severity of vasomotor symptoms of MS may reflect the severity of endothelial dysfunction in women of menopause age.
Study limitations
Our study attempted to find the cause of the relationship between the severity of menopausal disorders and an increased cardiovascular risk in women of menopause age.
One of the limitations of the study was the heterogeneity of the main clinical characteristics of patients in the studied groups (age, body weight, severity of abdominal obesity, severity of MS). This was primarily due to the differences in the menopausal status, which makes it practically impossible to select clinically comparable groups without introducing additional systematic errors. Therefore, we evaluated the studied indicators depending on the severity of MS, obesity, and IR disregarding the menopausal status of the women.
References
- Newson L. Menopause and cardiovascular disease. Post Reprod. Health. 2018; 24(1): 44-9. https://dx.doi.org/10.1177/2053369117749675.
- Baber R.J., Panay N., Fenton A.; IMS Writing Group. 2016 IMS Recommendations on women's midlife health and menopause hormone therapy. Climacteric. 2016; 19(2): 109-50. https://dx.doi.org/10.3109/13697137.2015.1129166.
- Юренева С.В., Ильина Л.М., Сметник В.П. Старение репродуктивной системы женщин: от теории к клинической практике. Часть I. Эндокринные и клинические характеристики стадий репродуктивного старения женщин. Акушерство и гинекология. 2014; 3: 21-7. [Yureneva S.V., Ilyina L.M., Smetnik V.P. Female reproductive system aging: From theory to clinical practice. Part 1. The endocrine and clinical characteristics of female reproductive system aging stages. Obstetrics and Gynecology. 2014; 3: 21-7. (in Russian)].
- Pinkerton J.V. Hormone therapy for postmenopausal women. N. Engl. J. Med. 2020; 382(5): 446-55. https://dx.doi.org/10.1056/NEJMcp1714787.
- Avis N.E., Crawford S.L., Greendale G., Bromberger J.T., Everson-Rose S.A., Gold E.B. et al. Study of Women's Health Across the Nation. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern. Med. 2015; 175(4): 531-9. https://dx.doi.org/10.1001/jamainternmed.2014.8063.
- Collins P., Webb C.M., de Villiers T.J., Stevenson J.C., Panay N., Baber R.J. Cardiovascular risk assessment in women – an update. Climacteric. 2016; 19(4): 329-36. https://dx.doi.org/10.1080/13697137.2016.1198574.
- Thurston R.C. Vasomotor symptoms: natural history, physiology, and links with cardiovascular health. Climacteric. 2018; 21(2): 96-100. https://dx.doi.org/10.1080/13697137.2018.1430131.
- Gast G.C., Pop V.J., Samsioe G.N., Grobbee D.E., Nilsson P.M., Keyzer J.J. et al. Vasomotor menopausal symptoms are associated with increased risk of coronary heart disease. Menopause. 2011; 18(2): 146-51.
- Biglia N., Cagnacci A., Gambacciani M., Lello S., Maffei S., Nappi R.E. Vasomotor symptoms in menopause: a biomarker of cardiovascular disease risk and other chronic diseases? Climacteric. 2017; 20(4): 306-12. https://dx.doi.org/10.1080/13697137.2017.1315089.
- Tuomikoski P., Savolainen-Peltonen H. Vasomotor symptoms and metabolic syndrome. Maturitas. 2017; 97: 61-5. https://dx.doi.org/10.1016/j.maturitas.2016.12.010.
- Svartberg J., von Mühlen D., Kritz-Silverstein D., Barrett-Connor E. Vasomotor symptoms and mortality: the Rancho Bernardo Study. Menopause. 2009; 16(5): 888-91. https://dx.doi.org/10.1097/gme.0b013e3181a4866b.
- Dam V., Dobson A.J., Onland-Moret N.C., van der Schouw Y.T., Mishra G.D. Vasomotor menopausal symptoms and cardiovascular disease risk in midlife: A longitudinal study. Maturitas. 2020; 133: 32-41. https://dx.doi.org/10.1016/j.maturitas.2019.12.011.
- Muka T., Oliver-Williams C., Colpani V., Kunutsor S., Chowdhury S., Chowdhury R. et al. Association of vasomotor and other menopausal symptoms with risk of cardiovascular disease: a systematic review and meta-analysis. PLoS One. 2016; 11(6): e0157417. https://dx.doi.org/10.1371/journal.pone.0157417.
- Szmuilowicz E.D., Manson J.E., Rossouw J.E., Howard B.V., Margolis K.L, Greep N.C. et al. Vasomotor symptoms and cardiovascular events in postmenopausal women. Menopause. 2011; 18(6): 603-10. https://dx.doi.org/10.1097/gme.0b013e3182014849.
- Harlow S.D., Gass M., Hall J.E., Lobo R., Maki P., Rebar R.W. et al.; STRAW 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause. 2012; 19(4): 387-95. https://dx.doi.org/10.1097/gme.0b013e31824d8f40.
- Вихляева Е.М. Руководство по эндокринной гинекологии. М.: МИА; 2006. 784с. [Vikhlyaeva E.M. Guide to endocrine gynecology. Moscow: MIA. 2006; 784 р. (in Russian)].
- Tuomikoski P., Ebert P., Groop P.H., Haapalahti P., Hautamäki H., Rönnback M. et al. Effect of hot flushes on vascular function: a randomized controlled trial. Obstetю Gynecol. 2009; 114(4): 777-85. https://dx.doi.org/10.1097/AOG.0b013e3181b6f268.
- Martínez Pérez J.A., Palacios S., Chavida F., Pérez M. Severity of menopausal symptoms and cardiovascular and osteoporosis risk factors. Climacteric. 2013; 16(2): 226-34. https://dx.doi.org/10.3109/13697137.2012.688077.
- Zhu D., Chung H.F., Dobson A.J., Pandeya N., Anderson D.J., Kuh D. et al. Vasomotor menopausal symptoms and risk of cardiovascular disease: a pooled analysis of six prospective studies. Am. J. Obstet. Gynecol. 2020; 223(6):898. e1-898. e16. https://dx.doi.org/10.1016/j.ajog.2020.06.039.
- Kaya C., Cengiz H., Yeşil A., Ekin M., Yaşar L. The relation among steroid hormone levels, lipid profile and menopausal symptom severity. J. Psychosom. Obste.t Gynaecol. 2017; 38(4): 284-91. https://dx.doi.org/10.1080/0167482X.2017.1321633.
- Thurston R.C., Chang Y., Barinas-Mitchell E., Jennings J.R., von Känel R., Landsittel D.P. et al. Physiologically assessed hot flashes and endothelial function among midlife women. Menopause. 2018; 25(11): 1354-61. https://dx.doi.org/10.1097/GME.0000000000001239.
Received 07.04.2021
Accepted 22.04.2021
About the Authors
Sergey N. Tolstov (Corresponding author), Dr. Med. Sci., Professor of the Department of Therapy with Courses in Cardiology, Functional diagnostics and Geriatrics,V.I. Razumovsky Saratov State Medical University, Ministry of Health of Russia; Head of the Vascular Center of the Regional Clinical Cardiological Dispensary.
Tel.: +7(8452)39-28-09. E-mail: tolstovsn@mail.ru. ORCID: 0000-0002-4546-9449.
112 B. Kazachya str., Saratov, 410012, Russian Federation; 16 Krymskiy proyezd str., Saratov, 410039, Russian Federation.
Igor A. Salov, Dr. Med. Sci., Professor, Head of Оbstetrics and Gynecology Department, V.I. Razumovsky Saratov State Medical University, Ministry of Health of Russia.
Tel.: +7(8452)51-51-21. E-mail: salov.i.a@mail.ru. ORCID: 0000-0002-1926-5418. 112 B.Kazachya str., Saratov, 410012, Russian Federation.
Andrey P. Rebrov, Dr. Med. Sci., Professor, Head of Hospital Therapy Department, V.I. Razumovsky Saratov State Medical University, Ministry of Health of Russia.
Tel.: +7(8452)49-14-37. E-mail: andreyrebrov@yandex.ru. ORCID: 0000-0002-3463-7734. 112 B. Kazachya str., Saratov, 410012, Russian Federation.
For citation: Tolstov S.N., Salov I.A., Rebrov A.P. Relationship between the severity of endothelial dysfunction and metabolic disorders and the severity of climacteric syndrome.
Akusherstvo I Gynecologia/Obstetrics and Gynecology (in Russian). 2021; 8: 119-126 (in Russian)
https://dx.doi.org/10.18565/aig.2021.8.119-126



