Relationship between the symptoms of vulvovaginal epithelial atrophy and the vaginal microbiota in postmenopausal women
Objective. To investigate the vaginal microbiota in postmenopausal women and its relationship to the symptoms of vulvovaginal atrophy (VVA).Eprikyan E.G., Yureneva S.V., Donnikov A.E., Ezhova L.S.
Subjects and methods. A total of 187 women, who had been postmenopausal for 1-20 years, were examined. All the patients signed informed consent forms. The inclusion criteria were the absence of menopausal hormone therapy for 6 months and systemic or local antibacterial therapy for 1 month before starting the study. The studies included cytological examination of vaginal wall smears, followed by estimation of the vaginal epithelium maturation index, vaginal pH value, and polymerase chain reaction for estimating the vaginal microcenosis.
Results. Bacterial community Type IV (BC-IV) is associated with the absence of lactobacilli. Vaginal dryness is not a specific symptom for atrophic changes in the vaginal mucosa. The presence or absence of this symptom seems to also depend on the composition of the vaginal microbiota in the postmenopausal period and to be associated with BC-IV.
Conclusion. Presentation of the relationship of vaginal symptoms not only to atrophy of the vaginal mucosa, but also to the specific features of its microflora will be able to personalize the management of postmenopausal women: those with BC-IV without atrophic changes in the vaginal mucosa from the standpoint of therapy for bacterial vaginosis, but if there are atrophic changes, local hormone therapy with estriol is the first-line treatment of choice.
Keywords
More than 50% of postmenopausal women experience the symptoms of vulvovaginal atrophy (VVA). The most common complaints are vaginal dryness, dyspareunia and discharge, which have a negative effect on the sexual health and overall quality of life of women [1]. To date, there is no unambiguous information on what is the root cause of these complaints: atrophic changes in the vaginal mucosa or a change in the microbiota of the vagina accompanied by decreased estrogen levels. The vaginal flora with a dominant number of lactobacilli is the key to vaginal and female health [2]. Lactobacillus spp. produce a hydrogen peroxide and lactic acid, which have an antimicrobial effect and play a key role in preventing colonization of the vagina by pathogenic and opportunistic bacteria [3]. The hormonal changes in a woman’s body at different age periods affect significantly the vaginal microflora. The low levels of estrogens in postmenopausal women induce structural and chemical changes in the urogenital tract, causing a cascade of events, starting with thinning of the vaginal epithelium. This leads to a decrease in the content of glycogen, the main substrate for the production of lactic acid, which provides the acidic environment of the vagina in the pH from 3.8 to 4.4. As a result, the pH level increases, the flora becomes alkaline and prevents the growth of lactobacilli [4]. The vaginal microbiota is colonized not only by opportunistic, but also by pathogenic microorganisms [2, 3, 5–7].
Vaginal microbiocenosis in postmenopausal women remains understudied: to date, the literature does not have enough information about the state of the vaginal microbiota in postmenopausal women. There are only a few studies on the composition of the vaginal microbiocenosis in postmenopausal women [8]. The unjustified prescription of antibiotic therapy to women during the postmenopausal period leads to a potential impairment of the very fragile microbiota.
The aim of the research was to study the microbiota of the vagina in postmenopausal women and its association with the symptoms of VVA.
Methods
The study included 187 women who had been postmenopausal for 1–20 years. All patients signed informed consent. The inclusion criteria were the absence of menopausal hormone therapy (MHT) for six months and systemic or local antibacterial therapy for one month before starting the study. The diagnosis of VVA was verified using the cytological method for examining and calculating the maturation index of the vaginal epithelium (ISEV = 0.5 × the number of intermediate cells (%) + 1 × the number of surface cells (%); (normal - ≥ 65%; <65 - vaginal atrophy)). The real-time polymerase chain reaction (PCR) method using the FEMOFLOR reagent kit (NPO DNA-Technology LLC, Russia) quantified the vaginal microbiocenosis. We studied the total bacterial mass (TBM), the amount and share of Lactobacillus spp.; Enterobacteriaceae; Staphylococcus spp.; Enterococcus spp.; Prevotella bivia/ Porphyromonas spp.; Eubacterium spp.; Sneathia spp./ Leptotrichia spp./ Fusobacterium spp; Megasphaera spp./ Veillonella spp./ Dialister spp.; Lachnobacterium spp./ Clostridium spp.; Mobiluncus spp./ Corynebacterium spp.; Peptostreptococcus spp.; Atopobium vaginae; Mycoplasma hominis; Ureaplasma urealyticum; Streptococcus spp.; Streptococcus agalactiae; Gardnerella vaginalis; Mycoplasma genitalium; Ureaplasma parvum; Actinomycetes spp.; Bifidobacterium; Anaerococcus spp.; S. aureus; E.coli; Candida spp.; Candida albicans.
Amplification was carried out in ‘real time’ mode using a DT-964 instrument (DNA-Technology, Russia). The fluorescence level was measured on each amplification cycle using the FAM, HEX, and ROX channels. Processing of the results was carried out automatically using the software. In our work, the bacterial vaginal microflora was structurally classified into five bacterial community state types (CST) [9, 10]. For this purpose, we studied the structure of lactoflora using additional evaluation with real time PCR: L. crispatus, L. acidophilus, L. iners, L. jensenii, L. gasseri, L.johnsonii, L.vaginalis.
Statistical data processing was performed using IBMSPSS STATISTICS 21 for Windows with the calculation of the arithmetic mean and its standard error (M ± m). To evaluate the intergroup differences, the non-parametric U Mann Whitney’s test was used for two independent samples. Differences between values were considered statistically significant at a confidence level of p < 0.05.
Results
A total of 187 postmenopausal women aged 40 to 75 years (mean age is 55.6 ± 5.9 years) who had been postmenopausal for 1-20 years (mean postmenopausal duration is 6.4 ± 5.1 years) were assessed on the basis of ISEV parameters. The patients were divided into two groups. The main group (group A) consisted of 120 (64.2%) women with VVA, and the comparison group (group B) included 67 (35.8%) patients. The main clinical characteristics of patients are presented in Table 1.
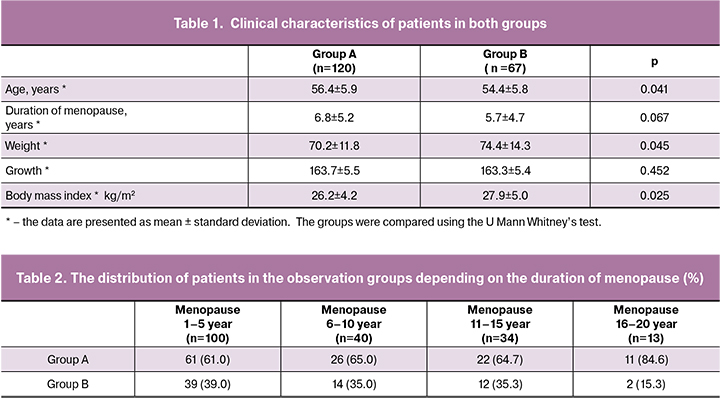
According to Table 1, the growth in the examined women in both groups did not have any significant differences, however, a body weight and BMI in patients in the main group A were significantly lower than in the comparison group. The women in the examined groups significantly differed in age. The postmenopausal period was shorter in group B, and these differences were borderline significant. To account for this potential confounder, the patients were stratified on the basis of the period of postmenopause (Table 2).
The data presented in Table 2 confirm an increase in the incidence of VVA as postmenopause progresses.
We conducted a survey of respondents: a comparative analysis of the results is presented in Table 3 taking into account the distribution of patients in the observation groups. The groups were compared using the U Mann Whitney’s test.

According to the data presented in Table 3, the predominant complaints in the observation groups were dryness (69.0%), burning (45.5%), irritation (35.8%) and itching (36.9%) in the vagina. In a smaller percentage of cases, women complained of pain (26.7%), discharge from the genital tract (29.4%) and the unpleasant odor of discharge from the genital tract (24.1%). The frequency of complaints was high in both groups, however, the patients of the main group complained of dryness in the vagina significantly more often than the patients in the comparison group, which confirms the specificity of atrophic changes in the vaginal epithelium during VVA.
The vaginal microflora in postmenopausal women was represented by lactobacilli, facultative anaerobes and aerobes: Enterobacteriaceae, Staphylococcus spp., Streptococcus spp. and obligate anaerobic microorganisms: Prevotella bivia/Porphyromonas spp., Eubacterium spp., Megasphaera spp./Veillonella spp./ Dialister spp., Lachnobacterium spp./ Clostridium, Mobiluncus spp./ Corynebacterium, Peptostreptococcus spp. DNA of Mycoplasma genitalium and Candida spp. was not detected in any of the samples.
 TBM in postmenopausal women was 106.3 (2.5-8.5) CFU/sample. In the structure of TBM, the highest values were detected in two groups of obligate anaerobic microorganisms - Eubacterium spp. (104.0(0-7.7) CFU/sample) and Prevotella bivia / Porphyromone spp. (102.7 (0-6.9 CFU/sample). Lactobacilli spp. were presented in the amount of 104.6(0-8.5 CFU/sample and were distributed as follows: CST I was predominantly represented by L. crispus (107.5(6.0-8.1) CFU/sample) and was found in 10% of postmenopausal women; CST II was present in 6% of women dominated by L. gasseri (106.6 (4.3-7.3) CFU/sample) and L. johnsonii (104.6 (2.5-5.4) CFU/sample); in CST III (14.9%) L. iners prevailed (107.2 (4.4-8.4) CFU/sample); CST IV (absence of lactobacilli) was diagnosed in 67.5% of postmenopausal women; CST V (1.6%) was represented by L. jensenii (106.5 CFU/sample). L. vaginalis was present in CST I, CST II, CST III, and CST V. L. acidophilus was not found in any of the microbiota community types. We excluded CST V due to its presence in only three women in both observation groups.
TBM in postmenopausal women was 106.3 (2.5-8.5) CFU/sample. In the structure of TBM, the highest values were detected in two groups of obligate anaerobic microorganisms - Eubacterium spp. (104.0(0-7.7) CFU/sample) and Prevotella bivia / Porphyromone spp. (102.7 (0-6.9 CFU/sample). Lactobacilli spp. were presented in the amount of 104.6(0-8.5 CFU/sample and were distributed as follows: CST I was predominantly represented by L. crispus (107.5(6.0-8.1) CFU/sample) and was found in 10% of postmenopausal women; CST II was present in 6% of women dominated by L. gasseri (106.6 (4.3-7.3) CFU/sample) and L. johnsonii (104.6 (2.5-5.4) CFU/sample); in CST III (14.9%) L. iners prevailed (107.2 (4.4-8.4) CFU/sample); CST IV (absence of lactobacilli) was diagnosed in 67.5% of postmenopausal women; CST V (1.6%) was represented by L. jensenii (106.5 CFU/sample). L. vaginalis was present in CST I, CST II, CST III, and CST V. L. acidophilus was not found in any of the microbiota community types. We excluded CST V due to its presence in only three women in both observation groups.
It is important to note that in postmenopause, regardless of its duration, the dominant community of the vaginal microbiota was CST IV with a predominance of obligate anaerobic microorganisms: Eubacterium spp. (105.2(0-7.7) CFU/sample), Prevotella bivia / Porphyromonas spp. (104.9(0-6.9) CFU/sample) and optionally anaerobic Streptococcus spp. (104.2(0-6.5) CFU/sample).
During the study we estimated the distribution of CSTs in the observation groups depending on the presence or absence of atrophic changes in the vaginal epithelium. It is worth noting that CST IV was found in 76% of cases in the main observation group, and in every second patient in the group without atrophy (p = 0.002). The vaginal microbiota was represented by L. crispatus, L. gasseri and L. iners (CST I – CST III) in 24% of postmenopausal women with VVA (Fig. 1). As postmenopause progresses, the distribution of CSTs within groups did not statistically significantly change.
When comparing the spectrum of microbial communities in the study groups, statistically significant differences in the composition of microbiocenosis were revealed (Table 4).

In CST IV, in the main observation group, TBM was significantly lower (p < 0.0001) compared with the women without atrophy. At the same time, the absolute number of some representatives of the facultative anaerobic (aerobic) vaginal flora was also significantly reduced: in CST III and CST IV Streptococcus spp. (p = 0.009, p = 0.048, respectively), and obligate anaerobic microorganisms in CST IV: Eubacterium spp. (p = 0.012), Megasphaera spp./ Veillonella spp./ Dialister spp. (p = 0.020). In 50% of patients in the group without atrophic changes in the vaginal mucosa and with CST IV, Gardnerella vaginalis (105.7(0.0-7.8)* CFU/sample) was detected in the vaginal microbiota (p < 0.0001).
When we were comparing the spectrum of microorganisms with CST in the study groups with atrophic changes in the vaginal epithelium and with an increase in menopause, the decrease in the absolute number of lactobacilli (p <0.0001) was observed: L. crispatus (p = 0.003), L. gasseri, L. iners , L. johnsonii (p = 0.001) (Table 5).

In our study, we investigated the acid-base state of the vaginal environment. The average pH was 5.7±0.9 (range from 4.2 to 7.5). When comparing the pH level in both groups, an increase in this indicator was revealed. In group A, the pH was 6.0±0.8 compared with the group without atrophy, which was 5.0±0.6 (p <0.0001). We conducted a ROC analysis to determine its critical level associated with VVA. The area under the ROC curve was AUC = 0.805 [95% CI 0.743-0.868], p <0.0001, which allowed us to evaluate it as very good (Fig. 3). The threshold pH was 6.0. The sensitivity and specificity of the proposed model in the area of the threshold value were 66 and 79%, respectively.
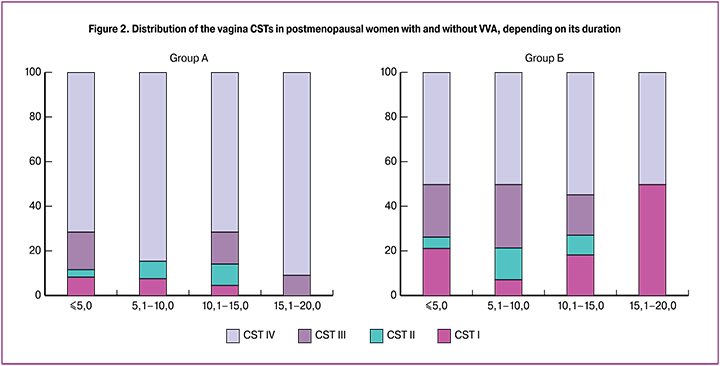
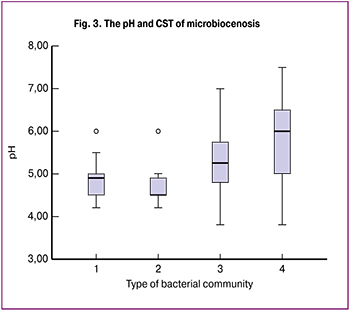 The analysis of the pH value of the vagina was also performed, depending on the type of community of the vaginal microbiocenosis. In CST I, the pH was 5.0 (4.8–5.0). This type of microbiocenosis is characterized by the predominance of L. Crispatus. In CST II (with the dominance of L. gasseri and L. Johnsonii), the pH was 4.8 (4.6–4.8), in CST III (L. Iners) 5.5 (5.0–6.0); in CST IV (absence of lactobacilli), the pH was the highest - 6.0 (5.0–6.0) (Figure 3).
The analysis of the pH value of the vagina was also performed, depending on the type of community of the vaginal microbiocenosis. In CST I, the pH was 5.0 (4.8–5.0). This type of microbiocenosis is characterized by the predominance of L. Crispatus. In CST II (with the dominance of L. gasseri and L. Johnsonii), the pH was 4.8 (4.6–4.8), in CST III (L. Iners) 5.5 (5.0–6.0); in CST IV (absence of lactobacilli), the pH was the highest - 6.0 (5.0–6.0) (Figure 3).
We also studied the prevalence of vaginal dryness and dyspareunia, depending on CST. As can be seen from Table 6, vaginal dryness was significantly associated with CST IV in postmenopausal women.
Discussion
To date, there are no common criteria for evaluating the vaginal microflora in postmenopausal women. Vaginal microbiota with a dominant number of lactobacilli is the key to vaginal and female health [2]. But due to hypoestrogenism accompanied by atrophic changes in the vaginal mucosa, there is a quantitative and qualitative compositional disorder of the microbiota and alkalization of the vaginal environment [4], which result in CST redistribution. Little is known about the relationship between vaginal microflora in postmenopausal women and complaints that occur during this age period. Apparently, atrophic changes in the vaginal mucosa and the CST redistribution are two mutually confounding, parallel processes in postmenopausal women. R. Hummelen et al. in their work [11] described the relationship of dryness, the quantitative ratio of lactobacilli and TBM in the postmenopausal period. The authors found that in the absence of complaints in women in the study groups, Lactobacillus spp. prevailed in the microbiota of the vagina, despite a decrease in TBM (p = 0.001), and in patients with complaints of dryness in the vagina, a decrease in the number of lactobacilli was detected with a predominance of obligate anaerobic microorganisms (Prevotella bivia, Porphyromonas spp.). Brotman et al. described the vaginal microbiota of 87 postmenopausal women according to five CSTs and associated with symptoms of VVA. In this work, a significant relationship was established between the symptoms of VVA and CST IV – A [10]. The researchers compared the relationship of symptoms with the subtypes of CST IV, which did not give any differences. They concluded that pain and dryness in the vagina, dyspareunia are associated with the predominance of anaerobic bacterial microflora and a low number of lactobacilli or their complete absence. In our work, we studied the prevalence of VVA symptoms, namely vaginal dryness and dyspareunia, depending on the prevalence of CST. Vaginal dryness in postmenopausal women with VVA was significantly associated with the predominant CST IV (p = 0.005). In 50% of postmenopausal women complaining of dryness, bacterial vaginosis was observed without atrophic changes in the vaginal mucosa. CST IV in postmenopausal women was significantly associated with a vaginal pH of 6.0 (p < 0.0001). Every second patient without atrophic changes having the prevalence of opportunistic flora, complained of unpleasant discharge from the genital tract with an odor. It is important to note that lactoflora persisted in 24% of postmenopausal women with VVA. Thus, we suggest that vaginal dryness is not a specific symptom for atrophic changes in the vaginal mucosa. Apparently, its presence or absence also depends on the composition of the microbiota of the vagina in the postmenopausal period and is associated with CST IV. This hypothesis is consistent with the results of a study by Hummelen R. et al., where vaginal dryness in postmenopausal women had an inverse correlation with the number of lactobacilli in the vaginal micribiota (p = 0.00141) [11].

Conclusion
Vaginal dryness is a specific symptom for CST in women with VVA, however, it is a common complaint in postmenopausal patients without atrophic changes in the vaginal mucosa associated with vaginal dysbiosis. The obtained data allow us to suggest that there is a possible association of the vaginal symptoms not only with the atrophy of the vaginal mucosa but also with the specific features of its microflora. These findings will enable us to personalize the management of postmenopausal patients with vaginal symptoms, namely the patients with CST IV without atrophic changes of the vaginal mucosa from the standpoint of therapy for bacterial vaginosis; but in case of atrophic changes, local hormone therapy with estriol is the first-line treatment.
References
- Sinha A., Ewies A.A. Non-hormonal topical treatment of vulvovaginal atrophy: an up-to date overview. Climacteric. 2013; 16(3): 305-12.doi: 10.3109/13697137.2012.756466
- Балан В.Е. Урогенитальные расстройства в климактерии (клиника, диагностика, заместительная гормональная терапия): дис. … д-ра мед. наук. М., 1998. 305 c. [Balan V.E. Urogenital disorders in menopause (clinic, diagnosis, hormone replacement therapy): dis. ... Dr. med. sciences. M., 1998. 305 p. (in Russ.)].
- Ворошилина Е.С., Тумбинская Л.В., Донников А.Е., Плотко Е.Э., Хаютин Л.В. Биоценоз влагалища с точки зрения количественной полилимеразной цепной реакции: что есть норма? Акушерство и гинекология. 2011; 1: 57-65. [Voroshilina E.S., Tumbinskaya L.V., Donnikov A.E., Plotko E.A., Khayutin L.V.Vaginal biocenosis with a view to quantitative polymerase chain reaction: what is its norm? Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2011; 1: 57-65. (in Russ.)].
- Sturdee D.W. Recommindations for the magement of postmenopausal vaginal atrophy. International menopause Society Writing Group. Climacteric. 2010; 13(6): 509-31. doi: 10.3109/13697137.2010.522875
- Сметник В.П., Юренева С.В., Ермакова Е.И., Глазунова А.В. Генитоуринарный менопаузальный синдром. Клинические рекомендации (проект). Менопауза. 2015; 1:18. [Smetnik V.P., Jureneva S.V., Ermakova E.I., Glazunova A.V. Genitourinar menopausal syndrome. Clinical recommendations (draft). Menopause. 2015; 1:18. (in Russ.)].
- Бурменская О.В. Молекулярно-генетические маркеры иммунного ответа при воспалительных заболеваниях органов женской репродуктивной системы: дис. … д-ра биол.наук. М., 2014. 249 c. [Burmenskaya O.V. Molecular genetic markers of the immune response during inflammatory diseases of the female reproductive system. Diss. M., 2014. 249 p. (in Russ.)].
- Балан В.Е., Ковалева Л.А., Амирова Ж.С., Рафаэлян И.В. Возможности гормональной терапии урогенитальной атрофии у женщин. Акушерство и гинекология. 2011; 6: 113-116. [Balan V.E., Kovaleva L.A., Amirova Zh.S., Rafaelyan I.V. Possibilities of hormonal therapy for female urogenital atrophy. Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2011; 6: 113-116.(in Russ.)].
- Есефидзе Ж.Т. Клиника, диагностика и лечение атрофического вагинита в постменопаузе. РМЖ; 9: 370. [Yesefidze J.T. Clinic, diagnosis, and treatment of atrophic vaginitis in postmenopausal women. Breast cancer; 9: 370. (in Russ.)].
- Ravel J., Gajer P., Abdo Z., Schneider G.M., Koenig S.S., McCulle S.L., Karlebach S., Gorle R., Russell J., Tacket C.O., Brotman R.M., Davis C.C., Ault K., Peralta L., Forney L.J. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108 Suppl 1: 4680-7. doi: 10.1073/pnas.1002611107
- Brotman R.M., Shardell M.D., Gajer P., Fadrosh D., Chang K., Silver M.I., Viscidi R.P. Burke A.E., Ravel J., Gravitt P.E. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause. 2014; 21(5): 450-8. doi: 10.1097/GME.0b013e3182a4690b
- Hummelen R., Macklaim J.M., Bisanz J.E., Hammond J.A., McMillan A., Vongsa R., Koenig D., Gloor G.B., Reid G. Vaginal microbiome and epithelial gene array in post-menopausal women with moderate to severe dryness. PLoS One. 2011; 6(11): e26602. doi: 10.1371/journal.pone.0026602
Received 20.05.2019
Accepted 21.06.2019
About the Authors
Elena G. Yeprikyan, postgraduate of gynecological endocrinology department, Federal State Budget Institution National Мedical Research Center for Obstetrics, Gynecology and Perinatology Ministry of Healthcare and Social Development of the Russian Federation. Тел.: + 7(915)3843020. e-mail: eldo989@rambler.ruSvetlana V. Yureneva, MD, general research staff of Federal State Budget Institution National Мedical Research Center for Obstetrics, Gynecology and Perinatology
Ministry of Healthcare and Social Development of the Russian Federation. Tel.: +7 9161797400, e-mail: syureneva@gmail.ru. Oparin street, 4, Moscow, Russia, 117997.
AndreyYe. Donnikov, PhD, Laboratory of Molecular Genetic Methods, Research Center for Obstetrics, Gynecology and Perinatology Ministry of Healthcare and Social Development of the Russian Federation. Tel.: +7 9036845247, e-mail: a_donnikov@oparina4.ru. Oparin street, 4, Moscow, Russia, 117997,
Larisa .S. Yezhova, PhD, Ist pathoanatomical department, Research Center for Obstetrics, Gynecology and Perinatology Ministry of Healthcare and Social Development of the Russian Federation. Tel.: +9154882383, e-mail: l.s.ezhova@yandex.ru. Oparin street, 4, Moscow, Russia, 117997.
For citations: Eprikyan E.G., Yureneva S.V., Donnikov A.E., Ezhova L.S. Relationship between the symptoms of vulvovaginal epithelial atrophy and the vaginal microbiota in postmenopausal women.
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2019; 11: 152-59.(In Russian).
https://dx.doi.org/10.18565/aig.2019.11.152-159



