Study of serum procalcitonin and lactate levels in women with preeclampsia
Objective: Assessment of serum procalcitonin (PCT) and lactate levels and analysis of the prevalence of polymorphic variants of thrombophilia and folate cycle genes in pregnant women with preeclampsia (PE).Semashchenko K.S, Vasilyeva E.V., Molokova N.N., Zhitkova O. Yu., Subbotina T.N.
Materials and methods: The study included 40 pregnant women with PE and 40 women with normal pregnancy in the control group. Along with standard clinical and biochemical tests, in all pregnant women serum PCT and lactate levels were measured, as well as genetic analysis of genes polymorphisms associated with thrombophilia: F2 (rs1799963, G20210A), F5 (rs6025, G1691A) and MTHFR (rs1801133, C677T) was performed.
Results: A significant increase in both standard clinical and biochemical parameters, and PCT and lactate levels was found in pregnant women with PE compared to the control group. The study also showed, that the development of severe PE was not associated with elevated serum PCT and lactate levels. There were no statistically significant differences between the studied groups in the prevalence of the studied polymorphisms in F2, F5 and MTHFR genes. It was found that the level of PCT in the group of women with PE with genotype 1 or 2 of T-allele of MTHFR gene C677T polymorphism (CT and TT genotypes) was lower than in the group of women with PE with CC genotype.
Conclusion: The results of the study showed that PCT and lactate tests in combination with standard clinical and laboratory parameters have clinical value in the early diagnosis of PE. However, the use of PCT and lactate combination as a marker for clarifying the disease severity is limited. The influence of polymorphisms in F2, F5, MTHFR genes on the development of PE in the studied groups was not confirmed.
Keywords
Preeclampsia (PE) is a multisystem disorder which may complicate the second half of pregnancy, and is characterized by arterial hypertension with proteinuria, edema and systemic inflammation [1]. Today, PE is one of the main causes of maternal and perinatal morbidity and mortality, because this disease has no significant diagnostic and prognostic signs at an early stage. The known developmental mechanisms of PE are complex and not fully understood; a large number of molecules are involved in the pathogenesis of the disease; this makes it difficult to determine the main causes of PE and select an effective treatment [2].
Some evidence suggests that activation of inflammation is an important factor in the development of PE [3, 4]. Procalcitonin (PCT) is considered to be one of the potential inflammatory markers of PE. PCT is a precursor of calcitonin, and is involved in calcium homeostasis. Normally, its amount in serum is minimal (<0.05 ng/ml), but during severe inflammation PCT level rapidly increases (up to 2–10 ng/ml). That is why PCT is a generally accepted prognostic and diagnostic marker of inflammation [5]. Recently, it has been found that PCT is associated with pregnancy complications, such as PE, since this condition is characterized by the production of a large number of proinflammatory cytokines, which can provoke an increase in PCT level [4]. Multiple studies showed that pregnant women with PE have higher level of PCT in serum than women with healthy pregnancy; this confirms the inflammatory status of the PE patients [3, 6]. Therefore, the need to assess the level of this indicator together with other markers of the disease is considered [7].
In addition to the activation of inflammation, PE can be caused by chronic placental hypoxia, which leads to endothelial dysfunction, inflammatory response and maternal hypoxia [8]. Under hypoxic conditions, the synthesis of lactate increases, and an increase in its concentration may lead to the development of PE.
Despite the confirmed diagnostic significance of PCT and lactate in PE, these indicators are still not included in the Russian standards for the diagnosis and treatment of PE, which makes the early detection and treatment of this disease more difficult.
Hemostasis disorders in pregnant women can also contribute to development of PE [9–11]. Risk factors for this pregnancy complication include polymorphic variants of genes responsible for development of thrombosis: F2 gene encoding coagulation factor II (prothrombin); F5 gene encoding coagulation factor V (Factor V Leiden); and MTHFR gene encoding methylenetetrahydrofolate reductase protein, responsible for conversion of folic acid into metabolically active forms. Study of these genes is important for treatment, because the mentioned gene polymorphisms lead to hypercoagulation, which, in turn, contributes to a decrease in placental perfusion due to micro clots in the blood vessels of the placenta and the development of placental ischemic disease and PE [12].
The aim of the study was to assess the level of PCT and lactate in serum and to analyze the prevalence of polymorphic variants of thrombophilia and folate pathway genes in pregnant women with PE.
Materials and methods
The study included 40 pregnant women with PE admitted to the intensive care unit of the City Maternity Hospital No. 5. PE was diagnosed according to symptoms and clinical and laboratory examination under the protocols of the Ministry of Health of the Russian Federation on hypertensive conditions during pregnancy [1]. Mean age of the patients in the studied group was 30.45 (3.23) years. The control group included 40 women with healthy pregnancy. The mean age of women in the control group was 30.4 (2.83) years.
Among the examined patients with PE, a mild form of the disease was observed in 13/40 women (32.5%), moderate – in 25/40 (62.5%), and severe – in 2/40 (5%). PE in previous pregnancies was registered in 14 of 40 patients, including 4/14 women (28.6%) with a mild disease and 10/14 (71.4%) with moderate disease.
All pregnant women underwent standard clinical and biochemical examinations: platelet counts in whole blood, measurement of fibrinogen level in plasma, bilirubin level in serum, the activity of ALT, AST, and LDH enzyme in serum, and the content of protein in the urine. Biochemical tests were performed using an automatic analyzer BS˗380 (Mindray, China), hematologic tests by analyzer BC-5800 (Mindray, China), and protein quantitation in urine by photometer “Photometer 5010 V5+” (Germany) using “Belok PGK Novo” reagent kit (Vector-Best, Russia).
In addition to standard clinical and laboratory tests, all studied pregnant women were tested for PCT and lactate levels, and underwent genetic analysis for the gene polymorphisms associated with thrombophilia: F2 (rs1799963, G20210A), F5 (rs6025, G1691A), and MTHFR (rs1801133, C677T).
Measurement of PCT amount in serum was performed by immunochromatographic method using “IC-procalcitonin-test” reagent kit (LLC “InVitroTest”, Russia). Serum lactate level was assessed on GEM Premier 3500 analyzer (IL Werfen, USA).
The F2, F5, and MTHFR gene polymorphisms were analyzed by genomic DNA isolated from leukocytes using a “DNA-Express-Blood” reagent kit (“Lytech” Co. Ltd.). PCR was performed with the isolated DNA samples using “SNP-express-RT” amplification reagent kit (“Lytech” Co. Ltd.) with real-time results.
Statistical analysis
Statistical processing of the results was performed using Statistica software package (Version 13.0) and MS Excel for Windows (2016). Statistical calculations of quantitative indicators included descriptive statistics: median (Me) and interquartile range in Me (Q1; Q3) were used when the distribution was other than normal. The normality of the distribution of values was evaluated using the Kolmogorov–Smirnov test. The Mann–Whitney U-test with Bonferroni correction was used to compare mean values of two independent samples in case of distribution other than normal. The Spearman rank correlation coefficient with subsequent calculation of the confidence interval was used to evaluate the strength of the correlation between the indicators. For qualitative indicators, the following parameters were calculated: the number of observations and proportion (in %) of the total number of patients or of the number of patients in the subgroup. The Fisher's exact test was used for categorical variables. Values were considered statistically significant at p<0.05.
Results and discussion
Сlinical and laboratory findings of the studied patients are presented in Table 1. Women with pregnancy complicated by PE had higher blood pressure (>140/90 mm Hg) compared with the group of women with healthy pregnancy (<120/80 mm Hg), and also had a higher level of urine protein, fibrinogen, bilirubin, and higher serum AST, ALT, and LDH enzyme activity compared to the control group; this is an evidence of the value of these markers as diagnostic criteria for the disease.
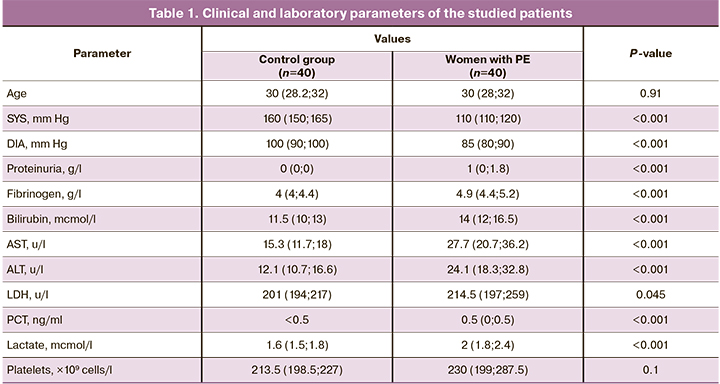
As publications state [13], one of the causes of PE is endothelial dysfunction, which easily damages the endothelium. To treat the resulting in micro clots formation and fibrin deposits on the vessel walls, impeding blood flow, especially in intrahepatic vessels. All this contributes to degeneration of liver cells followed by the release of AST, ALT, LDH, and bilirubin enzymes into the bloodstream. In response to systemic inflammation in PE, the level of fibrinogen, known as the protein of the acute phase of inflammation, elevates, and this can lead to an increased risk of thrombosis [14].
Proteinuria in PE is caused by changes in the renal glomeruli called “glomerular endotheliosis”, characterized by swelling and vacuolization of endothelial cells of nephrons, as well as fibrin deposition in the endothelium. Due to this, glomerular permeability increases, resulting the leakage of large amount of protein from the plasma into the primary urine [15].
In addition to increased levels of standard clinical and laboratory parameters, women with PE had increased levels of PCT and lactate compared with the control group. As noted earlier, PCT can be synthesized in the body under the action of proinflammatory cytokines, typical for PE. Thus, according to the obtained data, PCT is a biomarker essential for understanding the inflammatory profile of a pregnant women and can be used for diagnosing serious complications such as HELLP syndrome and eclampsia. An increase in the level of lactate in the serum is caused by an increase in LDH activity (particularly LDH-5 isoenzyme) as a result of degradation of hepatocytes and may indicate cellular hypoxia due to macro- or microcirculatory dysfunctions described above [7].
Although in PE, particularly in severe PE, thrombocytopenia is observed due to platelet depletion as a result of micro thrombs formation at endothelial damage [13], no statistically significant differences in platelet count between the groups of women with PE and women with a healthy pregnancy were found in our study. This can be explained by the small number of patients with severe PE, who could have contributed most to the total platelet count increase.
A comparison of clinical and laboratory parameters between the groups of women with different PE severity and the control group is presented in Table 2. It should be noted that the group of women with severe PE is represented by only 2 patients, which limits the search for associations between the studied parameters and the severity of the disease. The results of the study revealed a statistically significant increase only in the level of protein in the urine in the group with a moderate severity compared to the corresponding indicators in the group with a mild PE. It should be noted that in other countries proteinuria was excluded from the diagnostic criteria for PE, since the amount of protein did not correlate with the severity of the disease [16, 17].
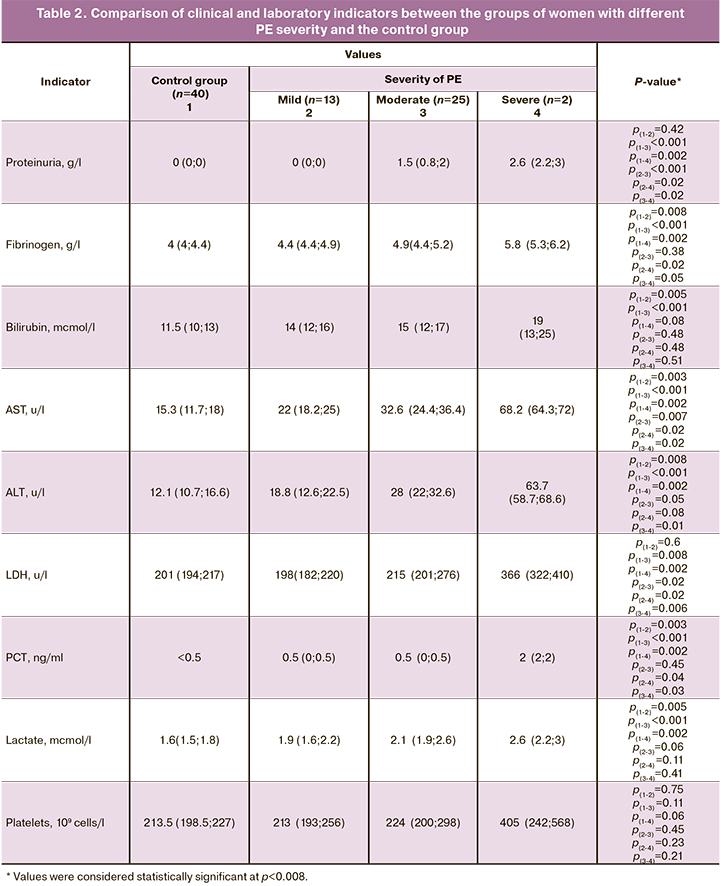
It was also found that the development of severe PE is not associated with an increase in the PCT level and lactate level in serum, which limits the use of these markers in determining the severity of PE.
A correlation analysis showed that women with PE, PCT and lactate levels had a positive correlation with fibrinogen levels in serum (r=0.38, p<0.05, CI [- 0.07; 0.53] and r=0.35, p<0.05, CI [-0.1; 0.51], respectively), as well as with protein content in urine (r=0.44, p<0.05 CI [-0.007; 0.58] and r=0.5, p<0.05, CI [0.07; 0.63], respectively). This also can be explained by endothelial dysfunction, inflammation, and swelling of endothelial cell of nephrons in this disease. Additionally, there was a weak positive correlation between the level of fibrinogen and the number of platelets in the blood (r=0.49, p<0.05, CI [0.06; 0.62]), as well as the protein content in the urine (r=0.43, p<0.05, CI [-0.02; 0.57]). However, such correlations were not observed in the control group. There was a strong positive correlation between AST and ALT levels (r=0.87, p<0.05, CI [0.97; 0.99] and r=0.86, p<0.05, CI [0.95; 0.99], respectively) in both groups of women with PE and healthy women. Moreover, correlations between ALT activity and LDH levels (r=0.37, p<0.05, CI [-0.08; 0.53]), and between ALT level and bilirubin (r=0.38, p<0.05, CI [-0.07; 0.53]) were found in women with PE. ALT level in women with PE positively correlated with platelet count in the blood (r=0.46, p<0.05, CI [0.02; 0.59]), while in the control group these indicators had an inverse negative correlation (r=-0.4, p<0.05, CI [-0.47; 0.15]).
Genetic analysis of nucleotide polymorphisms of the thrombophilia (F2, F5) and folate metabolism (MTHFR) showed the prevalence of different variants of genotypes among a group of pregnant women with PE and a group of women with a healthy pregnancy (Table 3). No statistically significant differences in the prevalence of polymorphisms G20210A (rs1799963) in the F2 gene, G1691A (rs6025) in the F5 gene, and C677T (rs1801133) in the MTHFR gene were found between the studied groups.
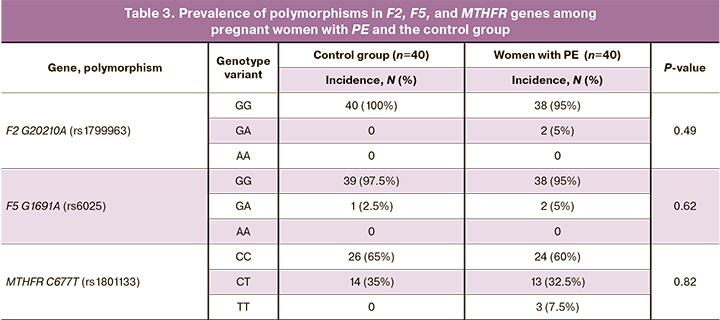
According to our data, the prevalence of GG, GA, AA genotypes of the G20210A polymorphism of the F2 gene among Russian women with PE is 67%, 22%, 11%, respectively; of GG, GA, AA genotypes of the G1691A polymorphism in the F5 gene – 57%, 41% and 4%, respectively; CC, CT, TT genotypes of the C677T polymorphism of the MTHFR gene – 46%, 37%, 17%, respectively [18].
Although minor alleles of the studied polymorphisms are associated with the risks of thrombosis and PE, the data on the correlation between the studied polymorphisms and the disease are contradictory. For example, some studies stated that G20210A polymorphism in the F2 gene, G1691A polymorphism in the F5 gene, and C677T polymorphism in the MTHFR gene were not associated with PE and pregnancy loss, suggesting that the development of this disease may be due to a possible multifactorial etiology, ethnic features of the studied population and involvement of other genes [19, 20].
It is worth noting that the PCT level in the group of women with PE who had the mutant T allele of the C677T polymorphism in the MTHFR gene in the heterozygous or homozygous variants (ST and TT) was lower than that in women with PE who had only normal C allele in the homozygous state (CC). To explain these findings, additional studies with a larger number of patients with PE are required.
Conclusion
The results of the study showed that in PE the levels of PCT and lactate increased, reflecting the presence of an inflammatory response and cellular hypoxia. This proves that the study of PCT and lactate together with standard clinical and laboratory parameters has clinical significance in the early diagnosis of PE. However, the use of PCT and lactate as markers to determine the severity of the disease is limited, since an increase in the severity of PE does not lead to an increase in the level of these parameters in the serum of pregnant women. The PCT level in the group of women with PE with the CT and TT genotypes of the C677T polymorphism in the MTHFR gene is lower compared with the corresponding indicators in the group of women with PE with the CC genotype.
References
- Адамян Л.В., Артымук Н.В., Башмакова Н.В., Белокриницкая Т.Е., Беломестнов С.Р., Братищев И.В., Вученович Ю.Д., Краснопольский В.И., Куликов А.В., Левит А.Л., Никитина Н.А., Петрухин В.А., Пырегов А.В., Серов В.Н., Сидорова И.С., Филиппов О.С., Ходжаева З.С., Холин А.М., Шешко Е.Л., Шифман Е.М., Шмаков Р.Г. Гипертензивные расстройства во время беременности, в родах и послеродовом периоде. Преэклампсия. Эклампсия. Клинические рекомендации (протокол лечения). М.; 2016. 40c. [Adamyan L.V., Artymuk N.V., Bashmakova N.V., Belokrinitskaya T.E., Belomestnov S.R., Bratishchev I.V. et al. Hypertensive disorders during pregnancy, childbirth and the postpartum period. Preeclampsia. Eclampsia. Clinical guidelines (treatment protocol). M.; 2016; 40p. (in Russian)].
- Головченко О.В. Молекулярно-генетические детерминанты преэклампсии. Научные результаты биомедицинских исследований. 2019; 5(4): 139-49. [Golovchenko O.V. Molecular genetic determinants of pre-eclampsia. Research Results in Biomedicine. 2019; 5(4): 139-49. (in Russian)]. https://dx.doi.org/10.18413/2658-6533-2019-5-4-0-11.
- Jannesari R., Kazemi E. Level of high sensitive C-reactive protein and procalcitonin in pregnant women with mild and severe preeclampsia. Adv. Biomed. Res. 2017; 6: 140. https://dx.doi.org/10.4103/2277-9175.218032.
- Mangogna A., Agostinis C., Ricci G., Romano F., Bulla R. Overview of procalcitonin in pregnancy and in pre-eclampsia. Clin. Exp. Immunol. 2019; 198(1): 37-46. https://dx.doi.org/10.1111/cei.13311.
- Vijayan A.L., Vanimaya, Ravindran S., Saikant R., Lakshmi S., Kartik R. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J. Intensive Care. 2017; 5: 51. https://dx.doi.org/10.1186/s40560-017-0246-8.
- Садыкова Г.К. Оптимизация прогностического алгоритма в диагностике тяжелого гестоза. Пермский медицинский журнал. 2008; 25(3): 44-7. [Sadykova G.K. Optimization of prognostic algorithm in diagnosis of severe gestosis. Perm Medical Journal. 2008; 25(3): 44-7. (in Russian)].
- Agostinis C., Rami D., Zacchi P., Bossi F., Stampalija T., Mangogna A. et al. Pre-eclampsia affects procalcitonin production in placental tissue. Am. J. Reprod. Immunol. 2018; 79(4): e12823. https://dx.doi.org/10.1111/aji.12823.
- Peguero A., Parra R.A., Carrillo S.P., Rjas-Suarez J., Figuras F. Association of plasma lactate concentration at admission of severe preeclampsia to maternal complications. Pregnancy Hypertens. 2019; 17: 89-93. https://dx.doi.org/10.1016/j.preghy.2019.05.003.
- Бен Амор Мариам, Гнатко Е.П., Турос Е.И., Брезицкая Н.В. Анализ показателей системы гемостаза при преэклампсии различной степени тяжести. ScienceRise: Medical Science. 2016; 8(4): 10-5. [Ben A.M., Gnatko E.P., Turos E.I., Brezitskaya N.V. Analysis of indicators of the hemostatic system in preeclampsia of varying severity. ScienceRise. Medical science. 2016; 8(4): 10-5. (in Russian)].
- Гнатко Е.П., Турос Е.И., Брезицкая Н.В., Бен Амор Мариам, Сидоренко Е.А. Молекулярно-генетическое исследование полиморфизма генов фолатного обмена и гемостаза при преэклампсии. Охрана материнства и детства. 2014; 2: 52-5. [Gnatko E.P., Turos E.I., Brezitskaya N.V., Ben A.M., Sidorenko E.A. Molecular genetic investigation of polymorphism in folate metabolism-related genes and hemostasis in preeclampsia. Maternal and child health. 2014; 2: 52-5. (in Russian)].
- Fong F.M., Sahemey M.K., Hamedi G., Eyitayo R., Yates D., Kuan V. et al. Maternal genotype and severe preeclampsia: a HuGE review. Am. J. Epidemiol. 2014; 180(4): 335-45. https://dx.doi.org/10.1093/aje/kwu151.
- Wang X., Bai T., Liu S., Pan H., Wang B. Association between thrombophilia gene polymorphisms and preeclampsia: a meta-analysis. PloS One. 2014; 9(6): e100789. https://dx.doi.org/10.1371/journal.pone.0100789.
- Макацария А.Д., Бицадзе В.О., Хизроева Д.Х. HELLP-синдром. Акушерство, гинекология и репродукция. 2014; 8(2): 61-8. [Makatsaria A.D., Bitsadze V.O., Khizroeva D.Kh. HELLP-syndrome. Obstetrics, Gynecology and Reproduction. 2014; 8(2): 61-8. (in Russian)].
- Медведев Б.И., Сюндюкова Е.Г., Сашенков С.Л. Клинико-биохимические предикторы развития преэклампсии. Акушерство и гинекология. 2013; 5: 30-5. [Medvedev B.I., Syundyukova E.G., Sashenkov S.L. Clinical and biochemical predictors for the development of preeclampsia. Obstetrics and Gynecology. 2013; 5: 30-5. (in Russian)].
- Башмакова Н.В., Цывьян П.Б., Чистякова Г.Н., Пестряева Л.А. Гагарина Е.М., Петрова М.М. Ангиогенные ростовые факторы и патогенез преэклампсии. Российский вестник акушера-гинеколога. 2017; 17(5): 7-12. [Bashmakova N.V., Tsyvyan P.B., Chistyakova G.N., Pestryaeva L.A., Gagarina E.M. Angiogenic growth factors and the pathogenesis of preeclampsia. Russian Bulletin of Obstetrician-Gynecologist. 2017; 17(5): 7-12. (in Russian)]. https://dx.doi.org/10.17116/rosakush20171757-12.
- Guida J.P., Parpinelli M.A., Surita F.G., Costa M.L. The impact of proteinuria on maternal and perinatal outcomes among women with pre-eclampsia. Int. J. Gynaecol. Obstet. 2018; 143(1): 101-7. https://dx.doi.org/10.1002/ijgo.12487.
- Tanacan A., Fadiloglu E., Beksac M.S. The importance of proteinuria in preeclampsia and its predictive role in maternal and neonatal outcomes. Hypertens. Pregnancy. 2019; 38(2): 111-8. https://dx.doi.org/10.1080/10641955.2019.1590718.
- Лоскутова Т.А. Анализ форм тромбофилии у беременных с акушерскими и перинатальными осложнениями при преэклампсии. Акушерство и гинекология. 2013; 10: 23-7. [Loskutova T.A. Analysis of the forms of thrombophilia in pregnant women with obstetric and perinatal complications in preeclamsia. Obstetrics and Gynecology. 2013; 10: 23-7. (in Russian)].
- Ahmed S.F., Ali M.M., Kheiri S., Elzaki S.E.G., Adam I. Association of methylenetetrahydrofolate reductase C677T and reduced-f carrier-1 G80A gene polymorphism with preeclampsia in Sudanese women. Hypertens. Pregnancy. 2020; 39(2): 77-81. https://dx.doi.org/10.1080/10641955.2020.1725037.
- Jääskeläinen E., Keski-Nisula L., Toivonen S., Romppanen E-L., Helisalmi S., Punnonen K. et al. MTHFR C677T polymorphism is not associated with placental abruption or preeclampsia in Finnish women. Hypertens. Pregnancy. 2006; 25(2): 73-80. https://dx.doi.org/10.1080/10641950600745137.
Received 18.06.2021
Accepted 02.07.2021
About the Authors
Ksenia S. Semashchenko, Postgraduate Student, the 1st course, Siberian Federal University, +7(933)329-13-32, kseniya.semashchenko@mail.ru,https://orcid.org/0000-0002-8735-271, 660041, Russia, Krasnoyarsk, Svobodny Prospekt, 79/10.
Ekaterina V. Vasilyeva, Postgraduate Student, the 2nd course, Siberian Federal University; medical laboratory technician, City Maternity Hospital No. 5, +7(913)513-45-48, katerinavas79@mail.ru, https://orcid.org/0000-0003-0009-8194, 660041, Russia, Krasnoyarsk, Svobodny Prospekt, 79/10.
Natalia N. Molokova, PhD (Bio), Biologist of Clinical Laboratory Diagnostics, City Maternity Hospital No. 5, +7(923)296-18-79, nataacadem@mail.ru,
https://orcid.org/0000-0002-1425-9692, 660100, Russia, Krasnoyarsk, Svobodny Prospekt, 73.
Olga Yu. Zhitkova, Head of Clinical Laboratory Diagnostics, City Maternity Hospital No. 5, +7(913)043-46-30, Zhitkova1973@list.ru, https://orcid.org/0000-0002-3856-680,
660100, Russia, Krasnoyarsk, Svobodny Prospekt, 73.
Tatyana N. Subbotina, PhD (Bio), Associate Professor at the Department of Medical Biology, Head of the Scientific and Practical Laboratory for Molecular Genetic Research Methods, Siberian Federal University; Senior Researcher, Federal Siberian Research Clinical Centre of the Federal Medical Biological Agency of Russia, +7(923)320-00-11, stn.25@mail.ru, https://orcid.org/0000-0001-7790-5033, 660041, Russia, Krasnoyarsk, Svobodny Prospekt, 79/10.
Authors’ contribution: Semashchenko K.S. – data collection, analysis and statistical processing, writing a part of the article; Vasilyeva E.V. – providing the materials for study, data processing, editing the text of the article; Molokova N.N. – the concept and design of the study, providing the materials for the study, editing the text of the article; Zhitkova O.Yu. – scientific editing and approval of the text of the article; Subbotina T.N. – the concept and design of the study, scientific editing and approval of the text of the article.
Conflicts of interest: The authors have no conflicts of interest.
Funding: The study was carried out without any sponsorship.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Semashchenko K.S., Vasilyeva E.V., Molokova N.N., Zhitkova O.Yu.,
Subbotina T.N. Study of serum procalcitonin and lactate levels in women with preeclampsia.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 10: 61-67 (in Russian)
https://dx.doi.org/10.18565/aig.2021.10.61-67



