В гинекологической и сексологической клинической практике у женщин репродуктивного и менопаузального возраста широко распространены жалобы на дискомфорт, диспареунию (боль при вагинальном проникновении, во время полового акта или после него), ощущение зуда, жжения в области вульвы, часто ассоциированные с сухостью влагалища [1]. Описанные вагинальные симптомы, которые относятся к основным клиническим проявлениям генитоуринарного менопаузального синдрома (ГУМС), включающего вульвовагинальную атрофию (ВВА), наблюдаются у 40–50% женщин после естественной и хирургической менопаузы, из них у 60% – умеренной или тяжелой степени выраженности [2, 3].
Субъективные симптомы ВВА коррелируют с объективными признаками ГУМС: установлено, что вагинальные симптомы ГУМС имеют линейную связь со снижением сексуального функционирования; в возрасте постменопаузы у женщин с сексуальной дисфункцией ВВА наблюдается в 3,84 раза чаще, чем у женщин без сексуальной дисфункции [4]. У 50% женщин, перенесших рак репродуктивных органов и прошедших его лечение, сухость влагалища и диспареуния ассоциированы с сексуальной дисфункцией [5].
Распространенными причинами диспареунии на фоне вагинальной сухости являются гормональный дисбаланс в пре- и постменопаузе, состояние после оперативных вмешательств на органах мочеполовой системы, инфекции мочевых путей и влагалища, иммунные расстройства, дерматологические заболевания, радиационная терапия, химиотерапия, прием некоторых лекарственных средств, в том числе антиэстрогенных препаратов, антидепрессантов, использование гигиенических средств [6–10].
Увлажненность вагинального эпителия в состоянии покоя и сексуального возбуждения обеспечивается молекулярными механизмами секреции вагинальной жидкости и выделением секрета парных малых (железы Скина) и больших (бартолиновы железы) желез преддверия влагалища. Также при сексуальном возбуждении у женщин происходит любрикация, которая обеспечивается нейрососудистой реакцией, приводящей к увеличению регионального кровотока, вазоконгестии, генитальному нагрубанию и активации вагинальной секреции [11].
Показаниями к применению любрикантов являются дискомфорт во время интимной близости при недостаточной готовности женщины к половому акту, диспареуния, возникающая вследствие сухости слизистой влагалища.
Вагинальный любрикант – это специальное средство, которое обеспечивает скольжение половых органов во время сексуального контакта. Любрикант помогает устранить сухость при недостатке естественного увлажнения, обеспечивает комфорт во время полового акта, защищает гениталии партнеров от микротравмы (натирания, появления микротрещин и раздражения).
Специальное средство можно считать любрикантом, если оно обеспечивает скольжение на протяжении всего полового акта, поддерживает приятные тактильные ощущения, не оказывает негативного влияния на состояние слизистой (раздражение, пощипывание, жжение и т.д.) и на микробиоту вульвы и влагалища.
Обнаружено, что использование слюны во время полового контакта увеличивает риск приобретения женщиной дрожжевой инфекции [12].
Основные типы химического состава и физиологические характеристики вагинальных любрикантов
Обычно вагинальный эпителий покрыт биологической пленкой, происходящей из субэпителиальной области стенки влагалища, содержащей плотную сеть капилляров, и состоящей из воды, электролитов, низкомолекулярных органических соединений (глюкозы, липидов и аминокислот), белков, ферментов, эпителиальных клеток, лейкоцитов и лимфоцитов [13].
Производители вагинальных любрикантов стремятся в значительной степени воспроизвести физиологические свойства естественного секрета. Качественные любриканты соответствуют по тактильным ощущениям вагинальной жидкости благодаря оптимальной степени мукоадгезии, увлажнения слизистой оболочки, а также влагоудерживающей способности.
Наиболее значимым является критерий мукоадгезии, которая определяет эффективность вагинального любриканта – равномерное распределение по поверхности и способность смачивания поверхности слизистой влагалища без впитывания. Мукоадгезия не имеет абсолютной количественной характеристики, обеспечивается пленкообразующими веществами (гидроксиэтилцеллюлоза, карбоксиметилцеллюлоза, альгинат натрия, нелетучие полисилоксаны), входящими в состав любрикантов.
При изготовлении любриканта преимущественно используют два типа составов: на водно-гликолевой/глицериновой и на силиконовой основе. В состав водорастворимых любрикантов включены вода, гелеобразователь, влагоудерживающая добавка, которая препятствует высыханию, улучшает распределение по поверхности слизистой полимерных молекул гелеобразователя, и консервант. В основе силикон-содержащих любрикантов используются силиконовые полимеры, обладающие достаточной мукоадгезией и свойством скольжения (таблица).
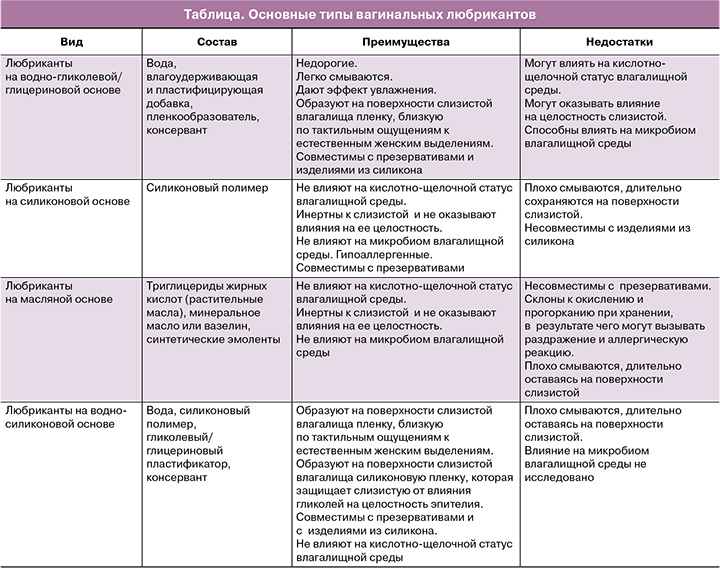
Главным фактором риска нарушения целостности эпителия во время полового акта является механическое трение. И поэтому основное назначение вагинального любриканта состоит в предотвращении натирания и повреждения половых органов партнеров во время сексуального контакта. Оно обеспечивается длительным сохранением смазывающего слоя во время полового акта. При этом от повреждений кожу и слизистую вульвы и влагалища в случае водорастворимых любрикантов предохраняет высокое содержание влагоудерживающих компонентов (например, глицерина). Защитное действие безводных любрикантов основано на отсутствии эффекта впитывания и испарения (например, за счет содержания силиконов).
Влагоудерживающие свойства любриканта – это способность длительно удерживать воду в составе скользящей пленки, препятствуя ее высыханию. Их количественная характеристика определяется скоростью испарения летучих компонентов, которая напрямую зависит от концентрации гигроскопичных веществ в составе любриканта. Чем выше концентрация, тем лучше влагоудерживающие свойства (что не применимо к безводному любриканту). Влагоудерживающие свойства любриканта обеспечиваются гигроскопичными веществами, такими как многоатомные спирты (глицерин, пропиленгликоль, полиэтиленгликоль, сорбит), мочевина, соли (хлорид натрия, хлорид магния), бетаин, гиалуроновая кислота.
Нарушение барьерной функции эпителиальной ткани может быть связано с увеличением восприимчивости к инфекциям, передаваемым половым путем (ИППП), с изменением микробиоты влагалища и возникновением бактериального вагиноза [14].
Немаловажным фактором риска нарушения целостности эпителия является высокая осмоляльность любриканта, за счет чего он способен повреждать эпителий слизистых оболочек, делая его более проницаемым.
Осмоляльность любриканта характеризует суммарную концентрацию всех растворенных частиц в воде, что не применимо к безводному любриканту. Чтобы не оказывать влияние на целостность эпителия, любрикант должен быть изоосмоляльным по отношению к вагинальным выделениям (370±40 мОсм/кг) [14], но тогда его смазывающие свойства будут подобны воде.
Обычно для гарантии защиты половых органов партнеров от натирания во время сексуального контакта производители вводят в состав любрикантов большое количество гликолей; однако была обнаружена связь между концентрацией увлажняющих компонентов в любриканте (гликоли, глицерин) и усилением секреторной реакции слизистой оболочки при осмоляльности более 2200 мОсм/кг [15]. Учитывая имеющиеся данные о способности таких интимных средств нарушать эпителиальную целостность слизистых оболочек и увеличивать риск заражения ИППП, Всемирная организация здравоохранения (ВОЗ) рекомендует значение осмоляльности для любриканта не более 1200 мОсм/кг [16].
Таким образом, в эффективном и безопасном водорастворимом любриканте должны соблюдаться оптимальный баланс между параметрами мукоадгезии, влагоудержания и осмоляльности.
Одним из важных показателей, характеризующих микробиоту, является рН влагалищной среды, который в норме поддерживается в диапазоне 3,8–4,5, что оптимально для жизнедеятельности лактобацилл [17]. Однако рН человеческой спермы имеет значение 7,2. Таким образом, кратковременное, во время полового акта, изменение кислотно-щелочного баланса влагалищной среды не оказывает влияния на жизнеспособность лактобацилл и не приводит к возникновению дисбиоза.
Любриканты не должны влиять на естественный баланс микробиоты влагалищной среды. В клинических исследованиях [18, 19] показано, что наилучшим образом с этой задачей справляются любриканты, в составе которых отсутствуют вещества, способные влиять на кислотно-щелочной баланс, а рН находится в диапазоне от 4 до 7.
Для фиксации рН на определенном уровне используются буферные системы из смеси закисляющих (молочная кислота, лимонная кислота) и защелачивающих (аргинин, триэтаноламин, гидроксид натрия) добавок. Физиологическим показателям рН соответствует, например, буферная смесь молочной кислоты и аргинина. Но, следует отметить, рекомендации по искусственному поддержанию рН в диапазоне 3,8–4,5 с помощью буферной системы лежат в области лечебных задач для женщин с существующим нарушением нормоценоза влагалищной флоры и не относятся к назначению любриканта.
В ряде исследований было убедительно показано, что большое значение для безопасности любрикантов, кроме показателей рН и осмоляльности, имеет собственная токсичность отдельных компонентов, входящих в их состав [18, 20–23]. Установлено, что целостность вагинального эпителия нарушают такие вещества, как поликватерний (катионные полимеры), хелатирующая добавка этилендиаминтетрауксусная кислота (ЭДТА) (более 0,1%), ноноксинол-9, бензалкониум хлорид (более 2%), хлоргексидин (более 2%), безводный глицерин, безводный пропиленгликоль, безводный полиэтиленгликоль. Их наличие в составе любриканта часто вызывает неприятное ощущение пощипывания в месте нанесения, а также увеличивает риск инфицирования ИППП.
Компоненты водорастворимого любриканта являются хорошей питательной средой для микроорганизмов. Для сохранения микробиологической чистоты продукта на протяжении всего срока годности любриканта в его состав включают только разрешенные для использования человеком консерванты. Самые распространенные из них: сорбат калия
(до 0,6%), бензоат натрия (до 0,5%), метилпарабен (до 0,4%), пропилпарабен (до 0,14%), феноксиэтанол (до 1%), диазолидинилмочевина (до 0,5%), o-кумен-5-ол (до 0,1%), хлоргексидин (до 0,3%) [24].
Для подавления жизнедеятельности разных видов микроорганизмов (бактерии, плесневые и дрожжевые грибы) требуются разные типы консервантов. Поэтому, как правило, в любрикантах используют смесь таких веществ. Любой консервант в силу своего назначения подавлять жизнедеятельность микроорганизмов обладает токсичностью; чем выше его эффективность, тем выше токсичность по отношению к живым организмам. В связи с этим разрешение к использованию любого консерванта регламентировано в области лекарственных, косметических средств и пищевых продуктов. Установлены максимально допустимые дозы использования консерванта в продукте, при которых риск негативного действия на здоровье человека минимален. Таким образом, соблюдается баланс между эффективностью и безопасностью консерванта. Например, сорбат калия и бензоат натрия оказывают низкое токсичное действие на человека, но при этом их способности защитить любрикант на базе гидроксиэтилцеллюлозы от микробной контаминации в процессе использования недостаточно. Риск нанесения ущерба здоровью при использовании микробиологически контаминированного любриканта из-за слабой эффективности консерванта в разы превышает подобный риск от присутствия консерванта. В связи с этим их комбинируют с более токсичным, но в то же время и более эффективным консервантом.
В последние десятилетия на фоне публикаций о негативном влиянии на здоровье человека консервантов в составе продуктов питания и средств для ухода за кожей проведено большое количество научных исследований, посвященных проблеме их безопасности.
Наиболее распространенными и хорошо изученными консервантами являются метилпарабен и пропилпарабен (эфиры гидроксибензойной кислоты); общепризнанна безопасность их использования в пищевой промышленности [25].
В квалификации канцерогенов Международного агентства по изучению рака (МАИР, англ. International Agency for Research on Cancer (IARC)) отсутствуют указания на канцерогенность метилпарабена и пропилпарабена для человека [26].
При разработке любрикантов особое внимание уделяется вопросам безопасности не только с позиций влияния на слизистую и микробиоту влагалища женщины, но и совместимость с половыми клетками, оплодотворением и эмбрионами для пар, не предохраняющихся от беременности, а также для применения медицинскими работниками во время манипуляций в программах вспомогательных репродуктивных технологий в клиниках репродукции [27, 28].
В связи с этим наиболее значимыми факторами являются осмоляльность (ниже 1200 мОсм/кг), эндотоксичность, влияние на подвижность сперматозоидов, целостность ДНК/хроматина сперматозоидов, на проникновение спермы в цервикальную слизь, проникновение спермы в любрикант [29]. Исследования любрикантов проводились на животной модели, оценивались результаты оплодотворения in vitro, последующее развитие эмбрионов и риски тератогенных эффектов [30]. Таким образом, были установлены вещества, входящие в состав любрикантов, не препятствующие зачатию и обладающие высоким уровнем безопасности, такие как вода, пропиленгликоль, глицерин, гидроксиэтилцеллюлоза, цетилгидроксиэтилцеллюлоза, гидроксипропилметилцеллюлоза, карбомер, фосфат натрия, фосфат калия, хлорид натрия, хлорид калия, хлорид магния, хлорид кальция, метилпарабен, пропилпарабен, фруктоза, галактоза, ксилоза, фенетиловый спирт, каприлил гликоль, экстракт мускатного шалфея, арабиногалактан.
Клинические ситуации и таргетные любриканты
Наиболее часто к ежедневному использованию любрикантов прибегают женщины в возрасте менопаузального перехода и постменопаузы, когда на фоне эстрогенного дефицита снижается трофическая и секреторная функция эпителия влагалища, что приводит к его истончению, развитию атрофических процессов, снижению продукции влагалищной жидкости, нарушению кислотно-щелочного баланса и появлению сухости, приносящей выраженный дискомфорт. При отказе от применения системных и топических эстрогенсодержащих препаратов при ВВА необходимая интенсивность увлажнения обеспечивается увлажняющими компонентами, введенными в состав используемого средства.
Проведено клиническое исследование [31] влияния гиперосмоляльного любриканта, содержащего 1% Д-пантенол, на рН влагалищной среды, состояние эпителия влагалища, количество вагинальных выделений у женщин. Авторами установлено, что в результате его ежедневного применения в течение 3 месяцев число женщин менопаузального возраста с явлениями ВВА сократилась с 66,7 до 20,0%. Секреторная функция вагинального эпителия улучшилась у 46,3% пациенток репродуктивного возраста и 60,1% пациенток менопаузального возраста. Влияние любриканта, содержащего 1% Д-пантенол, на рН влагалищной среды не установлено.
Представлены данные клинических исследований влияния гиперосмоляльных вагинальных гелей на состояние вагинального эпителия при ежедневном применении в течение 28 дней у женщин репродуктивного возраста [32] и женщин в постменопаузе [33]. Оценка индекса созревания клеток эпителия влагалища (MI), которая опосредованно характеризует сохранение барьерной целостности эпителия, у женщин в постменопаузе с симптомами ВВА, независимо от применяемого средства, через 56 дней признаков разрушения эпителия не выявила. Увеличение индекса вагинального здоровья свидетельствует о благоприятном влиянии увлажняющих средств на слизистую влагалища. Аналогичные результаты получены при клиническом исследовании гиперосмоляльных вагинальных гелей [34]. В течение 4-недельного периода не реже одного раза в неделю женщинам с сухостью влагалища и диспареунией было предложено использовать любриканты пяти видов с осмоляльностью 1000–5000 мОсм/кг. В результате использования любрикантов выраженные побочные эффекты не установлены, наблюдалось значимое улучшение сексуального функционирования согласно данным опросника «Индекс женской сексуальной функции».Таким образом, несмотря на риск нарушения целостности эпителия под влиянием гиперосмоляльного геля, интенсивное увлажнение слизистой влагалища устраняет явления сухости и связанного с ней дискомфорта. Эффект снижения симптомов ВВА напрямую зависит от концентрации увлажняющих добавок. Клинические данные эффективности изоосмоляльных гелей при сухости влагалища отсутствуют, что может объясняться их неэффективностью при данной клинической ситуации.
Для пациенток с симптомами ВВА любриканты являются альтернативой гормональной терапии; они при нанесении на ткани вульвовагинальной области устраняют явления сухости, обеспечивают увлажнение слизистой, повышают индекс вагинального здоровья. Эти эффекты любрикантов обеспечиваются рядом гигроскопичных компонентов: Д-пантенол (провитамин В5), мочевина, аминокислоты, гиалуроновая кислота, глицерин. Общими требованиями к выбору увлажняющих добавок являются их физиологичность (присутствуют в организме человека в норме), достаточная изученность фармакологического и токсикологического влияния, безопасность применения, низкая вероятность аллергической реакции.
Важное условие применения любрикантов связано с их влиянием на активность лактобацилл. Состав вагинальной микрофлоры может меняться под влиянием менструального цикла, во время беременности, при механическом разрушении бактериального слоя во время полового акта.
На жизнеспособность лактобацилл осмоляльность геля не влияет [14, 18, 20]. Также не установлено значимое негативное или положительное влияние на вагинальный микробиом рН любриканта [19]. Благоприятно влияет на сохранение нормоценоза влагалища входящий в состав любриканта пребиотик (например, инулин) [35], негативно – микробиоциды (хлоргексидин, мирамистин, ноноксинол, бензалкония хлорид и др. антисептики) [18]; как положительно, так и негативно могут влиять кислотно-щелочные добавки (молочная кислота, лимонная кислота, триэтаноламин, гидроксид натрия) [19]. Эти данные необходимо учитывать при рекомендации любриканта женщинам, склонным к дисбиозу, и беременным.
Необходимым условием при случайных половых контактах является предотвращение или снижение риска передачи инфекций. Способность любрикантов препятствовать разрыву презерватива во время полового акта, безусловно, снижает риск заражения ИППП [16].
Микробиоциды в форме любриканта – это наиболее удобный способ применения специальных антисептиков с доказанной активностью против ИППП. Однако установлена способность ряда компонентов в составе микробиоцидов усиливать восприимчивость влагалища к вирусным и бактериальным патогенам ИППП. В научном исследовании [20] показано, что ноноксинол-9 (2%), бензалкониум хлорид (2%), хлоргексидин (2%), ЭДТА (0,1%), пропиленгликоль (9900 мОсм/кг), полиэтиленгликоль (5700 мОсм/кг) увеличивают восприимчивость эпителиальной ткани к инфекции. Аналогичный результат показал любрикант на гликолевой основе (10 000 мОсм /кг). Так как восприимчивость к инфекции развивалась не сразу, а спустя время после воздействия продукта, то был сделан вывод о связи между разрушением целостности эпителия и появлением восприимчивости к инфекции. Не влияли на восприимчивость к инфекции метилпарабен, бензиловый спирт (1%), ЭДТА (0,02%), глицерин (10%, 1700 мОсм /кг), пропиленгликоль (10%, 1700 мОсм /кг).
В глобальном исследовании [18] 10 любрикантов на водной основе, 2 – на жировой основе и 2 силиконовых любрикантов установлены взаимосвязи между их составом и влиянием на целостность эпителия, на жизнеспособность лактобацилл и на репликацию ВИЧ-инфекции in vitro. В результате отсутствие токсичности во всех тестах на безопасность показали только два изоосмолярных геля и оба силиконовых любриканта. Линейная взаимосвязь между гиперосмолярностью используемого любриканта и увеличением риска репликации ВИЧ-инфекции не установлена. Выявлено, что любриканты на основе силикона, в отличие от любрикантов на водной основе, наиболее безопасны для эпителиальных клеток и тканей слизистой оболочки.
Посткоитальный цистит – часто рецидивирующее воспаление мочевого пузыря, возникающее в течение полутора суток после интимных отношений или влагалищных манипуляций. Проявляется учащенным болезненным мочеиспусканием, болями внизу живота, поллакиурией. Посткоитальный цистит развивается в результате восходящего инфицирования. При женской гипоспадии и наличии урогименальных тяжей наружное уретральное отверстие во время полового акта смещается в вагину и открывается. Под давлением, возникающим из-за движений полового члена, влагалищная флора забрасывается внутрь мочеиспускательного канала. С целью профилактики проникновения во время полового акта условно-патогенной микрофлоры влагалища в урогенитальный тракт женщины применяется хлоргексидин. В обзоре Касихиной Е.И. [36] описано, что интравагинальные аппликации хлоргексидина в концентрации, варьирующейся от 0,25 до 2%, каждые 4 ч не выявили нежелательных реакций в концентрации менее 1%. В случае повышения концентрации до 1% и более у 13% женщин появились жалобы на жжение или болезненные ощущения. Также на фоне применения гексикона (0,5% хлоргексидина биглюконата) существенно увеличивается высеваемость лакто- и бифидобактерий в сравнении с исходным уровнем (с 29, до 50,6%). Таким образом, использование любриканта с хлоргексидином до 0,5% в качестве антисептика делает оптимальным соотношение польза/риск у пациенток, склонных к посткоитальному циститу.
Существуют специальные добавки в составе некоторых любрикантов, назначение которых – усиление генитальной сексуальной реакции во время половых контактов. Для стимуляции возбуждения, вазоконгестии при оргазмической дисфункции, нарушениях генитальной реакции применяют в составе любриканта разогревающие вещества двух типов.
1) За счет усиления микроциркуляции крови (экстракты специй, производные никотиновой кислоты, ментол, ванилилбутиловый эфир). Однако при изменениях слизистой влагалища (вульвовагиниты, бактериальный вагиноз и пр.) их побочным эффектом может быть неприятное жжение.
2) Безводные гликоли/глицерин, обладающие высокой степенью гиперосмоляльности, побочным действием которых является разрушение эпителиального слоя слизистой влагалища и существенное повышение риска ИППП.
Заключение
Проведенный анализ научных данных о безопасности, переносимости, эффективности и особенностях применения вагинальных любрикантов подтверждает целесообразность их широкого использования при сексуальной дисфункции, в качестве увлажняющих средств при ВВА, а также возможность применения во время беременности, при посткоитальном цистите, для снижения риска инфицирования во время полового акта ИППП.
Необходимы дальнейшие исследования по поиску значимых молекулярных компонентов для любрикантов и увлажняющих интимных средств с целью устранения и предотвращения нарушений секреции, сохранения лактобацилл и микробиоты в целом, а также барьерной функции вагинального эпителия.



