Excess adipose tissue and chronic subclinical inflammation in patients with polycystic ovary syndrome
Objective: To analyze the serum levels of proinflammatory markers in patients with polycystic ovary syndrome (PCOS) and assess their association with body composition and metabolic parameters.Kirillova E.D., Vtorushina V.V., Krechetova L.V., Ivanets T.Yu., Chernukha G.E.
Materials and methods: This single-center cross-sectional study included 148 women with PCOS (mean age 25.1 (5.5) years). Of these, 118 had PCOS (mean age 25.1 (5.5) years), 14 were overweight and obese without PCOS (mean age 26.1 (7.4) years) and 16 were somatically healthy women (mean age 25.3 (5.1) years). All participants underwent comprehensive clinical, laboratory, and instrumental examination.
Results: Patients with PCOS had significantly higher levels of IL-6, TNF-α, and CRP than those in the control group; however, there were no statistically significant differences in the levels of IL-1, leptin, and adiponectin. PCOS patients had signs of chronic subclinical inflammation in the form of elevated IL-6 and CRP levels in 39% and 37% of the cases, respectively. Hidden obesity was diagnosed in 65.0% of PCOS patients with a normal BMI, manifesting as excess visceral adipose tissue in 46.2% of cases. This group was seven times more likely to have elevated CRP levels and nearly four times more likely to be insulin resistant than patients with normal total body fat percentage.
Conclusion: It is appropriate to recommend adipose tissue densitometry and assessment of levels of proinflammatory markers to identify risk groups for pregnancy complications, cardiovascular disease, and type 2 diabetes mellitus in patients with PCOS with normal BMI and for further correction of modifiable risk factors by lifestyle changes, drug therapy, and pre-pregnancy care.
Authors' contributions: Kirillova E.D., Chernukha G.E. – conception and design of the study, manuscript drafting; Kirillova E.D., Vtorushina V.V., Ivanets T.Yu. – data collection and analysis; Kirillova E.D. – statistical analysis; Chernukha G.E., Krechetova L.V. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: Research work “Clinical and pathogenetic aspects of polycystic ovarian syndrome in relation to the gut microbiota”.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Kirillova E.D., Vtorushina V.V., Krechetova L.V., Ivanets T.Yu., Chernukha G.E. Excess adipose tissue and chronic subclinical inflammation in patients with polycystic ovary syndrome.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (4): 111-119 (in Russian)
https://dx.doi.org/10.18565/aig.2023.56
Keywords
Polycystic ovary syndrome (PCOS) is a common endocrine disorder with a prevalence of up to 22.5% [1], depending on the diagnostic criteria. PCOS is characterized by insulin resistance (IR) and excess adipose tissue, which predisposes to the development of type 2 diabetes mellitus (DM), cardiovascular disease, and pregnancy complications [2]. Following the introduction of body composition methods into clinical practice, evidence has emerged that excess adipose tissue, including the most metabolically active visceral tissue, is present not only in obese and overweight patients but also in PCOS patients with a normal body mass index (BMI) [3]. It is worth noting that 20–30% of these patients have metabolic dysfunction in the form of impaired glucose tolerance (IGT), dyslipidemia (DLP), and IR, provoking compensatory hyperinsulinemia (HI) and hyperandrogenism [4].
In recent years, indirect signs of chronic subclinical inflammation in PCOS patients have been reported to be associated with a moderate increase in proinflammatory cytokines and C-reactive protein (CRP) [5, 6]. A positive correlation of CRP and IR with adipose tissue mass has also been reported [5–7]. It is unclear whether chronic subclinical inflammation is associated with PCOS or with BP and obesity. It is also debatable whether interleukin (IL)-6, the most active inducer of CRP production in the liver, is elevated in PCOS patients. For example, the results of a meta-analysis by Escobar-Morreale et al. showed no difference in serum IL-6 levels between patients with PCOS and healthy women, whereas several other studies have demonstrated a significant increase in IL-6 levels [6, 8–10].
The potential association between PCOS and increased levels of tumor necrosis factor-alpha (TNF-α) is also of interest because, as an important mediator of IR, it is associated with components of metabolic syndrome such as IGT, arterial hypertension, and DLP [11]. The results of the studies on TNF-α levels in PCOS are mixed. In addition to reports of elevated TNF-α levels compared to controls, even when BMI is normal [12, 13], there is evidence of no difference between the groups [7, 10].
Several studies have demonstrated an association between inflammation in adipose tissue and metabolic dysfunction [14–16]. However, while the relationship between inflammation and obesity is supported by literature, elevated proinflammatory markers in patients with PCOS, especially those with normal body weight, remain a matter of debate.
This study aimed to investigate the levels of serum proinflammatory markers in patients with PCOS and their association with body composition and metabolic parameters.
Materials and methods
This was a single-center cross-sectional study. PCOS was diagnosed according to Rotterdam criteria (2003), including clinical and/or biochemical hyperandrogenism, amenorrhea, anovulation or oligomenorrhea, and polycystic-appearing ovaries on ultrasound. The exclusion criteria were severe somatic diseases, systemic autoimmune diseases, and hormone therapy for less than 3 months before study inclusion (Fig. 1).
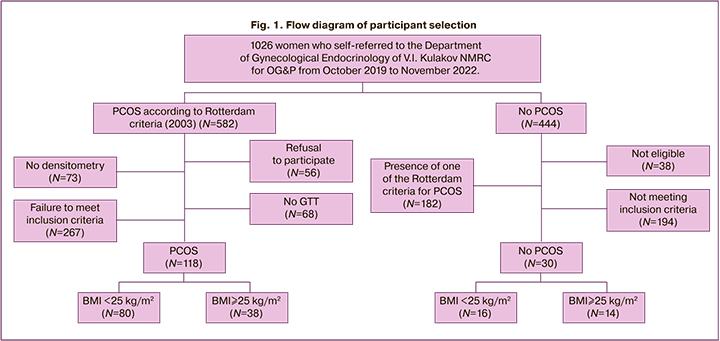
This study included 148 women aged between 18 and 40 years. Of these, 118 had PCOS (mean age, 25.1 (5.5) years; BMI, 24.1 (5.1) kg/m2). The comparison group included 14 overweight and obese women without PCOS (mean age 26.0 (7.4) years, BMI 29.1 (3.4) kg/m2]). The control group included 16 somatically healthy women (mean age 25.3 (5.1) years, BMI 20.4 (1.7) kg/m2). There were no statistically significant differences in age between groups (p>0.05). The study group was divided into BMI subgroups, including group 1 (n=80) with BMI <25 kg/m2 and group 2 (n=38) with BMI≥25 kg/m2 (because of the small number, it was decided to combine overweight and obese patients into one subgroup).
In accordance with clinical guidelines, a hormone profile on day 2–3 of the spontaneous or progesterone-induced menstrual cycle was carried out for the diagnosis of PCOS, and a pelvic ultrasound (US) was performed on day 5–7 of the cycle [17].
Serum levels of IL-1β, IL-6, and TNF-α were measured by enzyme immunoassay using the Vector-Best test systems (Russia). The results were recorded using an Infiniti F50 "TECAN" tablet spectrophotometer. Leptin and adiponectin levels were determined by enzyme immunoassay using Leptin ELISA (DBC, Canada) and Human Adiponectin ELISA (BioVendor, Czech Republic) commercial kits.
CRP and blood lipid spectra were determined by photometric and turbidimetric methods using a BA-400 automatic analyzer (Biosystems, Spain). Carbohydrate metabolism and GI disorders were diagnosed based on two-hour oral glucose tolerance test with 75 g of glucose. Glucose and immunoreactive insulin (IRI) levels were measured under fasting conditions and every 60 min during the 2-hour study.
The insulin sensitivity assessment model (HOMA-IR) was calculated as follows: fasting glucose (mmol/l) × fasting IRI (µED/ml)/22.5. A HOMA index greater than 2.7 was considered as a criterion for IR [18]. IGT was diagnosed based on glucose levels more than 7.8 mmol/l two hours post-glucose load or fasting glucose levels above 6.1 and below 7.0 mmol/l [19].
Body composition was assessed using dual-energy X-ray absorptiometry (densitometry) on a Lunar model 8743 machine (GE Medical Systems, Madison, WI, USA). The following parameters were analyzed: percentage of total adipose tissue (TAT), whole-body and trunk adipose mass, and android and gynoid fat distribution. A TAT percentage ≥30 was interpreted as excess adipose tissue or hidden obesity [20-22]. The volume and weight of visceral adipose tissue (VAT) were assessed using CoreScan, and excess VAT was diagnosed to be greater than 235 g [23].
Statistical analysis
Statistical analysis was performed using the SPSS software (IBM Statistical Package for the Social Sciences, version 21). Quantitative variables showing normal distribution were expressed as mean (M) and standard deviation (SD) and presented as M (SD); otherwise, the median (Me) with interquartile range (Q1; Q3) was reported. Categorical variables were reported as frequencies and percentages. ANOVA for normally distributed numeric parameters was applied to find differences between patient groups, followed by pairwise comparison of the groups using Student's t-test for two independent samples. Otherwise, comparisons of quantitative data were made by nonparametric Kruskal–Wallis tests (multiple groups), and then paired comparisons of groups were performed using the Mann–Whitney U-test for unpaired groups. For multiple comparisons, Bonferroni adjustment was applied. Categorical variables were compared using the chi-square test (χ2) with Yates correction; for pairwise comparisons between groups, Fisher's exact test for small samples was used. Correlation analysis was performed using Pearson's method (for normally distributed parameters) or nonparametric Spearman's test. The critical level of significance for statistical hypothesis testing was 0.05.
The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, and all participants signed an informed consent.
Results
In the PCOS group, 19/118 (16.1%) participants were diagnosed as overweight (BMI 25–29.9 kg/m2) and 19/118 (16.1%) as obese (BMI ≥30 kg/m2). Clinical signs of androgenism in the form of hirsutism and acne were found in 67/118 (56.8%) patients, and biochemical hyperandrogenism in 94/118 (79.7%) patients. All patients had menstrual irregularities:81.5% had oligomenorrhea, and 18.5% had primary or secondary amenorrhea.
Our analysis showed that the mean serum levels of IL-6, TNF-α, and CRP in PCOS patients were significantly higher than those in the control group (p<0.05) (Table 1). Elevated CRP and IL-6 levels were observed in 39.8% and 37.2% of PCOS patients, respectively, and none of the controls. No increase in TNF-α level was observed in either group. No statistically significant differences in IL-1, leptin, or adiponectin levels were observed between the groups. None of the mean values of the studied parameters, except for adiponectin, differed significantly between the comparison group and the PCOS group, but CRP and IL-6 levels were higher in the comparison group than in the control group (p=0.002 and p=0.03, respectively). There were no increases in CRP levels in the comparison group, whereas IL-6 levels exceeded normal values in 21% of cases, almost twice as rarely as in PCOS.
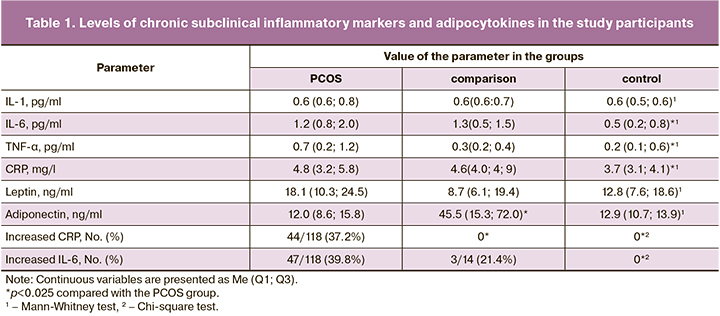
The analysis of chronic subclinical inflammatory markers categorized by BMI in Figure 1 showed no difference in the mean IL-6, TNF-α, and CRP levels between the comparison group (women without PCOS, BMI≥25 kg/m2) and subgroup 1 (PCOS, BMI<25 kg/m2). In subgroup 2 (PCOS at BMI≥25 kg/m2), these values were significantly higher than those in all other groups (Fig. 2).
The frequency of CRP elevation in subgroup 2 was 22/38 (60.5%), twice as high as that in subgroup 1 (20/80 (25%), (p<0.001). Elevated IL-6 levels were observed in subgroup 2 in 27/38 (71.1%) cases, more than 3-fold higher (p=0.002) than in subgroup 1 and the comparison group, with frequencies of 14/80 (17.5%) and 3/14 (21.0%), respectively (p>0.05).
The results of densitometry showed that, despite normal BMI, 65.0% of patients in subgroup 1 (BMI<25 kg/m2) had hidden obesity in the form of excess TAT, showing excess VAT in 46.2% (24/52) of cases (Table 2).
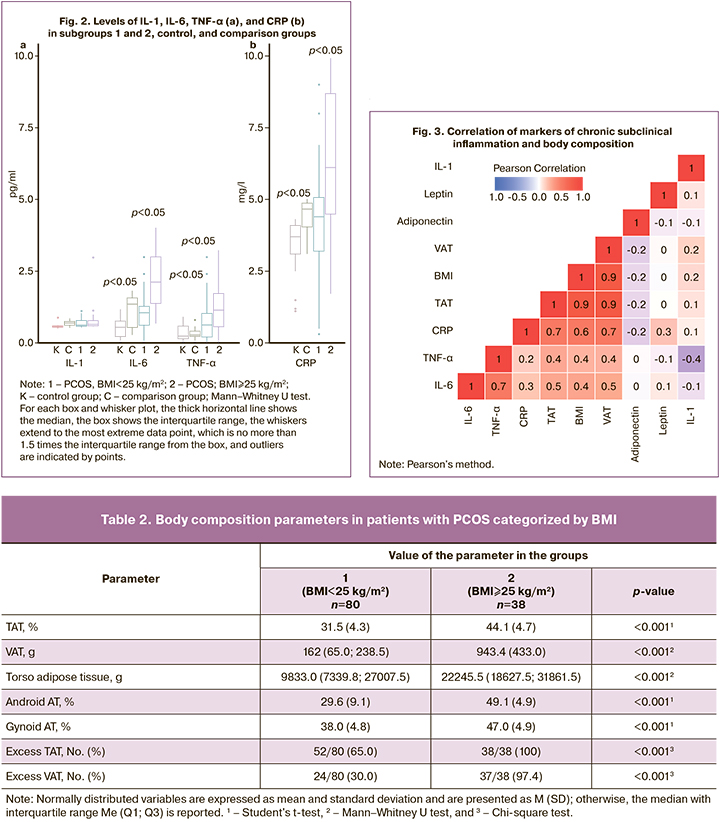
The findings of the correlation analysis shown in Figure 3 demonstrate a direct correlation between IL-6 and CRP levels, and body composition parameters. The strongest correlation was found with TAT (r=0.5, p<0.001; r=0.7, p<0.001) and VAT weight (r=0.5, p<0.001; r=0.7, p<0.001). The correlation of CRP with leptin and adiponectin was weaker (0.3 and -0.2, respectively, p<0.05). No correlation was found between the cytokine levels and androgenic profiles.
Given the correlation between pro-inflammatory markers and adipose tissue, as well as the presence of excess adipose tissue in 65.0% of patients with PCOS at BMI <25 kg/m2, body composition and cytokine scores were compared between patients with and without hidden obesity (Table 3).
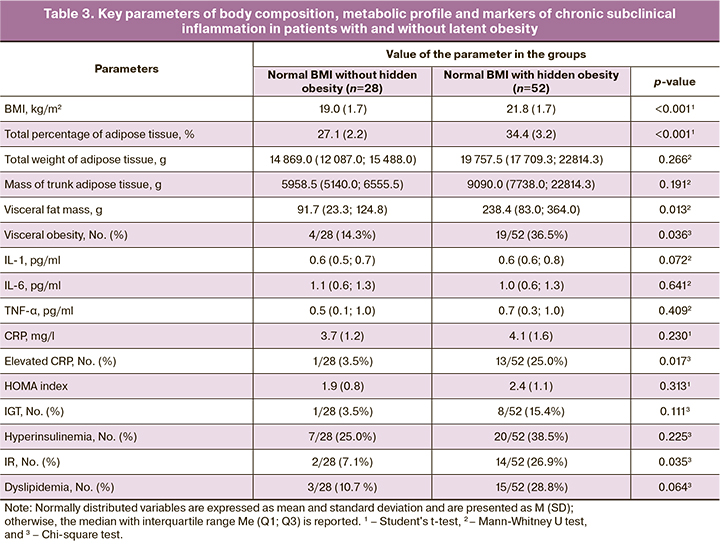
No statistically significant differences were found in the mean levels of the proinflammatory cytokines. However, patients with hidden obesity were seven times more likely to have elevated CRP levels (25.0%) and nearly four times more likely to have an elevated IR index (26.9%) than patients with normal TAT results (3.5% and 7.1%, respectively, p=0.017).
Discussion
In recent years, PCOS has been regarded not only as a major cause of anovulatory infertility and hyperandrogenism but also as a metabolic reproductive syndrome associated with chronic subclinical inflammation [6]. PCOS is considered a risk factor for cardiovascular disease and pregnancy complications (arterial hypertension, preeclampsia, gestational diabetes mellitus, fetal growth restriction), leading to increased perinatal morbidity and mortality due to IR, dyslipidemia, and excess adipose tissue [24]. However, the causal relationship between PCOS, obesity, and elevated levels of pro-inflammatory cytokines has not been clearly defined [25].
This study assessed the major proinflammatory cytokines and CRP levels in PCOS patients according to BMI and body composition. Levels of IL-6, TNF-α, and CRP were higher than those in controls. These data are comparable with the results of a meta-analysis of 31 clinical trials by Escobar-Moralle H.F. et al. (2011), in which the authors concluded that CRP levels were significantly higher in PCOS patients than in healthy women, which may reflect the relationship between PCOS and chronic subclinical inflammation [6]. No correlation was found between PCOS and IL-6 or TNF-α levels. However, there is considerable evidence of their elevation in PCOS patients [6, 8–10, 12, 13].
When the patients were divided into BMI groups, the levels of proinflammatory cytokines and CRP, as well as the frequency of IL-6 elevation, did not differ significantly between the PCOS patients without obesity and the obese and overweight women without PCOS. Despite a normal BMI, every 4th PCOS patient had an elevated CRP and every 5th had an elevated IL-6 level. This can be explained by the fact that, according to recent data, BMI cannot be considered a sufficiently informative indicator that reflects the amount of adipose tissue [4, 26]. Densitometry was therefore performed in 65% of patients with PCOS and normal BMI and showed hidden obesity associated with a sevenfold increase in CRP and a fourfold increase in IR compared with the group without hidden obesity. The presence of elevated CRP in all patients with PCOS and BMI≥25 kg/m2 was associated with evidence of chronic subclinical inflammation in 71.1% of the cases. There is a concept of metabolic inflammation, which results from adipocyte hypertrophy and the accumulation of immune cells secreting pro-inflammatory cytokines in adipose tissue in patients with excess adipose tissue [27]. This suggests that adipose tissue is a major source of pro-inflammatory cytokines that exacerbate the course of PCOS, confirming the utility of body composition assessment by adipose tissue densitometry in these patients. When this is not possible, BMI ≥23 kg/m2 can be considered a clinical marker of excess adipose tissue, increasing the risk of metabolic abnormalities associated with chronic subclinical inflammation [4].
Some researchers have linked the presence and extent of inflammation in PCOS patients to hyperandrogenism. In particular, this issue was discussed in a meta-analysis by Aboeldalyl S. et al. because CRP levels in non-androgenic PCOS phenotypes were comparable to those in controls [27]. In this study, there were no data on the relationship between androgens and markers of chronic subclinical inflammation in PCOS patients. However, proinflammatory cytokines and CRP levels were correlated with body composition parameters, especially VAT. The analysis of adipocytokines was ambiguous, presumably because the study included patients with relatively low BMI.
Thus, excess adipose tissue can exacerbate chronic subclinical inflammation associated with PCOS, leading to a vicious cycle of pathological conditions, metabolic complications, and increased cardiovascular risks. One-third of patients may have markers of subclinical atherosclerosis at a young age, manifesting as endothelial dysfunction, increased intima-media thickness, and carotid calcification [28]. Moreover, PCOS is known to increase the risk of pregnancy complications by 3–4 times [24]. Some authors attribute this to worsening IR during pregnancy and an increase in markers of chronic subclinical inflammation [29]. According to our findings, every third patient with PCOS had signs of chronic subclinical inflammation, which, if not corrected, may lead to pregnancy complications.
In recent years, there has been increasing evidence that chronic subclinical inflammation in PCOS is associated with a dysfunctional gut microbiota. Changes in the gut microbiota, such as decreased colonization rates of short-chain fatty acid-producing bacteria and increased lipopolysaccharide-producing bacteria, are thought to induce chronic subclinical inflammation through lipopolysaccharide penetration into the systemic bloodstream, causing an immune response through an increase in proinflammatory cytokines [30]. This highlights the need for lifestyle modifications and normalization of the gut microbiota in patients with PCOS and excess adipose tissue. This approach is particularly important in preparation for pregnancy because of the unfavorable metabolic background. Some studies indicate a positive effect of metformin and probiotics not only on IR, HA, menstrual rhythm regulation, and weight reduction but also on reducing IL-6 and CRP levels [31]. Thus, the presence of HSI is another argument for optimizing the management of patients with PCOS.
Conclusion
The results of this study suggest that one in three women with PCOS has laboratory evidence of chronic subclinical inflammation in the form of elevated CRP and/or IL-6 levels. Excess adipose tissue was associated with higher levels of proinflammatory markers not only in obese patients but also in those with normal BMI and hidden obesity, in whom CRP elevation was found in one in four cases. These findings allow these categories of patients to be classified as risk groups for the development of metabolic syndrome, pregnancy complications, and cardiovascular disease. They also confirmed the feasibility of correcting modifiable risk factors through lifestyle changes, drug therapy, and preconception care.
References
- Deswal R., Narwal V., Dang A., Pundir C.S. The prevalence of polycystic ovary syndrome: a brief systematic review. J. Hum. Reprod. Sci. 2020; 13(4): 261-71. https://dx.doi.org/10.4103/jhrs.JHRS_95_18.
- Goodman N.F., Cobin R.H., Futterweit W., Glueck J.S., Legro R.S., Carmina E.; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society. American association of clinical endocrinologists, American college of endocrinology, and androgen excess and pcos society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome - part 2. Endocr. Pract. 2015; 21(12): 1415-26. https://dx.doi.org/10.4158/EP15748.DSCPT2.
- Zhu S., Li Z., Hu C., Sun F., Wang C., Yuan H. et al. Imaging-based body fat distribution in polycystic ovary syndrome: a systematic review and meta-analysis. Front. Endocrinol. (Lausanne). 2021; 9(12): 697223.https://dx.doi.org/10.3389/fendo.2021.697223.
- Чернуха Г.Е., Мирошина Е.Д., Кузнецов С.Ю., Иванов И.А. Индекс массы тела, композиционный состав тела и метаболический профиль пациенток с синдромом поликистозных яичников. Акушерство и гинекология. 2021; 10: 103-11. [Chernukha G.E., Miroshina E.D., Kuznetsov S.Yu., Ivanov I.A. Body mass index, body composition, and metabolic profile of patients with polycystic ovary syndrome. Obstetrics and Gynecology. 2021; (10): 103-11. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.10.103-111.
- Kelly C.C.J., Lyall H., Petrie J.R., Gould G.W., Connell J.M.C., Sattar N. Low grade chronic inflammation in women with polycystic ovarian syndrome. J. Clin. Endocrinol. Metab. 2001; 86(6): 2453-5. https://dx.doi.org/10.1210/jcem.86.6.7580.
- Escobar Morreale H.F., Laque-Ramirez M., Gonzalez F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and meta-analysis. Fertil. Steril. 2011; 95(3): 1048-58. https://dx.doi.org/10.1016/j.fertnstert.2010.11.036.
- Mu L., Li R., Lai Y., Zhao Y., Qiao J. Adipose insulin resistance is associated with cardiovascular risk factors in polycystic ovary syndrome. J. Endocrinol. Invest. 2019; 42(5): 541-8. https://dx.doi.org/10.1007/s40618-018-0949-2.
- Ramamoorthy S., Bhuvaneswari K. A cross-sectional study on the status of inflammatory markers in polycystic ovary syndrome (PCOS) in Indian population. Biomed. Pharmacol. J. 2019; 12: 1975-83.https://dx.doi.org/10.13005/bpj/1829.
- Lin Y.S., Tsai S.J., Lin M.W., Yang C.T., Huang M.F., Wu M.H. Interleukin-6 as an early chronic inflammatory marker in polycystic ovary syndrome with insulin receptor substrate-2 polymorphism. Am. J. Reprod. Immunol. 2011; 66(6): 527-33. https://dx.doi.org/10.1111/j.1600-0897.2011.01059.x.
- Fulghesu A.M., Sanna F., Uda S., Magnini R., Portoghese E., Batetta B. IL-6 serum levels and production is related to an altered immune response in polycystic ovary syndrome girls with insulin resistance. Mediators Inflamm. 2011; 2011: 389317. https://dx.doi.org/10.1155/2011/389317.
- Borst S.E. The role of TNF-alpha in insulin resistance. Endocrine. 2004; 23(2-3): 177-82. https://dx.doi.org/10.1385/ENDO:23:2-3:177.
- Goswami S., Choudhuri S., Bhattacharya B., Bhattacharjee R., Roy A., Mukhopadhyay S. et al. Chronic inflammation in polycystic ovary syndrome: A case-control study using multiple markers. Int. J. Reprod. Biomed. 2021; 19(4): 313-20. https://dx.doi.org/10.18502/ijrm.v19i4.9057.
- Choi Y.S., Yang H.I., Cho S., Jung J.A., Jeon Y.E., Kim H.Y. et al. Serum asymmetric dimethylarginine, apelin, and tumor necrosis factor-α levels in non-obese women with polycystic ovary syndrome. Steroids. 2012; 77(13): 1352-8. https://dx.doi.org/10.1016/j.steroids.2012.08.005.
- De Luca C., Olefsky J.M. Inflammation and insulin resistance. FEBS Lett. 2008; 582: 97-105. https://dx.doi.org/10.1016/j.febslet.2007.11.057.
- Shoelson S.E., Lee J., Goldfine A.B. Inflammation and insulin resistance. J. Clin. Invest. 2006; 116(7): 1793-801. https://dx.doi.org/10.1172/JCI29069.
- Zatterale F., Longo M., Naderi J., Raciti G.A., Desiderio A., Miele C. et al. Chronic adipose tissue inflammation linking obesity to insulin resistance and Type 2 diabetes. Front. Psychol. 2020; 10: 1607. https://dx.doi.org/10.3389/fphys.2019.01607.
- Министерство здравоохранения Российской Федерации. Синдром поликистозных яичников. Клинические рекомендации М.; 2021. [Ministry of Health of Russian Federation. Clinical guidelines "Polycystic ovary syndrome". Moscow; 2021. (in Russian)].
- Sumner A.E., Cowie C.C. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis. 2008; 196(2): 696-703.https://dx.doi.org/10.1016/j.atherosclerosis.2006.12.018.
- Министерство здравоохранения Российской Федерации. Сахарный диабет 2 типа у взрослых. Клинические рекомендации М.; 2019. [Ministry of Health of Russian Federation. Clinical guidelines "Type 2 diabetes in adults". Moscow; 2019. (in Russian)].
- Sim S.J., Park H.S. The cut-off values of body fat to identify cardiovascular risk among Korean adults. Korean J. Obes. 2004; 13(1): 14-21.
- Dudeja V., Misra A., Pandey R.M., Devina G., Kumar G., Vikram N.K. BMI does not accurately predict overweight in Asian Indians in northern India. Br. J. Nutr. 2001; 86(1): 105-12. https://dx.doi.org/10.1079/bjn2001382.
- Goh V.H., Tain C.F., Tong T.Y., Mok H.P., Wong M.T. Are BMI and other anthropometric measures appropriate as indices for obesity? A study in an Asian population. J. Lipid Res. 2004; 45(10): 1892-8. https://dx.doi.org/10.1194/jlr.M400159-JLR200.
- Miazgowski T., Krzyżanowska-Świniarska B., Dziwura-Ogonowska J., Widecka K. The associations between cardiometabolic risk factors and visceral fat measured by a new dual-energy X-ray absorptiometry-derived method in lean healthy Caucasian women. Endocrine. 2014; 47(2): 500-5. https://dx.doi.org/10.1007/s12020-014-0180-7.
- Palomba S., de Wilde M.A., Falbo A., Koster M.P., La Sala G.B., Fauser B.C. Pregnancy complications in women with polycystic ovary syndrome. Hum. Reprod. Update. 2015; 21(5): 575-92. https://dx.doi.org/10.1093/humupd/dmv029.
- Abraham Gnanadass S., Divakar Prabhu Y., Valsala Gopalakrishnan A. Association of metabolic and inflammatory markers with polycystic ovarian syndrome (PCOS): an update. Arch. Gynecol. Obstet. 2021; 303(3): 631-43. https://dx.doi.org/10.1007/s00404-020-05951-2.
- Юренева С.В., Комедина В.И., Кузнецов С.Ю. Диагностические возможности антропометрических показателей для оценки ожирения у женщин в период менопаузального перехода. Акушерство и гинекология. 2022; 2: 72-9. [Yureneva S.V., Komedina V.I., Kuznetsov S.Yu. Diagnostic value of anthropometric characteristics of obesity in women during the menopause transition. Obstetrics and Gynecology. 2022; (2): 72-9. (in Russian)].https://dx.doi.org/10.18565/aig.2022.2.72-79.
- Aboeldalyl S., James C., Seyam E., Ibrahim E.M., Shawki H.E., Amer S. The role of chronic inflammation in polycystic ovarian syndrome-a systematic review and meta-analysis. Int. J. Mol. Sci. 2021; 22(5): 2734. https://dx.doi.org/10.3390/ijms22052734.
- Чернуха Г.Е., Блинова И.В. СПКЯ: кардиоваскулярные риски и влияние на них терапии сиофором. Трудный пациент. 2008; 6(1): 18-22. [Chernukha G.E., Blinova I.V. PCOS: cardiovascular risks and the impact of siofor therapy on them. Difficult Patient. 2008; 6(1): 18-22 (in Russian)].
- Palomba S., Falbo A., Chiossi G., Orio F., Tolino A., Colao A. et al. Low-grade chronic inflammation in pregnant women with polycystic ovary syndrome: a prospective controlled clinical study. J. Clin. Endocrinol. Metab. 2014; 99(8): 2942-51. https://dx.doi.org/10.1210/jc.2014-1214.
- Mukherjee A.G., Wanjari U.R., Kannampuzha S., Murali R., Namachivayam A., Ganesan R. et al. The implication of mechanistic approaches and the role of the microbiome in polycystic ovary syndrome (PCOS): a review. Metabolites. 2023; 13: 129. https://dx.doi.org/10.3390/metabo13010129.
- Custodero C., Mankowski R.T., Lee S.A., Chen Z., Wu S., Manini T.M. et al. Evidence-based nutritional and pharmacological interventions targeting chronic low-grade inflammation in middle-age and older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2018; 46: 42-59. https://dx.doi.org/10.1016/j.arr.2018.05.004.
Received 03.03.2023
Accepted 04.04.2023
About the Authors
Ekaterina D. Kirillova, Junior Reseasrcher at the Department of Gynecological Endocrinology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(917)533-48-00, emiroshina.md@gmail.com, https://orcid.org/0000-0002-3723-5052,117997, Russia, Moscow, Academician Oparin str., 4.
Lyubov V. Krechetova, Dr. Med. Sci., Head of the Laboratory of Clinical Imunology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-11-83, k_l_v_@mail.ru, https://orcid.org/0000-0001-5023-3476,
117997, Russia, Moscow, Academician Oparin str., 4.
Valentina V. Vtorushina, PhD, Immunologist at the Laboratory of Clinical Immunology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-11-83, v_vtorushina@oparina4.ru, https://orcid.org/0000-0002-8406-3206,
117997, Russia, Moscow, Academician Oparin str., 4.
Tatyana Yu. Ivanets, Dr. Med. Sci., Head of the Clinical Diagnostic Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(910)404-26-69, t_ivanets@oparina4.ru, https://orcid.org/0000-0002-7990-0276,
117997, Russia, Moscow, Academician Oparin str., 4.
Galina E. Chernukha, Dr. Med. Sci., Professor, Chief Researcher, Department of Gynecological Endocrinology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(985)999-60-00, c-galina1@yandex.ru, https://orcid.org/0000-0002-9065-5689,
117997, Russia, Moscow, Academician Oparin str., 4.



