Relationship between the umbilical cord blood acid-base status and neonatal hypoxic-ischemic encephalopathy
Aim. To investigate the relationship between umbilical cord blood acid-base balance (ABB) and the development of neonatal hypoxic-ischemic encephalopathy (HIE). Materials and methods. A retrospective case-control study included 180 women and their newborns. The levels of pH, lactate (Lac), base deficit (BD), partial oxygen pressure (pO2), and partial carbon dioxide pressure (pCO2) in umbilical cord arterial blood were determined using an ABL800 FLEX gas analyzer (Radiometer Medical ApS, Denmark). Results. Neonates with HIE had significantly lower pH [6.966 (0.309) versus 7.269 (0.074), p <0.001] and higher BD and Lac levels than neonates in the control group [18.1 (6.8) versus 7.9 (4.1), p <0.001 and 12.0 (5.1) versus 5.8 (2.7) mmol/L, p <0.001], respectively; pCO2 was significantly higher in newborns with HIE [59.7 (32.3) versus 41.7 (8.8), p=0.049], while pO2 was higher in the control group [28.1 (28.3) versus 21.3 (11.0), p <0.001]. The arterial umbilical cord blood pH was significantly lower, and BD was higher in newborns with grade II–II HIE than in those with grade I HIE [6.861 (0.309) versus 7.033 (0.163), p=0.04 and 21.7 (6.7) versus 16.2 (6.1)], p=0.02. Lac, pO2, and pCO2 levels were not associated with HIE severity. The predictors of moderate and severe HIE of hypoxic origin were pH <7.03 and BD> 19.2. The severity of HIE was associated with the incidence of adverse long-term outcomes. In 10 HIE cases (27.7%), ABB parameters at birth did not confirm the presence of metabolic acidosis. Conclusion. Determination of the pH and BD in umbilical cord arterial blood allows prediction of the development and severity of HIE. The proposed model for predicting moderate and severe HIE has a sensitivity and specificity of 94.7% and 90.9%, respectively. In 27.7% of cases, HIE was diagnosed in neonates with normal pH and BD in umbilical cord arterial blood, suggesting that there are causes of encephalopathy other than intrapartum fetal hypoxia.Prikhod'ko A.M., Romanov A.Yu., Tysyachnyi O.V., Baev O.R.
Keywords
Hypoxic-ischemic encephalopathy (HIE) remains the leading cause of neonatal neurological morbidity in term infants [1, 2]. The most common cause of birth asphyxia is intrapartum fetal hypoxia. Evaluation of blood gas parameters and acid-base balance (ABB) of umbilical cord blood allows an objective assessment of the neonatal condition and is a valuable tool to guide neonatal management and predict the prognosis [3, 4]. Normal intrapartum pH and base deficit (BD) indicate the absence of fetal hypoxemia, which rules out brain damage due to hypoxia, while low pH values and high BD indicate oxygen deficiency leading to acidosis. At the same time, according to the literature, there are significant differences in ABB parameters, indicating fetal hypoxia, which makes it difficult to predict the outcome and choose the treatment [5].
Given all of the above, this study aimed to investigate the predictive significance of umbilical cord blood ABB and its relationship with neonatal HIE development.
Materials and methods
This retrospective case-control study was conducted at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. The study included 180 female patients and their newborn infants. The study group comprised 36 patients and their children with HIE. The diagnosis of HIE was established postnatally based on a pediatric neurologist consultation, clinical and laboratory findings suggesting acute brain damage associated with difficulty initiating or maintaining respiration, abnormal tone, and reflexes, or seizures.
The severity of HIE was determined using a modified Sarnat N., Sarnat M. (1976) scale modified by Stoll B., Kliegman R. (2004). Long-term HIE outcomes were analyzed in 26 newborns. The diagnostic criterion for inclusion in the control group was umbilical cord arterial blood pH above 7.12 and BD below 12.4 mmol/L, which was defined as the absence of acidosis based on results of an earlier study evaluating the ABB parameters of umbilical cord arterial blood [4], but with no HIE (n=144). The control group of newborns was selected in a ratio of 4 to 1 for the patients of the study group, taking into account gestational age and the patients' age. The Research Ethics Committee of the V.I. Kulakov NMRC for OG&P approved this study.
Blood samples were taken by isolating the umbilical cord segment with three clamps. The cord was then cut between the 1st and 2nd clamps, and blood was drawn from the umbilical artery between the 2nd and 3rd clamps. The pH, lactate (Lac), base deficit (BE), oxygen partial pressure (pO2), and carbon dioxide (pCO2) levels were measured using an ABL800 FLEX blood gas analyzer (Radiometer Medical ApS, Denmark) no later than 10 minutes after sampling of the biomaterial, as described earlier [4, 6].
Statistical analysis
Statistical analysis and plotting were performed using GraphPad Prism (GraphPad Software, USA). Continuous variables were compared with the Student’s t-test (equality of variance was assessed by Levene's test) and the Mann–Whitney test for nonparametric data. Categorical variables were compared by the Chi-square test. The distribution of the data was assessed using the D’Agostino–Pearson normality test. Differences in the mean values between the three groups were analyzed using analysis of variance (ANOVA) with Tukey's test for multiple comparisons. Kruskal–Wallis test was used for comparing numerical data between three groups, followed by Dunn's post-test for multiple comparisons. Parametric data are presented as mean and standard deviation; nonparametric data are presented as median and interquartile range; categorical data are presented as counts and percentages. Differences were considered statistically significant at p<0.05. Logistic regression analysis is used to examine the association of independent variables with one binary dependent variable.
Using this method makes it possible to predict the probability of an event occurring for a specific event using the formula: Р = 1/(1 + e-z) where Р is the probability of the event, e is the base of the natural logarithm equal to 2.718281828, z is the linear regression equation. The dependent variable was HIE. PH and BD were independent variables (predictors).
Results
The HIE and control groups' patients were comparable in the age that averaged 30.1 (5.7) years. The body mass index of the patients was also similar and amounted to 27.0 (4.9) kg/m2 in the HIE group and 26.9 (3.4) kg/m2 in the control group (p=0.89). Four (11.1%) patients in the HIE group and 6 (4.2%) patients in the control group had a history of childbirth (p=0.12). The remaining patients included in the study were primiparous.
All study participants had a full-term delivery. The gestational age did not differ in the groups. Gestational age at delivery was 39.3 (1.12) and 39.6 (1.12) weeks in the study and control groups, respectively (p=0.09).
One (2.8%) patient in the study group and three (2.1%) patients in the control group had cesarean delivery before the onset of labor (p=0.59). Of the patients who entered labor, pre-induction of labor was used in 10 (31.6%) and 48 (33.3%) patients of the study and the control group, respectively (p=0.53).
The overall operative delivery rates differed between the groups (p=0.0012) and were 66.7% and 40% in the study and the control group, respectively. Vacuum delivery was more common in the study group and was 39.0% versus 22.9% in the control group (p=0.04).
The birth weight of newborns did not differ between groups. In the HIE group, it was 3279 (352) g versus 3350 (428) g in the control group (p=0.43). The body length of newborns also did not differ and was 51.3 (1.8) cm in the HIE group and 51.6 (2.3) cm in the control group (p=0.59).
Newborns in the study group had significantly lower first- and fifth-minute Apgar scores [3 (2–4.25) and 6 (4–6.25) versus 7 (6–8) points and 8 (8–9)], respectively (p <0.001 for both comparisons).
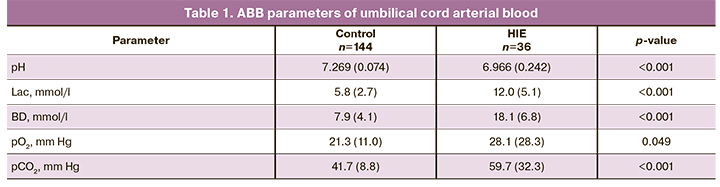
Umbilical cord arterial blood pH was significantly lower in newborns with HIE (p <0.001), while the levels of Lac and BD were substantially higher (p <0.001) than in the control group (Table 1). Umbilical cord arterial pCO2 was significantly higher in newborns with HIE (59.7 (32.3) versus 41.7 (8.8) mm Hg, p=0.049), while pO2 was higher in the control group (28.1 (28.3) versus 21.3 (11.0) mm Hg, p <0.001).
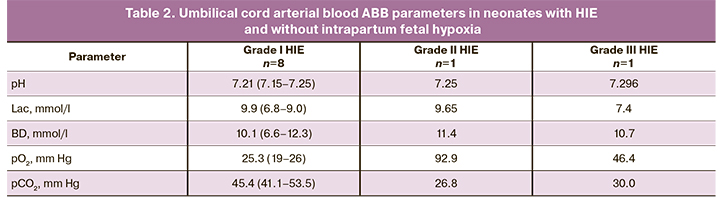
Among 36 children in the study group, 22 (61.1%), 8 (22.2%), and 6 (16.7%) newborns had grade I, II, and III HIE, respectively. It should be noted that eight newborns with grade I HIE, one with grade II, and one with grade III had umbilical cord arterial blood ABB parameters within the normal range, suggesting that their brain damage was not caused by intrapartum fetal hypoxia (Table 2).
HIE severity was not associated with patients' age, body mass index, parity, gestational age at delivery, and delivery model.
One (4.5%) patient with grade I HIE was delivered by cesarean section before labor. Six (27.3%), one (12.5%), and two (33.3%) patients with grade I, II, and III HIE underwent emergency intrapartum cesarean section initiated during labor (p = 0, 50). Five (22.7%), 4 (50%) and 3 (50%) patients had vacuum delivery (p = 0.06), respectively. Eleven (33%) infants with HIE had spontaneous delivery.
Birth weight and body length of neonates did not differ between subgroups. The birth weight of newborns was 3156 (345.6) g, 3341 (353.3) g, and 3379 (346.8) g in grade I, II, and III HIE subgroups, respectively (p=0.19). The body length of newborns was 51.6 (2.1), 51.1 (1.7) and 51.4 (1.7) cm, respectively (p=0.86).
Apgar scores were inversely proportional to the severity of HIE. First-minute Apgar scores were 4.5 (3–5), 3 (2–3.25), and 1 (1–3.25) (p=0.001); fifth-minute Apgar scores were 6.5 (5.75–7), 5 (4–6), and 3 (1–5.25) in neonates with grade I, II and III HIE subgroups, respectively (p <0.001).
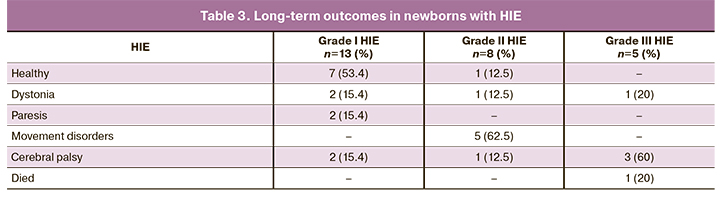
Long-term outcomes in 26 (72.2%) out of 36 children with HIE were also analyzed. Of these, 13, 8, and 5 had grades I, II, and III HIE (Table 3). No clinically measurable consequences at age three years were observed in 53.4% of children with grade I HIE. Two children of this subgroup developed cerebral palsy (15.4%); the rest had mild or moderate dystonia and paresis. Conversely, infants with grade II and III HIE were more likely to have pronounced manifestations of dystonia, paresis, and movement disorders. Only one child with grade II HIE was healthy at the age of 3. One child with grade III HIE died in the perinatal period. Considering that the consequences of HIE II and III degrees were more severe and similar in severity, these two groups were combined for outcome analysis. Since in 10 cases, according to ABB, there was no acidosis in labor, their outcomes were analyzed separately. Out of 10 infants, in 2 (20%), the outcomes were unknown, 5 (50%) were healthy, and one (10%) had cerebral palsy; in other cases, 3 (30%) had mild dystonia.
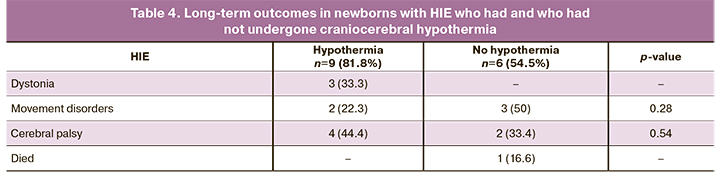
Eleven (50%) neonates underwent craniocerebral hypothermia due to severe asphyxia. Fifteen out of 22 (68.2%) children were available for assessing long-term outcomes (Table 4); no significant differences were found between the groups. Umbilical cord artery blood pH was significantly lower in grade II–III HIE [6.861 (0.309)] than in grade I HIE [7.033 (0.163)], p=0.04 (Table 5). The BD level was higher in grade II–III HIE [21.7 (6.7) mmol/L] than in grade I HIE [16.2 (6.1) mmol/L], p=0.02. Lac, pCO2, pO2 levels were not associated with HIE severity.
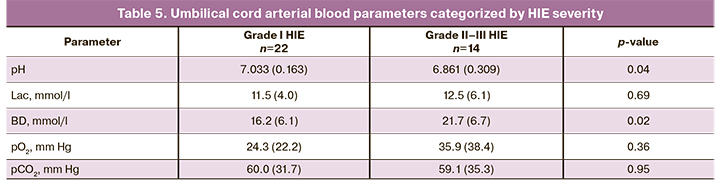
Significant differences were found in umbilical cord blood pH, BD, and pCO2 levels in neonates with HIE depending on the presence of acidosis during labor (Table 6).
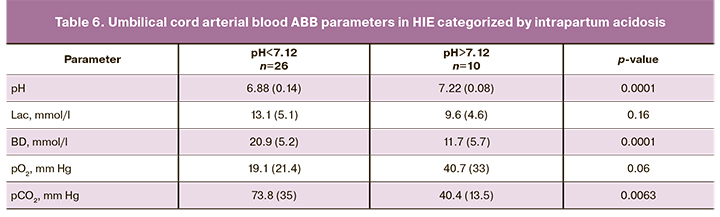
Considering the association between HIE severity and the levels of pH and BD, the predictive value of these data was studied.
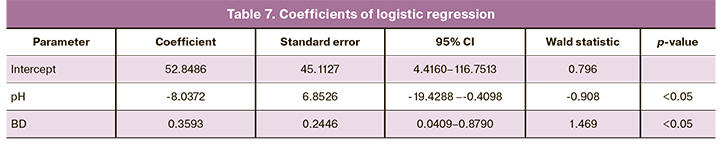
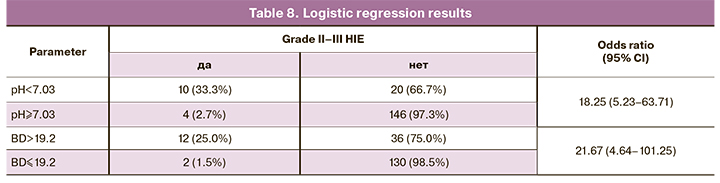
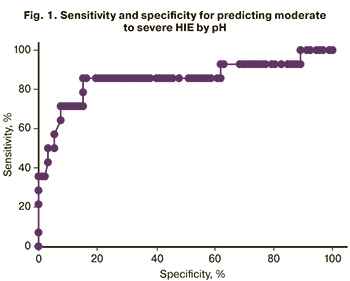 The analysis showed that a cutoff pH value for predicting moderate and severe HIE was <7.03 (Fig. 1, Tables 7, 8). The area under the ROC curve was 85.2%, the sensitivity and specificity of the model were 71.4% and 88.0%, respectively.
The analysis showed that a cutoff pH value for predicting moderate and severe HIE was <7.03 (Fig. 1, Tables 7, 8). The area under the ROC curve was 85.2%, the sensitivity and specificity of the model were 71.4% and 88.0%, respectively.
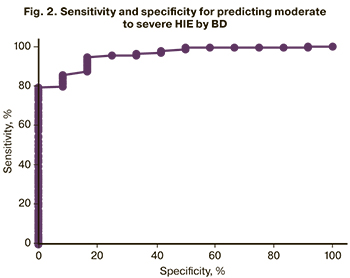
The analysis also showed that the BD > 19.2 mmol/L allows predicting moderate and severe HIE (Fig. 2, Tables 7, 8). The area under the ROC curve was 89.4%, the sensitivity and the specificity of the model were 85.7% and 78.3%, respectively.
These results showed that the logistic regression predicts the probability of developing moderate and severe HIE based on the combination of the identified factors using the following formula:
Р = 52.8486 – 8.0372×pH + 0.3593×|BD|
- With a P value between 0 and 1.9, the likelihood of HIE is high.
- With a P value between 0 and -1.9, the probability of HIE is low.
- If P is greater than 2, the likelihood of HIE is extremely high.
- With a P value of less than -2, the probability of HIE is extremely low.
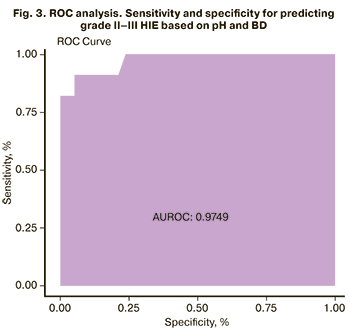
 We also performed a ROC analysis for this model. The area under the curve was 97.5%, the sensitivity and specificity were 94.7% and 90.9%, respectively (Fig. 3).
We also performed a ROC analysis for this model. The area under the curve was 97.5%, the sensitivity and specificity were 94.7% and 90.9%, respectively (Fig. 3).
Discussion
Umbilical cord blood ABB is used to assess intrapartum fetal acidosis [7]. In this study, we analyzed umbilical cord arterial blood ABB parameters to identify values associated with HIE development in newborns after childbirth. The Apgar score was, as expected, lower in babies with HIE than without, which confirms the role of asphyxia in the development of HIE [8].
Many recent studies have focused on searching for markers of HIE development and predictors of its severity [7–10].
 According to a systematic review by Graham E.M. et al. (2008), for non-anomalous term infants, the incidence of an umbilical arterial pH<7.0 at birth is 0.37%, of which 17.2% survived with neonatal neurologic morbidity, 16.3% had seizures, and 5.9% died during the neonatal period. HIE incidence for term infants born with cord pH< 7.0 was 23.1% 7.0 was 2.5 per 1000 live births. The proportion of cerebral palsy associated with HIE was 14.5%. However, the vast majority of cerebral palsy cases in full-term infants were not related to intrapartum hypoxia-ischemia. [11].
According to a systematic review by Graham E.M. et al. (2008), for non-anomalous term infants, the incidence of an umbilical arterial pH<7.0 at birth is 0.37%, of which 17.2% survived with neonatal neurologic morbidity, 16.3% had seizures, and 5.9% died during the neonatal period. HIE incidence for term infants born with cord pH< 7.0 was 23.1% 7.0 was 2.5 per 1000 live births. The proportion of cerebral palsy associated with HIE was 14.5%. However, the vast majority of cerebral palsy cases in full-term infants were not related to intrapartum hypoxia-ischemia. [11].
Our study found a relationship between the development of HIE and changes in all umbilical cord arterial blood ABB parameters (pH, Lac, BD, pO2, pCO2). However, only pH and BD were associated with HIE severity. Thus, pH and BD are the most significant umbilical cord arterial blood ABB predictive markers for the development and severity of HIE, which is consistent with current literature [12].
Of particular note are observations of HIE in babies who had normal intrapartum umbilical cord pH and BD. According to Yatham S.S. et al. (2019), brain damage causes may be non-hypoxic (inflammatory and metabolic). In such cases, cardiotocography, Apgar score, and umbilical cord blood ABB are not predictive for the state of the newborn [13]. Besides, M.S. Panova and Panchenko A.S. (2017) identified a relationship between HIE occurrence, fetoplacental insufficiency, and chronic intrauterine fetal hypoxia. Analysis of maternal somatic and infectious comorbidities showed that the urinary system's diseases and acute respiratory viral infection during pregnancy turned out to be statistically significant. More than half of babies with HIE were delivered by cesarean section. Every 4th child with HIE has a concomitant pathology of the respiratory system [14].
The initial signs of fetal decompensation are associated with the centralization of blood circulation by peripheral vasospasm and the switch from aerobic cellular respiration to anaerobic glycolysis with the development of metabolic acidosis. With continued fetal hypoxia and depletion of compensatory mechanisms against progressing acidosis's backdrop, NO synthase activation leads to peripheral vasodilation, resulting in a decrease in cerebral blood flow and then hypoxic-ischemic brain damage [15]. However, in subacute hypoxia, due to intermittent compression of the umbilical cord vessels, a decrease in fetal blood pressure and damage to brain structures occurs without significant changes in umbilical cord blood ABB [13].
In addition to hypoxia, metabolic disorders play a significant role in the development of HIE. This is seen in the ABB analysis of the umbilical cord blood of newborns. A meta-analysis by G.L. Malin (2010) demonstrated that low umbilical cord arterial blood pH was associated with HIE and cerebral palsy [16].
In this study, we showed that HIE is associated with low pH and other abnormalities of umbilical cord arterial blood ABB, including increased levels of Lac, BD, pCO2, and pO2. Of these indicators, only the pH and BD were associated with HIE severity, which suggests the predominance of the metabolic component in severe HIE development. The use of craniocerebral hypothermia was not associated with the long-term HIE outcomes due to the small sample size. Further studies are needed to clarify the interpretation of these results.
Conclusion
This study analyzed characteristics of umbilical cord arterial blood ABB and their association with the development of HIE. The relationship between brain damage in newborns and metabolic acidosis and hypoxia was confirmed. Predictors of moderate and severe HIE of hypoxic origin are pH <7.03 and BD> 19.2 mmol/L. Based on our findings, the likelihood of developing moderate to severe HIE in newborns may be determined using binary logistic regression with pH and BD as predictors. Cerebral palsy developed in 60% of children with grade III HIE. Nevertheless, in 27.7% of cases, HIE was diagnosed in neonates with normal umbilical cord arterial blood pH and BD, suggesting that there are causes of encephalopathy other than intrapartum fetal hypoxia.
References
- Martin R.J., Avroy A.F., Walsh C.M. Fanaroff and Martin's neonatal-perinatal medicine: diseases of the fetus and infant. Saunders; 2014. 1936 p.
- Chiang M.C., Jong Y.J., Lin C.H. Therapeutic hypothermia for neonates with hypoxic ischemic encephalopathy. Pediatr. Neonatol. 2017; 58(6): 475-83. https://dx.doi.org/10.1016/j.pedneo.2016.11.001.
- Hankins G.D., Speer M. Defining the pathogenesis and pathophysiology of neonatal encephalopathy and cerebral palsy. Obstet. Gynecol. 2003; 102(3): 628-36. https://dx.doi.org/10.1016/s0029-7844(03)00574-x.
- Приходько А.М., Романов А.Ю., Шуклина Д.А., Баев О.Р. Показатели кислотно-основного равновесия и газовый состав артериальной и венозной пуповинной крови в норме и при гипоксии плода. Акушерство и гинекология. 2019; 2: 93-7. [Prikhodko A.M., Romanov A.Yu., Shuklina D.A., Baev O.R. The indicators of acid-base balance and the gas composition of umbilical cord arterial and venous blood in health and fetal hypoxia. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; 2: 93-7. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.2.93-97.
- Strijbis E.M.M., Oudman I., van Essen P., MacLennan A.H. Cerebral palsy and the application of the international criteria for acute intrapartum hypoxia. Obstet. Gynecol. 2006; 107(6): 1357-65. https://dx.doi.org/10.1097/01.AOG.0000220544.21316.80.
- Приходько А.М., Баев О.Р. Определение кислотно-основного состояния пуповинной крови. Показания и техника. Акушерство и гинекология. 2018; 5: 127-31. [Prikhodko A.M., Baev O.R. Determination of umbilical cord blood acid-base status. Indications and techniques. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; 5: 127-31. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.5.127-131.
- Chalak L.F., Sánchez P.J., Adams-Huet B., Laptook A.R., Heyne R.J., Rosenfeld C.R. Biomarkers for severity of neonatal hypoxic-ischemic encephalopathy and outcomes in newborns receiving hypothermia therapy. J. Pediatr. 2014; 164(3): 468-74. e1. https://dx.doi.org/10.1016/j.jpeds.2013.10.067.
- Douglas-Escobar M., Weiss M.D. Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr. 2015; 169(4): 397-403. https://dx.doi.org/10.1001/jamapediatrics.2014.3269.
- Блинов Д.В. Диагностическое значение ЭЭГ и биохимических маркеров повреждения мозга при гипоксически-ишемической энцефалопатии. Эпилепсия и пароксизмальные состояния. 2016; 8(4): 91-8. [Blinov D.V. Diagnostic value of EEG and biochemical markers of brain damage in hypoxic-ischemic encephalopathy. Epilepsy and paroxysmal conditions. 2016; 8(4): 91-8. (in Russian)]. https://dx.doi.org/10.17749/2077-8333.2016.8.4.091-098.
- Приходько А.М., Киртбая А.Р., Романов А.Ю., Баев О.Р. Биомаркеры повреждения головного мозга у новорожденных. Неонатология. 2018; 7(1): 70-6. [Prikhod'ko A.M., Kirtbaya Anna R., Romanov A.Yu., Baev O.R. Biomarkers of brain damage in newborns. Neonatology: News, Opinions, Training. 2018; 7 (1): 70-6. (in Russian)]. https://dx.doi.org/10.24411/2308-2402-2018-00009.
- Graham E.M., Ruis K.A., Hartman A.L., Northington F.J., Fox H.E. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am. J. Obstet. Gynecol. 2008; 199(6): 587-95. https://dx.doi.org/10.1016/j.ajog.2008.06.094.
- Daboval T., Ouellet P., Charles F., Booth R.A., MacLean G., Roeper R. et al. Comparisons between umbilical cord biomarkers for newborn hypoxic-ischemic encephalopathy. J. Matern. Fetal Neonatal Med. 2019; Nov 25: 1-14. https://dx.doi.org/10.1080/14767058.2019.1688292.
- Yatham S.S., Whelehan V., Archer A., Chandraharan E. Types of intrapartum hypoxia on the cardiotocograph (CTG): do they have any relationship with the type of brain injury in the MRI scan in term babies? J. Obstet. Gynaecol. 2020; 40(5): 688-93. https://dx.doi.org/10.1080/01443615.2019.1652576.
- Панова М.С., Панченко А.С. Факторы риска гипоксически-ишемической энцефалопатии у доношенных новорожденных детей. Забайкальский медицинский вестник. 2017; 4: 84-9. [Panova M.S., Panchenko A.S. Risk factors for hypoxic-ischemic encephalopathy in full-term newborns. Transbaikal Medical Bulletin. 2017; 4: 84-9 (in Russian)]. https://www.elibrary.ru/download/elibrary_32269197_23010232.pdf
- Yıldız E.P., Ekici B., Tatlı B. Neonatal hypoxic ischemic encephalopathy: an update on disease pathogenesis and treatment. Expert Rev. Neurother. 2017; 17(5): 449-59. https://dx.doi.org/10.1080/14737175.2017.1259567.
- Malin G.L., Morris R.K., Khan K.S. Strength of association between umbilical cord pH and perinatal and long term outcomes: systematic review and meta-analysis. BMJ. 2010; 340: c1471. https://dx.doi.org/10.1136/bmj.c1471.
Received 21.10.2020
Accepted 16.02.2021
About the Authors
Andrey M. Prikhod'ko, Ph.D., Physician at the 1st Maternity Department, Teaching Assistant at the Department of Obstetrics and Gynecology, Researcher at the Department of Innovative Technologies, Institute of Obstetrics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(495)438-30-47. E-mail: a_prikhodko@oparina4.ru.4 Oparina str., Moscow, 117997, Russian Federation.
Andrey Yu. Romanov, Ph.D. Student, Specialist at the R&D Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel. +7(903)158-94-00.
E-mail: romanov1553@yandex.ru. 4 Oparina str., Moscow, 117997, Russian Federation.
Oleg V. Tysyachnyi, Ph.D., Researcher at the Department of Innovative Technologies, Institute of Obstetrics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)438-11-88. E-mail: olti23@mail.ru. 4, Oparina str., Moscow, 117997, Russian Federation.
Oleg R. Baev, Dr. Med. Sci., Professor, Head of the 1st Maternity Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia; Professor at the Department
of Obstetrics, Gynecology, Perinatology and Reproductology, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University). Tel.: +7(495)438-11-88.
E-mail: o_baev@oparina4.ru. 4 Oparina str., Moscow, 117997, Russian Federation.
For citation: Prikhod'ko A.M., Romanov A.Yu., Tysyachnyi O.V., Baev O.R. Relationship between the umbilical cord blood acid-base status and neonatal hypoxic-ischemic encephalopathy.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 4: 90-97 (in Russian)
https://dx.doi.org/10.18565/aig.2021.4.90-97



