Злокачественные новообразования (ЗНО) являются одной из ведущих социально-экономических проблем в современном мире. Согласно данным систематического анализа GBD (Global Burden of Disease Study 2019), среди 22 групп заболеваний и травм онкологические процессы занимают второе место после сердечно-сосудистых заболеваний по количеству смертей, потерянных лет жизни и нетрудоспособности [1]. По оценкам авторов исследования GBD, в 2019 г. зарегистрировано 23,6 млн новых случаев рака во всем мире; при этом с 2010 г. отмечен рост заболеваемости на 26,3% [1]. В Российской Федерации в 2021 г. впервые выявлено 580 415 случаев ЗНО (в том числе 315 376 у пациентов женского пола). Рост данного показателя, по сравнению с 2020 г., составил 4,4% [2]. Наибольший удельный вес в структуре онкологической заболеваемости женщин имеют ЗНО органов репродуктивной системы, которые составляют 40,1% [2]. Рак молочной железы (22,1%) является ведущей онкологической патологией у женского населения, далее следуют ЗНО тела матки (8,1%), шейки матки (4,9%), яичника (4,2%) [2].
Учитывая современные возможности диагностики рака на ранних стадиях и повышение качества и эффективности проводимого лечения, особенно в странах с высоким уровнем жизни, выживаемость и продолжительность жизни у женщин с онкологическими заболеваниями репродуктивной системы в анамнезе увеличилась примерно на 38% за последние 25 лет [3, 4]. В связи с вышеизложенным, одной из основных задач в сфере российского здравоохранения является повышение качества жизни и трудоспособности у данной категории пациентов [4]. Низкое качество жизни пациенток с ЗНО органов репродуктивной системы в анамнезе в первую очередь обусловлено развитием климактерического синдрома. В многочисленных исследованиях установлено, что климактерические проявления при ятрогенной менопаузе развиваются значительно быстрее и протекают более тяжело по сравнению с симптомами, возникающими после естественной менопаузы, особенно у пациенток репродуктивного возраста [5, 6]. Это обстоятельство связано с рядом факторов [5–7]:
- быстрым и внезапным развитием дефицита половых стероидов после радикальных оперативных вмешательств на органах малого таза;
- химио- и лучевой терапией, обладающей гонадотоксическим действием;
- длительной гормональной терапией (тамоксифеном или ингибиторами ароматазы) у пациенток с раком молочной железы, направленной на развитие стойкой гипоэстрогении.
Вторыми по частоте проявлениями менопаузы после вазомоторных являются симптомы генитоуринарного менопаузального синдрома (ГУМС): сухость слизистой вульвы и влагалища, диспареуния, зуд, жжение, контактные кровянистые выделения, рецидивирующие инфекции мочевых путей, недержание мочи. Тяжелое течение ГУМС приводит к резкому снижению сексуальной активности, снижению качества жизни в целом и является одной из причин прекращения эндокринной терапии у пациенток с гормонпозитивными опухолями репродуктивной системы, что приводит к снижению безрецидивной выживаемости [8].
Эстрогензависимые опухоли репродуктивной системы являются абсолютным противопоказанием к использованию как системной, так и локальной менопаузальной гормонотерапии [9]. Поэтому данной категории пациенток необходимо подбирать альтернативные негормональные методы коррекции симптомов ГУМС [9]. В качестве негормональной терапии вульвовагинальной атрофии (ВВА) используют различные лубриканты. В систематическом обзоре 2021 г. [10] указано, что вагинальные лубриканты, отпускаемые без рецепта, достоверно облегчают симптомы ВВА. Однако их химические составы сильно различаются, и некоторые из них вызывают нежелательные эффекты и повреждения слизистой из-за нефизиологического pH, осмоляльности и добавок. Всемирная организация здравоохранения (ВОЗ) определила критерии безопасности вагинальных лубрикантов [11]:
- осмоляльность предпочтительно должна быть менее 1200 мОсм/кг (оптимально 200–600 мОсм/кг). Это достигается за счет ограничения содержания глицерина (массовая доля должна быть 9,9% и менее), пропилена (массовая доля до 8,3%), смеси гликолей (массовая доля до 8,3%);
- рН должен быть в диапазоне от 4,5 до 6. Увлажняющие средства с рН более 7 не рекомендованы к применению;
- в составе лубрикантов должны отсутствовать поликватерний, парабены (особенно бутилпарабен), хлоргексидин.
Вагинальные лубриканты могут быть изготовлены на разной основе: водной, масляной, силиконовой или на основе гиалуроновой кислоты (ГК). Эффективность и безопасность препаратов на основе ГК изучались в нескольких клинических испытаниях, в том числе и у пациенток с эстрогензависимыми онкозаболеваниями репродуктивной системы [12–14]. Систематический обзор 2021 г. [15] включал 5 рандомизированных клинических испытаний, в которых оценивалась эффективность вагинальных лубрикантов на основе ГК в сравнении с гелем или кремом с эстрадиолом 0,5 мг, вагинальными таблетками с эстрадиолом 25 мг 1 раз в 14 дней, конъюгированными эстрогенами 0,625 мг в день. Обобщенные результаты позволили предположить, что ГК имеет профили эффективности, безопасности и переносимости, сопоставимые с вагинальными эстрогенами для лечения сухости влагалища и диспареунии.
Отдельную группу негормональных средств для коррекции симптомов ГУМС/ВВА представляют локальные препараты с фитоэстрогенами. Рандомизированные клинические исследования показывают, что современные препараты с содержанием фитоэстрогенов не только увлажняют слизистую влагалища, уменьшают болезненность при половой жизни, но и повышают упругость и эластичность тканей, снижают рН влагалища и нормализуют локальную микрофлору [16–18]. В систематическом обзоре, посвященном влиянию системных и локальных фитоэстрогенов на урогенитальные симптомы, указано, что многочисленные клинические испытания с участием женщин в менопаузе не показали увеличения риска рака молочной железы или гиперплазии эндометрия даже на фоне длительного системного и локального применения фитоэстрогенов [18].
Продукт, использованный в исследовании
Крем дозированный «Эстрогиал» (свидетельство о государственной регистрации СГР ТС № В У.70.06.01.001.Е 003744.09.16) представляет собой комбинацию низкомолекулярной ГК и фитокомплекса (состав: полиэтиленгликоль, вода, экстракт клевера, экстракт календулы, экстракт хмеля, натрия гиалуронат (натриевая соль ГК)). Основой крема является натриевая соль ГК, которая агрегирует на себе молекулы воды и обеспечивает выраженный увлажняющий эффект. Фитокомплекс «Эстрогиала» представлен экстрактами клевера, календулы и хмеля, обладающими тонизирующим, регенерирующим, противовоспалительным, антиоксидантным эффектами, а также стимулирующим процессы неоколлагенеза. Крем «Эстрогиал» сформирован в виде свечей, каждая содержит дозу применения, что облегчает его введение во влагалище. Крем «Эстрогиал» имеет безопасный состав по критериям ВОЗ [11]. Согласно аннотации по применению, «Эстрогиал» не противопоказан пациенткам с гормонзависимыми онкозаболеваниями органов репродуктивной системы [19].
Цель исследования: оценить эффективность терапии симптомов ГУМС/ВВА негормональным кремом с ГК и фитокомплексом у пациенток с гормонзависимыми опухолями органов репродуктивной системы в анамнезе.
Материалы и методы
В наблюдательное проспективное исследование, которое проводилось на базе отделения гинекологической эндокринологии ФГБУ «НМИЦ АГиП им. В.И. Кулакова» МЗ РФ в период с августа 2022 г. по май 2023 г., включены 30 пациенток с симптомами ГУМС/ВВА в возрасте от 30 до 60 лет со следующими заболеваниями.
1. Женщины в постменопаузе с раком молочной железы в анамнезе (n=13):
- после оперативного лечения, закончившие химио- и/или лучевую терапию не ранее чем за 3 месяца до включения в исследование и в настоящее время получающие адъювантную терапию тамоксифеном или ингибиторами ароматазы (n=8);
- после оперативного лечения, в настоящее время получающие адъювантную терапию тамоксифеном или ингибиторами ароматазы в течение 6 месяцев и более (n=5).
2. Женщины в постменопаузе с раком эндометрия в анамнезе (n=17):
- после оперативного лечения, закончившие химио- и/или лучевую терапию не ранее чем за 3 месяца до включения в исследование (n=10);
- через 3 месяца и более после радикального оперативного лечения (n=7).
На момент включения в исследование все пациентки не имели клинических признаков прогрессирования онкозаболевания, подтвержденных данными компьютерной томографии.
На визите скрининга после оценки состояния женщины получали крем «Эстрогиал», который использовали по следующей схеме: по 1 дозе на ночь во влагалище ежедневно в течение 4 недель, затем по 1 дозе через день в течение 8 недель, затем по 1 дозе 2 раза в неделю в течение 12 недель.
Эффективность и безопасность лечения оценивались по следующим критериям.
Адаптированная шкала для оценки вульвовагинальных симптомов (табл. 1). Участники исследования оценивали симптомы по 4-балльной шкале (0 – нет; 1 – легкая степень; 2 – умеренная; 3 – тяжелая), затем данные усреднялись для получения сводных оценок по визуально-аналоговой шкале (ВАШ). Более высокие показатели указывали на большее количество и тяжесть симптомов [20].
Гинекологический осмотр с определением pH влагалища. рН-метрию содержимого заднего свода влагалища проводили с помощью индикаторных полосок Кольпо-тест рН («Биосенсор АН», Россия) путем сопоставления окраски сенсорного элемента тест-полосок с соответствующими цветовыми полями шкалы. Цветовая шкала на этикетке содержит 12 цветовых полей, соответствующих значениям pH в ед.: 3,0; 3,5; 3,7; 4,0; 4,2; 4,5; 4,8; 5,0; 5,5; 6,0; 6,5 и 7,0.
Определение индекса вагинального здоровья (ИВЗ) по G. Bachmann. Пять показателей ИВЗ оценивались по 5-балльной шкале: количество и качество отделяемого влагалища, pH, увлажненность, эластичность и толщина влагалищного эпителия. Результаты оценивались следующим образом: 20–25 баллов – норма, 15–20 баллов – незначительные атрофические изменения, менее 15 баллов – ВВА, менее 10 баллов – выраженные атрофические изменения (табл. 2) [21].
Оценка микробиоценоза влагалища. Для оценки микробиоценоза влагалища была использована тест-система «Фемофлор-16», основанная на методе полимеразной цепной реакции (ПЦР) с детекцией результатов в режиме реального времени. В основу данного способа положена комплексная количественная оценка микрофлоры влагалища с проведением сравнительного анализа конкретных представителей нормо- и условно-патогенной микрофлоры с общим количеством микроорганизмов для выявления дисбаланса микробиоты и степени его выраженности.
Дневники нежелательных явлений (НЯ). Дневники НЯ выдавались на каждом визите. Пациентки указывали симптомы, время их возникновения, длительность и тяжесть.
Статистический анализ
В процессе статистической обработки данных использовались:
- для обработки и визуализации данных – табличный процессор Microsoft Excel 2015;
- для построения регрессионных моделей влияния индивидуальных характеристик пациентов на динамику целевых показателей – программный пакет для эконометрического анализа gretl;
- для обработки данных, тестирования значимости изменения целевых показателей и визуализации данных – язык программирования Python (библиотеки pandas, scipy, matplotlib, seaborn).
Построение регрессионных моделей в gretl проводилось на основе метода наименьших квадратов. Выводы о значимости сделаны на основе теста Стьюдента. В качестве уровня значимости было принято значение 5%.
Результаты
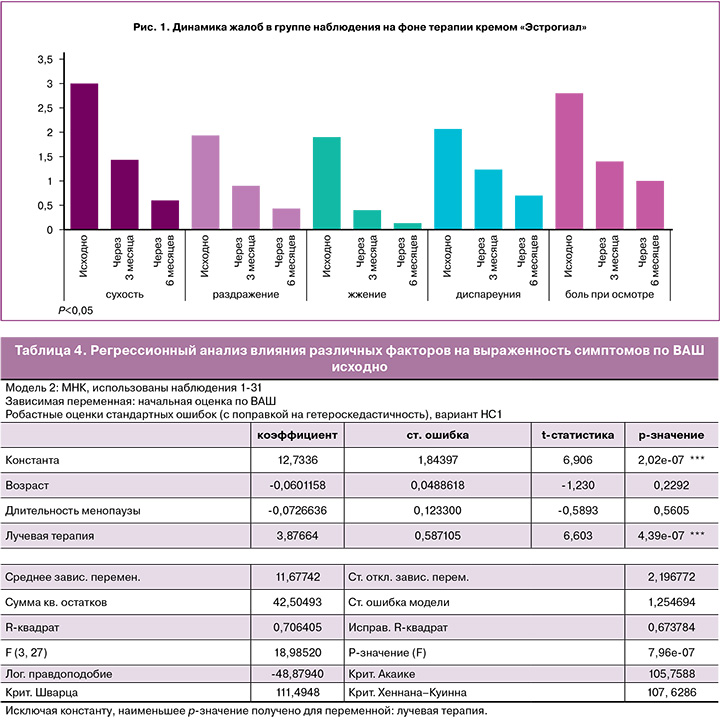
Все 30 участниц, включенных в исследование, завершили 6-месячный курс лечения. Контроль эффективности и безопасности терапии вагинальным кремом «Эстрогиал» проводился через 12 и 24 недели после визита скрининга (табл. 3).
Средний возраст пациенток, принявших участие в исследовании, составил 46±7 лет, средний возраст менопаузы – 4,0±2,7 года (от 1 года до 14 лет). 16/30 (53,3%) женщин были замужем и имели регулярную половую жизнь, 6/30 (20%) женщин отмечали наличие нерегулярных сексуальных контактов, и 8/30 (26,7%) пациенток не имели половых партнеров.
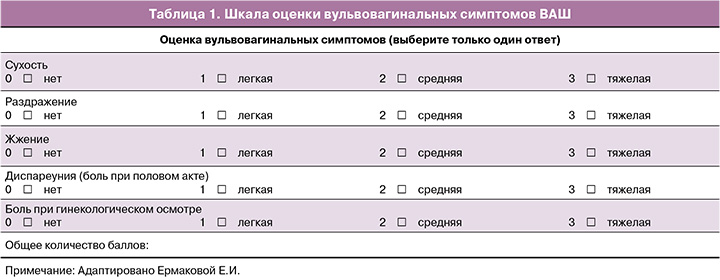
Согласно анкетированию, ведущей жалобой пациенток на визите скрининга являлась сухость во влагалище; все 30 женщин (100%) оценили исходно этот симптом максимально в 3 балла. К концу периода наблюдения 14/30 (46,7%) участниц не сообщали о наличии сухости во влагалище, остальные 16/30 (53,3%) пациенток отмечали заметное уменьшение симптома и оценивали его в 1 балл. 22/30 (73,3%) сексуально активные женщины отмечали боли при половом акте и периодические контактные кровянистые выделения. По данным ВАШ, 15 участниц из 22 оценивали исходно диспареунию в 3 балла, остальные 7 – в 2 балла. Через 6 месяцев локальной терапии только 2 пациентки оценивали данный симптом в 2 балла, 11 женщин отметили отсутствие болей при половом акте, остальные 9 указывали на значительное уменьшение болевого фактора и оценивали его по ВАШ в 1 балл. Кроме того, 24/30 (80%) пациентки жаловались на жжение во влагалище и оценивали данный симптом исходно в 1–3 балла; 17/30 (56,7%) женщин отмечали раздражение в области вульвы и влагалища (оценка от 1 до 3 баллов) и 25/30 (83,3%) участниц указывали на боль при гинекологическом осмотре и предупреждали об этом врача. На фоне проводимой терапии все пациентки отмечали положительную динамику от выраженного уменьшения до полного отсутствия данных симптомов. Динамика жалоб по ВАШ в течение периода наблюдения представлена на рисунке 1.
Динамика средних показателей ВАШ свидетельствует о статистически значимом уменьшении субъективных симптомов: исходно – 11,7, через 3 месяца – 5,3, через 6 месяцев – 2,8; р<0,001 (рис. 2).
Регрессионный анализ влияния таких факторов, как возраст, длительность менопаузы, диагноз, варианты лечения онкозаболевания (лучевая, химиотерапия, гормонотерапия), на выраженность субъективных симптомов по ВАШ показал, что при прочих равных условиях у женщин, перенесших лучевую терапию, изначальная оценка ВАШ в среднем выше на 3,9. То есть, несмотря на возраст и длительность менопаузы, у пациенток, подвергшихся лучевой терапии, симптомы ВВА были более выраженными, чем у женщин без нее (табл. 4).
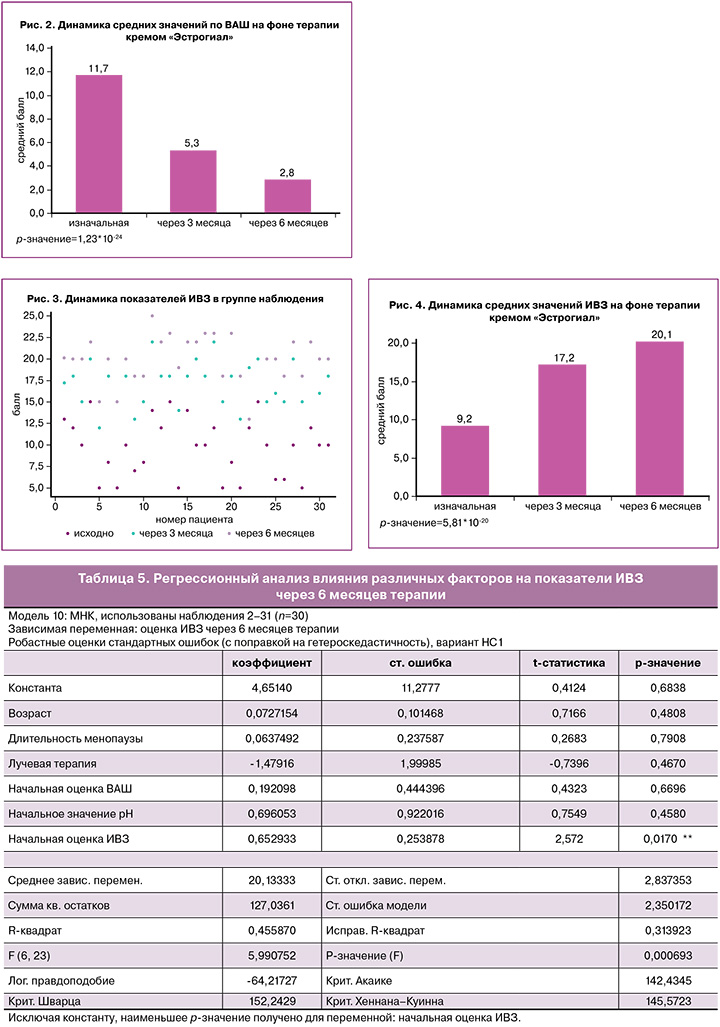
Объективные симптомы и признаки ГУМС/ВВА оценивались на всех визитах, и определялось значение ИВЗ по G. Bachmann. Исходно показатели ИВЗ находились в диапазоне от 5 до 15 баллов; через 6 месяцев терапии кремом «Эстрогиал» показатели ИВЗ статистически значимо повысились до 15–25 баллов, что свидетельствует об улучшении состояния слизистой оболочки вульвы и влагалища, ее эластичности и увлажненности. Динамика параметров ИВЗ по каждой пациентке представлена на рисунке 3.
Сравнение средних значений ИВЗ через 3 и 6 месяцев лечения с исходным уровнем показало статистически значимое изменение: исходно – 9,2, через 3 месяца – 17,2, через 6 месяцев – 20,1, р<0,001, что, безусловно, свидетельствует об эффективности проводимой терапии (рис. 4).
Одним из объективных и достоверных признаков ВВА является повышение pH содержимого заднего свода влагалища. Исходно значения pH у пациенток исследуемой группы находились в диапазоне от 4,8 до 7,0; через 6 месяцев терапии показатели pH снизились до 4,2–6,0. Высокий pH более 5,0 исходно определялся у 23/30 (76,7%) пациенток, к концу периода наблюдения – у 10/30 (33,3%) пациенток. Динамика значений pH по каждой пациентке представлена на рисунке 5.
Сравнение средних значений pH демонстрирует статистически значимое снижение на фоне лечения: исходно – 6,1, через 3 месяца – 5,4, через 6 месяцев – 5,1; р<0,001 (рис. 6).
C помощью метода наименьших квадратов (МНК) построена регрессионная модель, определяющая влияние таких факторов, как возраст пациента, длительность менопаузы, предшествующая терапия (лучевая, химиотерапия), гормонотерапия, на объективные симптомы ВВА и ИВЗ на фоне проводимой терапии (табл. 5).
Анализ данных показал, что на значения ИВЗ через 6 месяцев терапии статистически значимо влияет только исходная оценка ИВЗ. В связи с вышеизложенным, можно сделать вывод, что терапия негормональным кремом «Эстрогиал» позитивно влияет на параметры ИВЗ, в том числе на pH влагалища вне зависимости от других факторов и индивидуальных характеристик пациенток.
Оценка микробиоценоза влагалища методом ПЦР проводилась на визите скрининга и через 6 месяцев терапии. До начала лечения у 11/30 (36,7%) пациенток Lactobacillus spp. отсутствовали (в абсолютных значениях -1000 копий/образец), у остальных женщин титр Lactobacillus spp. был снижен и колебался от 102,3 до 105,5 копий/образец. К концу периода наблюдения у всех 30/30 (100%) участниц исследования определялась лактофлора в абсолютных значениях от 103,4 до 107,2 копий/образец.
Перед началом лечения микрофлора у большинства пациенток (n=19) была представлена в основном облигатно-анаэробными микроорганизмами (Gardnerella vaginalis/Prevotella bivia/Porphyromonas spp., Eubacterium spp., Megasphaera spp./Veillonella spp./Dialister spp., Lachnobacterium spp./Clostridium, Mobiluncus spp./Corynebacterium, Peptostreptococcus spp.), реже преобладали факультативно-анаэробные микроорганизмы (семейство Enterobacteriaceae; Staphylococcus spp.; Streptococcus spp.). У 5/30 (16,7%) женщин микрофлора влагалища была представлена единичными Lactobacillus spp. и условно-патогенными микроорганизмами, при этом общая бактериальная масса (ОБМ) составляла менее 3–5 копий/образец.
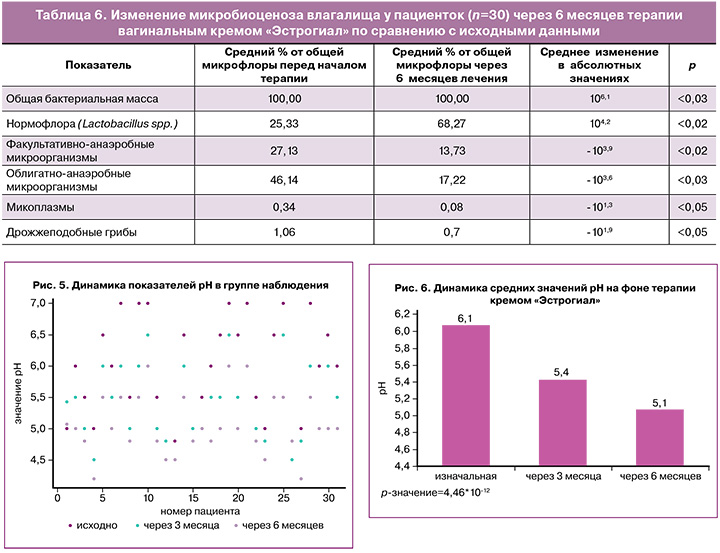
Для объективного восприятия данных был рассчитан средний процент Lactobacillus spp. и представителей условно-патогенной микрофлоры от ОБМ исходно и через 6 месяцев терапии, а также определены средние изменения полученных значений (табл. 6).
Согласно данным таблицы 6, к концу периода наблюдения отмечено статистически значимое увеличение количества Lactobacillus spp. (исходно средний процент от ОБМ – 25,33, через 6 месяцев терапии – 68,27, среднее изменение – 104,2; р<0,02), а также, статистически значимое снижение факультативно-анаэробной (исходно средний процент от ОБМ – 27,13%, через 6 месяцев терапии – 13,73%, среднее изменение – -103,9, р<0,02) и облигатно-анаэробной микрофлоры (исходно средний процент от ОБМ – 46,14%, через 6 месяцев терапии – 17,22%, среднее изменение – -103,6, р<0,03).
Через 3 и 6 месяцев терапии проводилась оценка нежелательных явлений. У 5/30 (16,7%) пациенток отмечалось жжение во влагалище легкой или умеренной степени после введения крема в течение первых 3–4 недель терапии, затем данный симптом проходил без применения дополнительных лечебных средств. У одной пациентки 48 лет с раком молочной железы в анамнезе, получающей тамоксифен в течение 3 лет, отмечался быстрый рост эндометрия на 6–8-й неделе терапии с 6 мм до 12 мм. Ей проведены гистероскопия и раздельное диагностическое выскабливание. По результату гистологии: железисто-фиброзный полип эндометрия без атипии. Данное нежелательное явление расценено как не связанное с локальной терапией кремом «Эстрогиал», а, вероятно, связанное с приемом адъювантной гормонотерапии тамоксифеном. Пациентка продолжила участие в исследовании, и при дальнейшем использовании крема «Эстрогиал» роста эндометрия по данным ультразвукового исследования не было отмечено.
Выводы
Проведенное нами исследование позволило сделать выводы, что локальная терапия ГУМС/ВВА в течение 6 месяцев негормональным дозированным кремом «Эстрогиал» у пациенток с раком эндометрия и раком молочной железы в анамнезе приводит:
- к редукции субъективных симптомов (сухости, диспареунии, жжения и т.д.) более чем в 4 раза, по сравнению с исходными данными (оценка ВАШ исходно – 11,7, через 6 месяцев – 2,8, р<0,001);
- достоверному улучшению объективных симптомов и повышению ИВЗ более чем в 2 раза по сравнению с исходными значениями (ИВЗ исходно – 9,2, через 6 месяцев – 20,1; р<0,001);
- статистически значимому снижению pH на фоне лечения: исходно – 6,1, через 6 месяцев – 5,1, р<0,001;
- повышению количества лактофлоры во влагалищном биотопе более чем в 2,5 раза (исходно средний процент от ОБМ – 25,33, через 6 месяцев терапии – 68,27) и снижению более чем в 2 раза факультативно-анаэробной (исходно средний процент от ОБМ – 27,13%, через 6 месяцев терапии – 13,73%) и облигатно-анаэробной микрофлоры (исходно средний процент от ОБМ – 46,14%, через 6 месяцев терапии – 17,22%).
Полученные результаты свидетельствуют о высокой эффективности крема «Эстрогиал» даже у такой сложной категории пациентов с ЗНО органов репродуктивной системы.
Заключение
Реабилитация пациентов с гормонзависимыми онкологическими заболеваниями репродуктивной системы – одна из основных задач российского здравоохранения. Радикальные оперативные вмешательства на органах малого таза, лучевая и химиотерапия, длительная гормонотерапия у женщин с раком молочной железы в анамнезе приводят к развитию тяжелых климактерических расстройств. Симптомы ГУМС/ВВА значимо снижают качество жизни, особенно у молодых сексуально активных женщин. Учитывая, что локальные эстрогены противопоказаны данной категории пациентов, вагинальные лубриканты в этом случае могут служить эффективной альтернативой.
Проведенное нами исследование показало, что комбинированный крем дозированный «Эстрогиал» с ГК и фитокомплексом эффективно редуцирует симптомы ВВА, улучшает состояние слизистой влагалища, повышает ее эластичность, транссудацию, достоверно снижает рН влагалища и способствует росту нормальной микрофлоры. Крем «Эстрогиал» обладает хорошей переносимостью и имеет безопасный состав по критериям ВОЗ, что обеспечивает возможность его длительного применения.



