Association between the level of specific urinary SERPINA1 protein in pregnant women and the severity of preeclampsia and perinatal outcomes
Aim: The study was aimed to identify association between SERPINA1/α1-antitrypsin (A1AT) and the severity of preeclampsia and perinatal/neonatal outcomes.Karavaeva A.L., Zubkov V.V., Starodubtseva N.L.,Timofeeva L.A., Kan N.E., Tyutyunnik V.L., Baev O.R., Nizyaeva N.V.
Materials and methods: The analysis of medical histories, specific features of the course of pregnancy in 79 women, childbirth, postpartum period, and the state of health of their newborns was performed. Urine samples for testing were collected before 34 weeks of gestation, when pregnant women were admitted to hospital. All patients were divided into 3 groups: group 1 (the main group) included 41 pregnant women with preeclampsia; group 2 (the comparison group) – 17 women with chronic arterial hypertension; group 3 (the comparison group) – 18 women with normal pregnancy. Depending on the detected level of urinary SERPINA1 in pregnant women, group 1 was divided into 2 subgroups: 1a (n=23) – preeclampsia, SERPINA1 positive (PE SER+) and 1b (n=21) – preeclampsia, SERPINA1 negative (PE SER-).
Results: The study confirmed the adverse effect of preeclampsia on the condition of the fetus and newborn. It was demonstrated, that determination of peptides of SERPINA1 (α1-antitrypsin) in the urine of pregnant women predicts a severe course of preeclampsia and was associated with a high incidence of growth retardation and fetal hypoxia. In infants born to women preeclampsia, SERPINA1 positive, there was a negative correlation with the body weight, Apgar score and necessity for resuscitation, as well as the duration of the newborn's stay in hospital.
Conclusion: The study of the urine peptide profile – determination of SERPINA1 peptides contributes to the differential diagnosis of the severity of preeclampsia and other hypertension disorders during pregnancy helping to determine obstetric tactics and prognostically significant risk factors for the newborns.
Keywords
Hypertensive disorders are the most сommon complications of pregnancy, which have a pronounced effect on health of a mother, fetus and newborn [1, 2]. Among all hypertensive disorders, the most formidable pathological condition is preeclampsia, the rate of which remains high throughout the world and does not tend to decrease. According to the published data, the rate of preeclampsia in Europe is 2–5%, in the developing countries – 12–15% [3–6]. In Russia, according to the data of the Russian Ministry of Health, the rate of preeclampsia is 3–7%. At the same time, the development of this complication is associated with high rates of maternal morbidity and mortality, as well as severe perinatal and neonatal outcomes [7–10].
The foregoing data point out that the search for various biomarkers for preeclampsia remains relevant, allowing not only to predict the timing of development and the severity of this pathological condition, but also to assess their impact on the growth, development of the fetus and newborn’s health.
In recent years, new methods of non-invasive diagnostics based on molecular genetic studies have been widely introduced. The researchers focus attention on the study of urine peptidome of pregnant women as a more accessible non-invasive method [11–13]. It was proposed to consider Collagen Type I Alpha 1 Chain, collagen Type III Alpha 1 Chain, uromodulin and peptides of SERPINA1 (α1-antitrypsin) as early markers for preeclampsia. The last one is the most promising among peptide markers for preeclampsia [7, 14–16] and can be used as highly specific diagnostic test correlating with the severity of preeclampsia.
N. Starodubtseva et al. [7] noted that detection of SERPINA1 in the urine of pregnant women is associated with the severity of ischemic and hypoxic damage to placental villous tree, which causes fetal growth retardation. Therefore, it is interesting to study the relationship between the detection of specific urine protein α1-antitrypsin (SERPINA1) in pregnant women and the features of the course of the neonatal period. This will make possible to predict the development of pathological conditions in newborns in order to optimize approaches to treatment, observation and nursing.
The aim of the study was to identify association between SER-PINA1/α1-antitrypsin (A1AT) and the severity of preeclampsia and perina-tal/neonatal outcomes.
Materials and methods
The analysis of medical histories, specific features of the course of pregnancy, childbirth, postpartum period, and the condition of the fetuses and newborns born to 79 women, who underwent surveillance in the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Health of Russia in the period from 2018 to 2019. All patients were divided into 3 groups: group 1 (the main group) included 41 pregnant women with preeclampsia (PE); group 2 (the comparison group) – 17 women with chronic arterial hypertension (CAH); group 3 (the comparison group) – 18 women with normal pregnancy. The analysis of urinary peptidome profile showed that the main group was heterogeneous. Depending on the detected level of urinary SERPINA1 in pregnant women, group 1 was divided into 2 subgroups: subgroup 1a (n=23) – women with preeclampsia, SERPINA1 positive (PE SER+) and subgroup 1b (n=21) – women with preeclampsia, SERPINA1 negative (PE SER-).
The pregnant women were included in the study using matched pairs design. The inclusion criteria were: singleton pregnancy at 22–40 weeks of gestation, the age of women from 18 to 5 years, the presence of preeclampsia (group 1), chronic arterial hypertension (group 2). Exclusion criteria were: multiple pregnancies, acute clinical manifestation of diabetes mellitus, organ transplantation, autoimmune and oncological diseases, chronic kidney disease and fetal congenital malformations.
The patients have signed the informed consent, which was approved by the Ethics Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Urinary specimens were collected from all patients before 34 weeks of gestation, when they were admitted to different departments of the Center. The patients did not receive specific therapy aimed at correction of pathological conditions of pregnancy at the time of collection of urinary specimens for analysis.
Urinary specimens were centrifuged for 10 min at 2000 g at 4°C. The supernatant was stored at -80°C until the analysis. The urine peptide profile was isolated by gel filtration, and HPLC-tandem mass spectrometry (MS/MS) analysis was performed in full compliance with the protocols developed by the authors [13]. The analysis of the peptide fraction and gradient elution was performed with 1100 nanoflow chromatograph (Agilent Technologies, USA) and using 7-T LTQ-FT hybrid mass spectrometer (Thermo Electron, Germany) on 75 μm×12 cm column, Reprosil-Pur C18 3 μm beads (Dr. Maisch HPLC GmbH, Germany). MS and MS/ MS data were acquired in the data-dependent manner using Xcalibur software (Thermo Finnigan, San José, California, USA). Scan MS/MS spectra of isolated precursor ions (in the range of m/z 300–1600 amu) was obtainted with FTICR mass analyzer at resolution R=50000 at m/z 400 amu (the amount of accumulated ions was 5×106). MS data analysis was performed using PEAKS Studio software.
Assessment of health status of newborns included anthropometric measurements, the need for intensive and resuscitation care, the Apgar score at 1st and 5th minutes, as well as the duration of their hospital stay.
Statistical analysis
The obtained data were recorded in the specially produced thematic map and entered into MSExcel spreadsheets. Statistical data processing was performed using software package SPSS 26.0 Statistics for Windows. Before conducting a comparative analysis of qualitative data in the groups under the study, the conformity to the normal distribution was assessed (graphical data analysis, the Kolmogorov–Smirnov test). To describe quantitative data with a normal distribution, the arithmetic mean (M) and standard deviation (SD) were used. For the parameters, that did not follow a normal distribution, the method of nonparametric statistics was used. The quantitative parameters in the absence of normal distribution were presented as median (Ме) and interquartile range (Q1; Q3). The Kruskal–Wallis test for comparison of three or more independent groups with Bonferroni correction for multiplicity and Mann–Whitney test for pairwise comparisons were used. Pearson's χ2 test was used for comparison of binary variables, and 2×2 contingency table was constructed. Fisher’s exact test was used for small samples. The assessment of the influence of risk factors was calculated using the odds ratio (OR) and 95% confidence interval (95% CI). The differences were statistically significant at р<0.05. When comparing three groups, the critical value of the significance level (p) was considered as 0.017, and when comparing four groups – 0.008.
Results
At the first stage, the clinical and anamnestic data of the women included in the study were analyzed (Table 1).
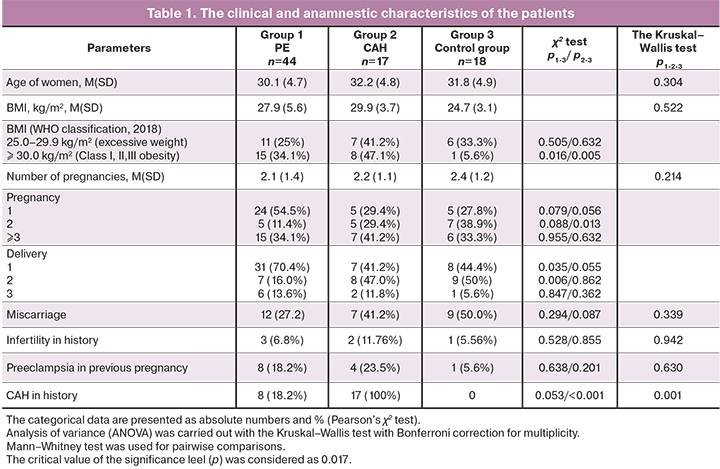
It is known that the predisposing factors for preeclampsia include: moth-er's age less than 20 and over 40 years, primigravity, BMI> 30kg/m², a long intergravid period (more than 10 years), the presence of preeclampsia in previ-ous pregnancy, chronic arterial hypertension (CAH), kidney and urinary tract diseases [4, 5].
As it can be seen from Table 1 above, there was no age difference be-tween the patients (р>0.1). Statistically significant differences in body mass in-dex (BMI) at the time of conception occurrence were not found: BMI (M (SD)) in group I was 27.9 (5.6) kg/m², in group II – 29.9 (3.7) kg/m², in group III – 24.7 (3.1) kg/m², р1-2-3 =0.522. However, impaired lipid metabolism (Class I, II, III obesity) was observed mainly in women with chronic arterial hypertension and preeclampsia compared to the control group (15/44 (34.1%), 8/17 (47.1%) and 1/18 (5.6%), respectively, р1-3= 0.016, р2-3 =0.005).
As has been reported [3, 4, 15], the proportion of primiparas prevailed in group 1 (PE) – 31/44 (70.4%) and was higher compared to group 2 (CAH) – 7/17 (41.2%) (OR1-2=3.4 (95% CI 1.1–10.9), p=0.035) and group 3I – 8/18 (44.4%) (OR1-3=2.9 (95% CI 1.0–9.3), р=0.055).
The course of pregnancy and delivery was analyzed. The significant complications of pregnancy, such as gestational diabetes mellitus, fetal growth retardation, amniotic fluid disorders were noted predominantly in group 1 (р1-2-3<0.05). Moreover, impaired fetal growth was detected only in group 1 – 10/44 (22.7%), and it was not noted in groups 2 and 3 (р1-3=0.028, р1-2=0.032).
Blood pressure (BP) indices were higher in patients in group 1 compared to the group of women with CAH. Apparently, this was due to elective thera-py of women with hypertensive disorders in group 2 at the stage of preparing for pregnancy and correction of maintenance therapy starting from the early stages of pregnancy.
At the time of admission to hospital, systolic blood pressure (SBP) (M(SD)) in women in group 1 was 154.8 (9.4) mmhG, in group 2 – 141.2 (8.6) mmhG, in group 3 – 114.0 (7.7) mmhG (р1-2-3<0.001). Diastolic blood pressure (DBP) was 98.3 (5.7) mmhG in group 1, 93.6 (4.4) mmhG in group 2 and 72.7 (6.6) mmhG in group 3 (р1-2-3<0.001).
Hematologic disorders (anemia and thrombocytopenia) were observed mainly in the third trimester of pregnancy. Anemia was more common in pa-tients in group 3 – 5/18 (27.8%), compared to the group with CAH – 1/17 (5.98%), OR2-3 =6.2 (0.6–59.5), р2-3=0.086, and the group with PE – 2/44 (4.5%), OR1-2=8.1 (95% CI 1.4–46.7), р1-3 =0.009. While thrombocytopenia was more common in patients in group 1: 14/44 (31.8%), 1/17 (5.9%) and 2/17 (11.1%), OR1-2=7.5 (0.9–62.1), р1-2 =0.035, OR1-3 = 3.7 (95% CI 0.8–18.5), р1-3 =0,091.
Laboratory tests showed that the level of proteinuria was significantly higher in the group with PE compared to the control group and the group with CAH. The level of proteinuria (M (SD)) was 2.03 (1.79) g/l in group 1, 0.039 (0.007) g/l in group 2 and 0.002 (0.001) g/l in group 3 (р1-2-3<0.001).
Statistically significant differences in the mode of delivery in the groups were not found. Cesarean delivery rate was 9/17 (52.9%) in group 2, 10/18 (55.6%) in group 3 and 33/44 (75.0%) in group 1; OR1-2=2.7 (95% CI 0.8–8.6), р1-2=0.096, OR1-3=2.4 (95% CI 0.7–7.6), р1-3=0.132.
Live-births were delivered in all studied groups. The length of gestation at delivery was longer in the control group M (SD) – 272.8(7.66) days com-pared to 262.1 (24.6) days in the group with CAH – and 246.9 (45.2) days in the group with PE (р1-2-3<0.001).
At the second stage of the study, peptide analysis of urine samples col-lected from 79 women was performed (group 1 with PE, n=44, group 2 with CAH, n=17 and group 3 (the control group), n=18).
A total of 1552 urine peptides were found. In urine peptidome, SERPI-NA1 peptides (n=50) ranked second after collagen-alpha-1 peptides. As has been shown in earlier publications [11], these peptides, were associated with hypertensive disorders of pregnancy. However, only 7 of these peptides were specific for preeclampsia and were not detected in the control group and the group with chronic arterial hypertension.
It was found that specific peptides associated with preeclampsia were detected not in all patients in group 1. Due to this, the group of pregnant women with preeclampsia was divided into 2 subgroups: subgroup 1a (n=23) – PE, SERPINA1 positive (PE SER+) and subgroup Ib (n=21) – PE, SERPINA1 negative (PE SER-). Moreover, it was found that the patients in group 1, who had preeclampsia on the background of chronic arterial hypertension (PE+CAH) were negative for SERPINA1 (subgroup Ib).
The obtained results were different in patients with preeclampsia regarding the clinical manifestation and severity of preeclampsia, blood pressure indices and SFLT-1/PLGF ratio, the degree of proteinuria and thrombocytopenia, as well as the timing of delivery (Table 2).
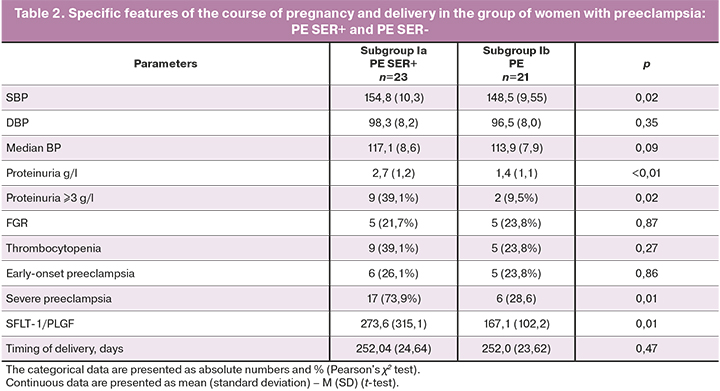
According to literature [3, 12], there is evidence of a more favorable course of preeclampsia on the background of chronic arterial hypertension. However, it is not always possible to make a differential diagnosis between various hypertensive disorders during pregnancy and/or their combination. Absent urinary SERPINA1 peptides may be a predictor of favorable outcomes in pregnant women with preeclampsia and, accordingly, will determine the obstetric tactics of management of their condition and possible prolongation of pregnancy and/or the need for early delivery.
The detection of SERPINA1 peptides in urine of pregnant women in subgroups 1a (PE Ser+) and 1b (PE Ser-) was associated with high blood pressure (154.8(10.3) mmhG and 148.5(9.55) mmhG, accordingly, р=0,02), proteinuria (2.7 (1.2) g/l and 1.4 (1.1) g/l, (р<0.01), the values of the SFLT-1/PLGF ratio (273.6±315.1 and 167.1±102.2, р<0.01), as well as the severity of preeclampsia (17/23 (73.9%) and 6/21 (28.6%), р<0.01). Thus, the obtained data showed that the diagnostic value of SERPINA1 in urine of pregnant women was high.
At the third stage, we made a comparative analysis of the newborns’ condition born to mothers in the studied groups. The infants born to mothers in groups II and III were term infants. The length of gestation in group II was 37.4 (2.9) weeks, in group 3 – 38.96 (1.1) weeks, in group 1 – the length of gestation was shorter – 36.0 (3.49), р1-2-3<0.001. 24/44 (54.5%) preterm infants were born to mothers with preeclampsia and 2/17 (11.7%) – to mothers with CAH, OR1-2 =9.0 (95% CI 1.8–44.1), р=0.003. All infants in group 3 were full-term babies, 14/23 (60.9%) newborns born to mothers in subgroup Ia and 10/21(47.6%) newborns in subgroup Ib needed admission to intensive care unit and primary resuscitation care in the delivery block. There were no statistically significant differences between subgroups Ia and Ib regarding the timing of delivery – (M (SD) 36.0 (3.5) weeks and 36.0 (3.4) weeks, accordingly, р=0.467. However, the birth of babies with a low body weight was observed mainly in the group of mothers with preeclampsia Ser+.
As can be seen from the data presented in Table 3, the birth weight of newborns in subgroup 1a was 2211.65 (783.87) g, in subgroup 1b – 2397.43 (779.16) g, in group 2 (CAH) – 2921.65 (602.62) g, in group 3 – 3361.78 (491.48) g. The birth weight of infants born to mothers in the group with preeclampsia was lower compared to group 2 with CAH (р<0.001) and group 3 (р<0.001), as well as of infants in group 2 compared to group 3 (р=0.014). There were no statistically significant differences between subgroups 1a and 1b (р=0.378).
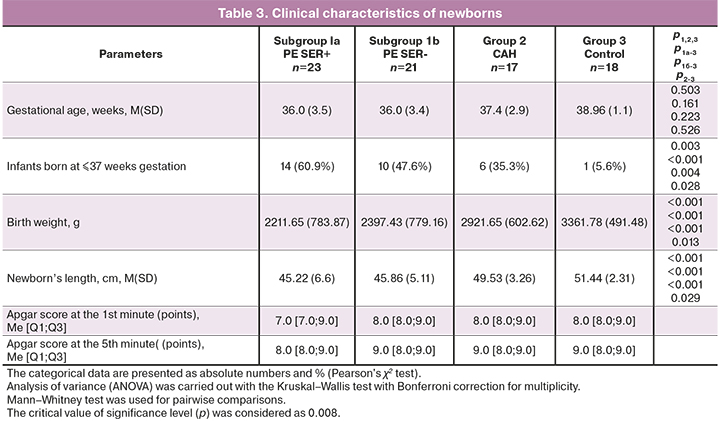
Severe and moderate asphyxia was only in infants born to mothers in the group with preeclampsia 10/44 (22.7%). Despite the available information about the prognostically mild course of preeclampsia on the background of chronic arterial hypertension [12], approximately similar number of cases of severe and moderate asphyxia was in newborns in subgroups 1a and 1b (in subgroup 1a – 5/23 (21.7%), in subgroup 1b – 5/21 (23.8%), (р=0.87).
There was an inverse relationship between detection of SERPINA1 peptides in the urine of pregnant women and the 1-minute Apgar score: 7.0 [7;9] points in the group of women with PE SERr+, 8.0 [8.0;9.0] points in the group of women with PE SER-, 8.0 [8.0;9.0] points in the group of women with CAH and 8.0 [8.0;9.0] points in the control group.
Neonatal death on the 13th day of a live-born infant was in subgroup 1a and amounted to 4.3%. The reason was a severe course of early neonatal sepsis, accompanied by disseminated intravascular coagulation (DIC) with multiple organ failure on the background of extreme prematurity and extremely low birth weight babies (ELBW) (gestational age was 28 weeks, body weight – 745 g).
In the structure of morbidity among the newborns in the studied groups, the main causes were respiratory disorders, infectious pathology, hematologic and metabolic disorders in newborns in group 1.
It is known from literature, that the predisposing risk factors in preeclampsia leading to preterm birth and/or intrauterine growth retardation are placental ischemia and placental hypoperfusion [4, 13]. It was noted that in the group with PE SER+ 11/23 (47.8%) newborns were small for gestational age. In this group this index was higher compared to the group with PE SER- –3/21 (14.%), OR1а-1б=5.5 (95% CI 1.3–23.9), р=0.018, group with CAH – 1/17 (5.6%), OR1а-2=14.7 (95% CI 1.7–129.7), р=0.002, and the control group – 1/18 (5.6%), OR1а-3=15.6 (95% CI 1.8–137.4), р=0.003.
Respiratory disorders were most common in newborns in group 1а (PE SER+) – 4/23 (17.4%), subgroup 1b – 1/21 (4.8%), р=0.207, group 2 – 1/17 (5.9%), р=0.228. In the control group, respiratory disorders did not require respiratory therapy in the early neonatal period, р1а-3=0.063. Repiratory disorders that developed in newborns in the studied groups were predominantly transient.
Infectious and inflammatory diseases prevailed in group 1 – 17/44 (38.6%). There were no significant differences in the rate of congenital infections among the newborns in subgroups 1a and 1b: in 8/23 (34.8%) and 9/21 (42.9%) infants, respectively, (р=0.583). The incidence of congenital infectious diseases in group 2 was lower than in group 1 and amounted to 2/17 (11.8%), OR1-2=3.9 (95% CI 0.8–19.2), р=0.082. The data on infectious diseases in the infants born to mothers with normal pregnancy were not obtained, р1-3=0.002, р2-3=0.134. Necrotizing enterocolitis (NEC) was only in newborns in group 1 with 0approximately similar percentage in the subgroups: 1/23 (4.4%) in subgroup 1a and 1/21 (4.8%) in subgroup 1b, р1а-1b=0.948.
The central nervous system (CNS) pathology was in 13/44 (29.5%) infants born to women with preeclampsia and the number of cases was higher (OR1-2 =3.1 (95% CI 0.6–15.7), р1-2=0.149) compared to the group of women with CAH – 2/17 (11.8%) and group 3– 1/18 (5.6%), OR1-3= 7,1 (95% CI 1.1–59.2), р1-3=0.041. It should be noted, that significant disorders (grade II-III intraventricular hemorrhage, intracranial hemorrhage) were detected only in subgroup 1a (in mothers with PE, SERPINA1-positive) in 3/23 (13.0%) cases, this is most likely associated with the development of thrombocytopenia as one of manifestations of PE.
Hypoglycemia that developed after birth and required parenteral correction in the first hours of life was in 5/44 (11.4%) newborns in group 1. According to published data [14], metabolic disorders, such as hypoglycemia, are typical for infants born to mothers with preeclampsia. However, in this study neonatal hypoglycemia was only in newborns in subgroup 1a and was not present in subgroup 1b in the incidence of preeclampsia on the background of chronic arterial hypertension. In group 2 (CAH), hypoglycemia was in 1 case (5.9%), OR1а-2=4.4 (95% CI 0.5–42.2), р1а-2=0.146. There were no metabolic disorders in newborns in the control group, р1-3=0.035.
Hematologic disorders were most common in infants born to 14/23 (60.9%) mothers with PE SER1+ compared to 7/21(33.3%) women with PE SER1-, OR1а-1б= 3.1 (95% CI 0.9–10.7), р1а-1б=0.068), 3/17 (17.6%) women in the group with CAH, OR1а-2=7.3 (95% CI 1.6–32.6), р1а-2=0.007, and in 1/18 (5,6%) woman in the control group, OR1а-3=26.4 (95% CI 3.0–234.8), р1а-3<0.001.
Among the hematologic disorders in the studied groups, congenital anemia was in 2/23 (8.7%) cases in subgroup 1a, in 3/21 (14,3%) in subgroup 1b; in 2/17 (11,8%) in group 24; polycythemia of newborns was in 4/23 (17,4%) cases in subgroup 1a; in 2/21 (9,5%) in subgroup 1b; in 1/17 (5,9%) in group 2; thrombocytopenia was in 6/23 (26.1%) cases in group 1а, in 1/18(5,6%) in group 3; neutropenia was in 2/23 (8,7%) cases in subgroup 1a and in 2/21 (9,5%) in subgroup 1b. The prevalence of anemia was observed among the group of newborns born to mothers who had chronic arterial hypertension in pregnancy, while there was an increased incidence of polycythemia and thrombocytopenia among infants born to mothers with preeclampsia, SERPINA1-positive, as can be seen from the above data.
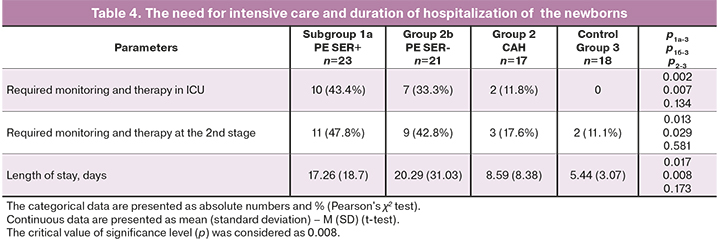
As can be seen from the data presented in Table 4, the length of stay was higher in the group of infants born to mothers with preeclampsia (р<0,01): 17.26 (18.7) days in subgroup 1a and 20.29 (31,03) days in subgroup 1b; 8.59 (8.38) days in group 2 and 5.44 (3.07) days in the control group. The length of newborns’ stay in the Department of Physiology was comparable: 3.71 (2.89) days in subgroup 1a, 4.93 (1.6) days in subgroup 1b; 4.8 (1.42) days in group 2 and 4.0 (1,2) days in the control group. The infants born to mothers with preeclampsia SERPINA1-positive had a longer period of nursing care and monitoring in the Neonatal Pathology Department, and the infants born to mothers with preeclampsia SERPINA1- negative required a longer monitoring in the Intensive Care Unit.
Discussion
Preeclampsia is still the most formidable pathological condition associated with pregnancy, and is the main cause of maternal morbidity and mortality [1–4]. Increasing severity of preeclampsia significantly increases the risk of adverse pregnancy outcomes, worsening of perinatal and neonatal outcomes [4, 8, 15].
According to the WHO [3], in preeclampsia, the priority objective is to prolong gestational age until maturity, as it increases the infant's chance for survival. However, prolongation of pregnancy, especially with early-onset preeclampsia (≤ 34 weeks of pregnancy) is often associated with the risk of rapid progression of pathological processes in pregnant woman and development of formidable obstetric complications (eclampsia, HELLP syndrome, pulmonary edema, severe form of fatty hepatosis, multiple organ failure, etc.) [2–4].
The only effective treatment for preeclampsia (especially severe forms) is delivery. However, the risk of preterm birth and the prognosis of severe perinatal and neonatal outcomes dictate the need to search for available and effective methods for predicting the development of preeclampsia, the severity of this condition for making a difficult decision on obstetric management of patients, as well as criteria for determining indications for emergency delivery. However, the risk of giving birth preterm and the prognosis of severe perinatal and neonatal outcomes dictates the necessity to search accessible and effective methods for prediction of preeclampsia, detection of severity of this disorder in order to find solutions for obstetric management of patients, as well as criteria for emergency delivery.
Various biological media, such as plasma or urine are used as research material for preeclampsia [6, 7, 16, 8–10]. V.N. Serov et al. [16] demonstrated that SFLT1/PIGF ratio in pregnant women is one of the highest sensitive predictor for severity of preeclampsia.
In recent years, the new methods of non-invasive diagnostics based on molecular genetic studies have been widely introduced. The researchers directed attention to the study of urine peptidome of pregnant women as a more accessible non-invasive diagnostic method [6–11].
In the pathogenesis of preeclampsia, one of the key points is inadequate trophoblast invasion and impaired spiral artery remodeling, that lead to placental ischemia and impaired spiral artery remodeling [4]. The expression of SERPINA1 (alpha-1-antitrypsin) may be a physiological mechanism involved in the regulation of trophoblast invasion. In preeclampsia with accompanying hypoxia, it increases threefold, especially in cyto- and syncytiotrophoblasts, as well as in the decidua [6]. At the same time, large amounts of fibrinoid deposits – coagulants aiding in plasma clotting – that accumulate in placenta in response to injury also accumulate SERPINA1 [6], resulting in the intervillous space reduction, structural "blocking" of trophoblast, limitation of trophoblast invasion into the uterine spiral arteries. It also contributes to feto-placental blood flow impairment. Increased concentration of SERPINA1 is considered to be a defense mechanism in preeclampsia in response to injury and placental hypoxia due to its indirect participation in the prevention of collagen degradation [6, 7], and systemic inflammatory response [7, 13] due to inhibition of anti-inflammatory cytokines production. A proven fact is apoptosis of trophoblast cells and placental villous stroma in preeclampsia, most likely leading to the release of SERPINA1 granules from placental structures [7, 16]. N. Starodubtseva et al. [7] found, that the destruction of trophoblast and release of SERPINA1 proteins leads to their increase in the urine of pregnant women with PE and is associated with prognostic factors of the disease severity.
As was shown in our study, the detection of SERPINA1 peptides in urine, was associated with a more severe course of preeclampsia. For substantive reasons this allows to distinguish between 2 types of preeclampsia: a true type of severe preeclampsia (or preeclampsia with a high risk of severe course) and preeclampsia on the background of CAH, which is characterized by a mild course and more favorable outcomes for the mother and her newborn baby.
We have found SERPINA1-positive women have higher indices of arterial blood pressure, severity of proteinuria and SFLT1/PIGF. Severe preeclampsia developed 2.6 times more common in subgroup 1a (ПЭ SER+).
Women in group 1a 1.3 times more common delivered preterm babies who had lower birth weight indices, compared to the group with PE SER–, and this was consistent with literature data.
In our study, a severe cause of preeclampsia in subgroup 1a led to childbirth with low Apgar scores, hematologic diseases (such as thrombocytopenia and polycythemia), which resulted in detection of severe intracranial hemorrhages in newborns, as well as hypoglycemia, requiring parenteral correction in the early neonatal period and necessity for resuscitation care for the newborn in the delivery room, as well as ICU follow-up and long-term nursing care in the departments at the second stage.
According to literature [7, 16], destruction/apoptosis of trophoblast cells occurs with development of severe PE. At the same time, intrauterine symptoms of hypoxia become worse, and greater amount of highly toxic substances enters fetal blood flow (products of lipid peroxidation, Schiff bases and diene conjugates, etc.), apparently, also resulting in hematologic and metabolic disorders in early neonatal period. According to the published data [15], thrombocytopenia in newborns is associated with the phenomenon of intrauterine hypoxia and suppression of granulocyte colony-stimulating factor, although, of course, this statement needs additional study.
In chronic arterial hypertension, placental membrane composed of trophoblasts remains intact. The changes in the structure of terminal villi, dystrophic changes, as well as the amount of fibrinoid deposits is less pronounced [7, 11]. It is most likely, that anemia in newborns manifests in response to chronic intrauterine hypoxia in the presence of CAH.
Detection of SERPINA1 peptides in the urine of pregnant women may be considered to be an additional diagnostic criterion for development of severe preeclampsia, as well as a predictor of severe neonatal outcomes in newborns.
Conslusion
It is commonly recognized, that preeclampsia have a negative impact on health of a mother, fetus and newborn. In this connection, the search for diagnostic markers allowing topredict development of severe preeclampsia for the timely initiation of therapy, correction of pathological disorders in pregnant women and prevention of adverse perinatal/neonatal outcomes, remains relevant. We have found association between the detection of SERPINA1 peptides in the urine of pregnant women and severe course of preeclampsia. SERPINA1-positive pregnant women have a high risk having preterm infants with low birth weight and low Apgar scores. These infants need resuscitation care and long nursing in ICU and in the Department of Pathology of Newborns. Preeclampsia development in a mother is a high risk factor for hematologic and metabolic disorders, development of severe intracranial hemorrhages in a newborn in early neonatal period.
Thus, detection of SERPINA1 peptides in the urine of pregnant women should be considered as an additional diagnostic criterion, a predictor of low birth weight and/or premature newborn with the risk of asphyxia at birth, and due to it the necessity for resuscitation care, readiness for correction of patho-logic conditions (such as hypoglycemia, polycythemia, thrombocytopenia) and the need for follow-up in the Departments of Resuscitation, Intensive Care and Pathology of Newborns.
In conclusion, it should be noted that, confirmation of the results obtained in this study need further studies of statistical power of residual variance and large sample size.
References
- Hutcheon J., Lisonkova S., Joseph K. Epidemiology of preeclampsia and the other hypertensive disorders of pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2011; 25(4): 391-403. https://dx.doi.org/10.1016/j.bpobgyn.2011.01.006.
- Mhiri R., Mvogo A., Kamga A., Yassinguezo S., Fagla H., Dotou D., Kallel H. Epidemiology and maternal prognosis of hypertension disorders of pregnancy in French Guiana. Pregnancy Hypertens. 2020; 20: 96-101. https://dx.doi.org/10.1016/j.preghy.2020.03.010.
- WHO recommendations for prevention and treatment of preeclampsia and eclampsia. WHO; 2011.
- Гипертензивные расстройства во время беременности, в родах и послеродовом периоде. Преэклампсия. Эклампсия: Федеральные клинические рекомендации (протокол лечения). М.; 2016. 72с. [Hypertensive disorders during pregnancy, childbirth, and the postpartum period. Preeclampsia. Eclampsia: Federal clinical guidelines (treatment protocol). Moscow, 2016. 72 p. (in Russian)].
- Paré E., Parry S., McElrath T.F., Pucci D., Newton A., Lim K.H. Clinical risk factors for preeclampsia in the 21st century. Obstet. Gynecol. 2014; 124(4): 763-70. https://dx.doi.org/10.1097/AOG.0000000000000451.
- Serrano-Pérez B., Almería S., Mur-Novales R., López-Helguera I., Garcia-Ispierto I., Alabart J.L. et al. Uterine serpin (SERPINA 14) correlates neg-atively with cytokine production at the foetal-maternal interface but not in the corpus luteum in pregnant dairy heifers experimentally infected with Neospora caninum. Reprod. Domest. Anim. 2018; 53(2): 556-8. https://dx.doi.org/10.1111/rda.12937.
- Starodubtseva N., Nizyaeva N., Baev O., Bugrova A., Gapaeva M., Muminova K., Kononikhin A., Frankevich V., Nikolaev E., Sukhikh G. SERPINA1 peptides in urine as a potential marker of preeclampsia severity. Int. J. Mol. Sci. 2020; 21(3): 914. https://dx.doi.org/10.3390/ijms21030914.
- Law K.P., Han T.-L., Tong C., Baker P.N. Mass spectrometry-based proteomics for preeclampsia and preterm birth. Int. J. Mol. Sci. 2015; 16(5): 10952-85. https://dx.doi.org/10.3390/ijms160510952.
- Guo H.X., Zhu Y.B., Wu C.P., Zhong M., Hu S.W. Potential urine biomarkers for gestational hypertension and preeclampsia. Mol. Med. Rep. 2019; 19(4): 2463-70. https://dx.doi.org/10.3892/mmr.2019.9911.
- Buhimschi I.A., Zhao G., Funai E.F., Harris N., Sasson I., Bernstein I. et al. Proteomic profiling of urine identifies specific fragments of serpina-1. Am. J. Obstet. Gynecol. 2009; 199(5):551. e1-16. https://dx.doi.org/10.1016/j.ajog.2008.07.006.
- Стародубцева Н.Л., Бугрова А.Е., Кононихин А.С., Вавина О.В., Широкова В.А., Наумов В.А., Гаранина И.А., Лагутин В.В., Попов И.А., Логинова Н.С., Ходжаева З.С., Франкевич В.Е., Николаев Е.Н., Сухих Г.Т. Возможность прогнозирования и ранней диагностики преэклампсии по пептидному профилю мочи. Акушерство и гинекология. 2015; 6: 46-52. [Starodubtseva N.L., Bugrova A.E., Kononikhin A.S., Vavina O.V., Shirokova V.A., Naumov V.A., Garanina I.A., Lagutin V.V., Popov I.A., Loginova N.S., Khodzhaeva Z.S., Frankevich V.E., Nikolaev E.N., Sukhikh G.T. The possibility of predicting and early diagnosis of preeclampsia by the peptide profile of urine. Obstetrics and gynecology. 2015; 6: 46-52 (in Russian)].
- Rana S., Lemoine E., Granger J., Karumanchi S.A. Preeclampsia: pathophysiology, challenges, and perspectives. Circ. Res. 2019; 124(7): 1094-112. https://dx.doi.org/10.1161/CIRCRESAHA.118.313276.
- Parikh N.I., Gonzalez J. Preeclampsia and hypertension: courting a long while: time to make it official. JAMA Intern. Med. 2017; 177(7): 917-8. https://dx.doi.org/10.1001/jamainternmed.2017.1422.
- Ton T.G., Bennett M., Incerti D., Peneva D., Druzin M., Stevens W. et al. Maternal and infant adverse outcomes associated with mild and severe preeclampsia during the first year after delivery in the United States. Am. J. Perinatol. 2020; 37(4): 398-408. https://dx.doi.org/10.1055/s-0039-1679916.
- Backes C.H., Markham K., Moorehead P., Cordero L., Nankervis C.A., Giannone P.J. Maternal preeclampsia and neonatal outcomes. J. Pregnancy. 2011; 2011: 214365. https://dx.doi.org/10.1155/2011/214365.
- Серов В.Н., Кан Н.Е., Тютюнник В.Л. Прогностическое значение отношения растворимой fms-подобной тирозинкиназы-1 к плацентарному фактору роста у беременных с преэклампсией. Акушерство и гинекология. 2016; 6: 5-10. [Serov V.N., Kan N.E., Tyutyunnik V.L. Prognostic value of the ratio of soluble fms-like tyrosine kinase-1 to placental growth factor in pregnant women with preeclampsia. Obstetrics and gynecology. 2016; 6: 5-10. https://dx.doi.org/10.18565/aig.2016.6.5-10. (in Russian)].
Received 27.04.2021
Accepted 07.07.2021
About the Authors
Anna L. Karavaeva, doctor, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(926)279-46-39, a_karavaeva@oparina4.ru, 117997, Russia, Moscow,Ac. Oparina str., 4.
Victor V. Zubkov, PhD, Director of the Institute of Neonatology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(963)750-48-77, v_zubkov@oparina4.ru, https://orcid.org/00000001-8366-5208, 117997, Russia, Moscow, Ac. Oparina str., 4.
Nataliya L. Starodubtseva, PhD, Head of the Laboratory of human reproduction proteomics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(916)463-98-67, n_starodubtseva@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Leila A. Timofeeva, PhD, Head of the Neonatal Unit, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(925)253-44-44, l_timofeeva@oparina4.ru,
117997, Russia, Moscow, Ac. Oparina str., 4.
Natalia E. Kan, MD, PhD, Professor, Deputy Director of Science, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(926)220-86-55, kan-med@mail.ru, Researcher ID: B-2370-2015, SPIN-код: 5378-8437, Authors ID: 624900, Scopus Author ID: 57008835600, https://orcid.org/0000-0001-5087-5946,
117997, Russia, Moscow, Ac. Oparina str., 4.
Victor L. Tyutyunnik, MD, PhD, Professor, Leading Researcher of Research and Development Service, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
+7(903)969-50-41, tioutiounnik@mail.ru, Researcher ID: B-2364-2015, SPIN-код: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500,
https://orcid.org/0000-0002-5830-5099, 117997, Russia, Moscow, Ac. Oparina str., 4.
Oleg R. Baev, MD, PhD, Professor, Head of the Maternity Department, Head of the Department of Obstetrics and Gynecology, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia, +7(495)438-11-88, o_baev@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Natalia V. Nizyaeva, PhD, Head of the Pathoanatomical Department V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(926)248-28-93, niziaeva@gmail.com, https://orcid.org/0000-0001-5592-5690, SPIN-код: 9893-2630, 117997, Russia, Moscow, Ac. Oparina str., 4.
Authors’ contributions: Karavaeva A.L., Zubkov V.V., Starodubtseva N.L., Timofeeva L.A., Kan N.E., Tyutyunnik V.L., Baev O.R., Nizyaeva N.V. – design of the study, obtaining data for analysis, review of publications on the topic of the article, statistical analysis of the obtained data, writing the text of the manuscript.
Conflicts of interest: The authors declare that they have no conflicts of interest.
Funding: The study was conducted without any sponsorship.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Karavaeva A.L., Zubkov V.V., Starodubtseva N.L.,Timofeeva L.A.,Kan N.E., Tyutyunnik V.L., Baev O.R., Nizyaeva N.V. Association between the level of specific urinary SERPINA1 protein in pregnant women and the severity of preeclampsia and perinatal outcomes.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2021; 9: 50-=59 (in Russian)
https://dx.doi.org/10.18565/aig.2021.9.50-59



