Clinical and morphological features of the placenta in acute intrauterine hypoxia during childbirth
Objective. To evaluate the clinical and morphological features of the placenta in the development of acute intrauterine hypoxia during childbirth. Subjects and methods. The retrospective study enrolled 62 women. All the cases were divided into two groups: a study group included 35 women in whom fetal hypoxia verified by the umbilical cord arterial pH value (<7.12) developed during childbirth; a comparison group consisted of 27 women with a pH of ≥7.12. All the patients underwent pathomorphological examination of the placenta. Results. The pathomorphological study analyzed more than 30 umbilical cord and placental parameters associated with the risk of fetal hypoxia during childbirth. There were significant differences in the detection rate of the signs of parenchymal placental insufficiency (p = 0.05), in the frequency of combined changes with the predominance of moderate and severe terminal villous hypercapillarization and pathological villus tree immaturity (p <0.03). Conclusion. In physiological pregnancy, compensatory placental capabilities contribute to the high resistance of a fetus to acute oxygen deficiency. Placental morphofunctional disorders that may have no clinical manifestations in the antenatal period and in the stressful situation of childbirth are a determinant of decompensation with the development of clinically important fetal hypoxia.Nizyaeva N.V., Prikhodko A.M., Evgrafova A.V., Tysyachnyi O.V., Baev O.R.
Keywords
Hypoxia is a pathological process characterized by absolute or relative insufficient supply of energy to organs and tissues. The initial cause of hypoxia is the oxygen deficiency with subsequent impairment of cell function followed by its death [1].
Fetal hypoxia is a condition associated with a lack of energy supply to the organs and tissues of the fetus during pregnancy and childbirth. Acute hypoxia develops over a short period of time and is usually characterized by rapid progression. Chronic hypoxia develops for a long time, and could lead to fetal growth retardation. Hypoxia leads to the disturbances of tissue metabolism, an increase in the level of anaerobic glycolysis, which may be accompanied by a decrease in blood pH and an increase in lactate concentration [2, 3].
The exchange between mother and fetus is provided by the placenta. Hypoxia may be divided into three subtypes: pre-placental (maternal), utero-placental and post-placental (disorders in the fetus).
Thus, the placenta is the central element in the system that determines the mechanisms for compensating oxygen deficiency.
Currently, there are a lot of studies devoted to the investigation of the placenta in chronic fetal hypoxia. However, the role of the placenta and its compensatory mechanisms in the genesis of acute fetal hypoxia remains understudied.
The aim of the study was to reveal the clinical and morphological features of the placenta in the development of acute fetal hypoxia during childbirth.
Materials and Methods
The study included 62 primiparous and multiparous women who delivered at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology from 2018 to 2019. All observations were divided into two groups. Fetal hypoxia developed during childbirth in 35 women who were included in the study group, hypoxia in this group was verified by the pH level of arterial umbilical cord blood (<7.12). The comparison group consisted of 27 women (pH≥7.12) [4, 5].
Inclusion criteria were patients’ age from 18 to 40 years, spontaneous singleton pregnancy, vertex presentation of the fetus, full-term pregnancy. Informed consent to participate in the study was obtained from all patients. The study was approved by the local ethics committee.
Exclusion criteria were severe pathology, complicated pregnancy, abnormalities of the uterus, congenital fetal malformations, laboratory-confirmed intrauterine infection of the newborn.
To determine the indicators of the acid-base state and the gas composition of cord blood, the gas analyzer ABL800 FLEX was used (Radiometer Medical ApS, Denmark) [6].
Pathomorphological study of the placenta was performed in all the patients [2]. In order to clarify the role of the placenta in hypoxia etiology, placental mass, size of the placental disc, length and attachment of the umbilical cord (central, paracentral, marginal), as well as the degree of umbilical coiling were determined [6, 7]. For histological examination, placental tissue samples of patients from the study and comparison groups were obtained after operative delivery. Fragments of placental tissue size of 1.5x1.0x0.3cm were dissected from the paracentral zone (including the villous chorion, basal and chorionic plates). The obtained fragments were fixed in 10% solution of buffered neutral formalin (Biovitrum, Russia) for 24 hours, then they were enclosed in paraffin. The paraffin-embedded sections with a thickness of 4 μm were stained with hematoxylin and eosin. The condition of the villous tree was evaluated in accordance with the maturity scale of the villous tree [8]:
- 22 points – a fully mature normally capillarized villous tree with a predominance of terminal villi and the presence of mature intermediate villi;
- 33 points – the predominance of branching terminal villi with multiple syncytial knots (Tenney-Parker changes), a great number of small capillaries in transverse sections of terminal villi, the absence of immature intermediate villi;
- 32 points – a less pronounced form than in 33 (moderately pronounced branched angiogenesis).
- 23 points – the prevalence of branched mild angiogenesis that reflects the presence of compensatory mechanisms.
Branched angiogenesis of 23-33 points is characteristic of the placental villous tree (in terminal villus there are normally about 3-5 capillaries) [8, 9]. Increased number of blood vessels and syncytial knots in villous tree is suggestive of compensatory processes in the placenta.
- 12 points – retarded maturation of the villous tree, the predominance of mature intermediate villi, terminal villi are single, immature intermediate villi are in the form of clusters (typically characteristic of 34-36 weeks);
- 21 points - the balance between mature intermediate and terminal villi;
- 24, 42, 44 points – unbranched angiogenesis (mild, moderate, severe). The predominance of small threadlike terminal villi with long wide capillary loops; a significant difference in diameter between small terminal and large stem villi.
Statistical processing of the results was carried out using the software package IBM SPSS Statistics 22.0. The hypothesis of normal distribution was tested using the Shapiro-Wilk test. Under normal distribution, the mean value (M) and standard deviation (SD) were calculated, and the t-test was used to determine statistical significance. In the distribution of signs other than normal, the Mann-Whitney test was used. The data were presented as the median and interquartile range. Frequencies were calculated and Fisher’s exact test was used for qualitative data and the determination of statistical significance. Differences between the compared values were considered statistically significant at p <0.05 (95% level of statistical significance).
Results
The comparison of the patients’ groups did not reveal any significant differences in body mass index, age, obstetric and gynecological history, parity, gestational age, duration of labor, weight and length of newborns, as well as the frequency of cesarean section (Table 1).
In the study group, 12 (34.28%) women required delivery by vacuum extraction of the fetus versus 1 (3.70%) patient in the comparison group, p = 0.02. Indications for fetal vacuum extraction were acute fetal hypoxia and weak contractions in women from the study group, and weak contractions in patients from the comparison group. The frequency of cesarean section did not differ in the groups (p = 0.44). Indications for cesarean section were prolonged labor, namely 1 (9.1%) in the study group versus 3 (27.27%) in the comparison group (p = 0.30), cephalopelvic disproportion - 0 (0%) versus 3 (27.27%), respectively (p = 0.18), acute fetal hypoxia according to cardiotocography – 10 (90.9%) versus 5 (45.45%), respectively (p = 0.04).
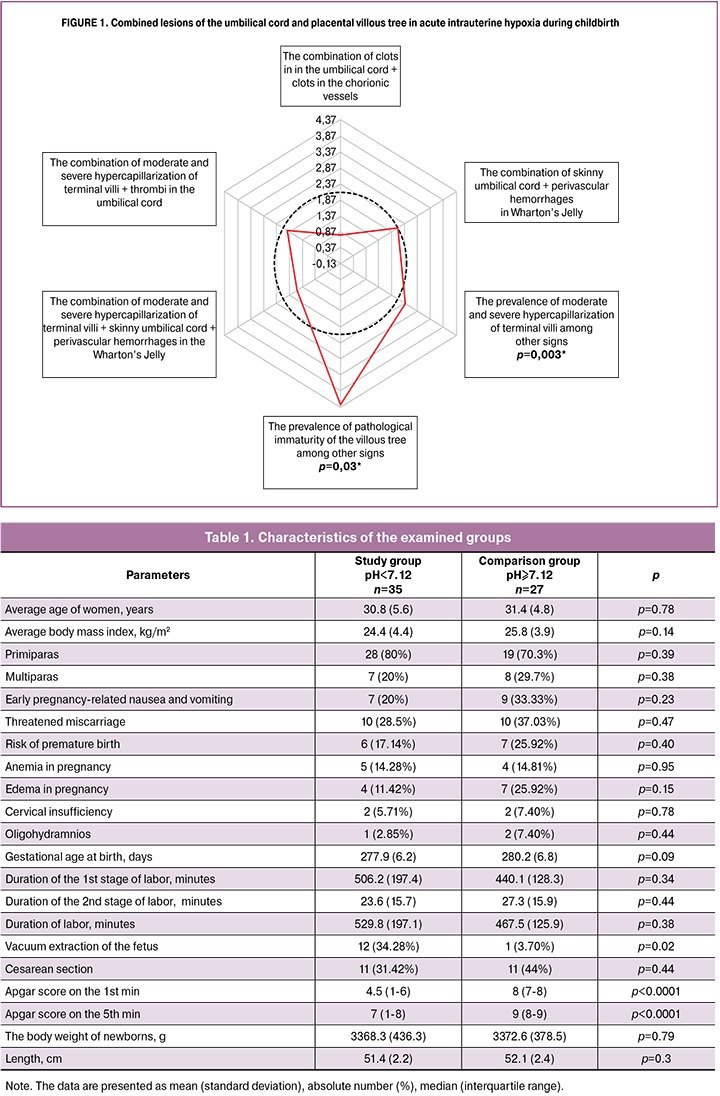
We analyzed more than 30 parameters of the umbilical cord and placenta associated with the risk of fetal hypoxia in childbirth (Table 2, Fig. 1, Fig. 2 A-K).
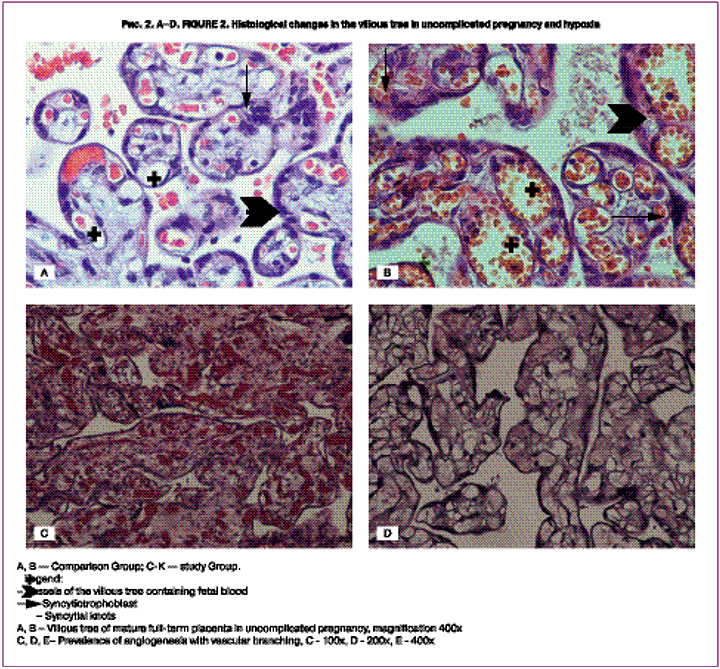
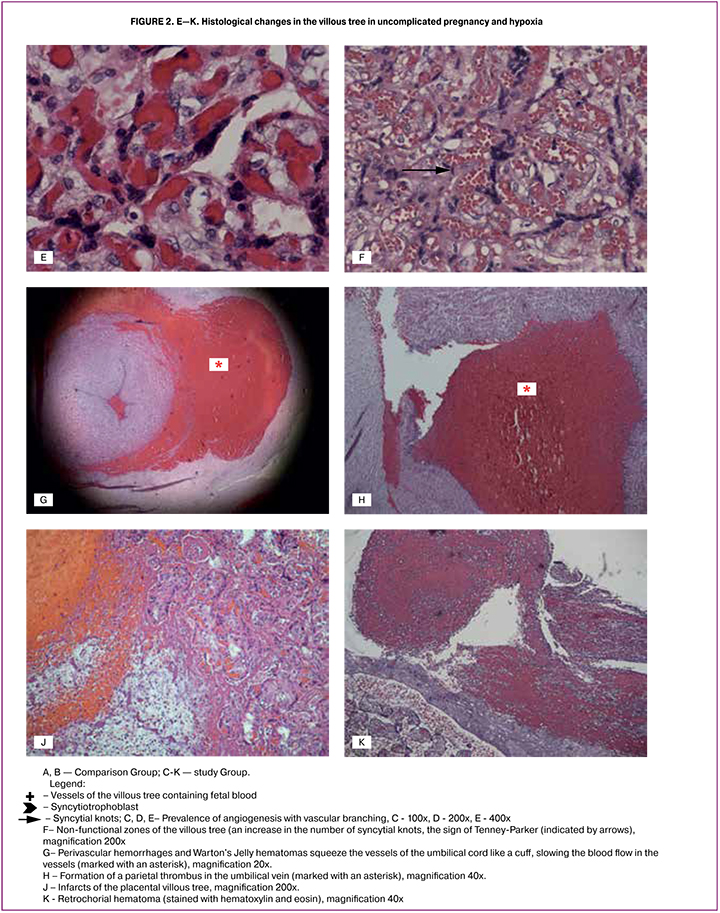
The analysis of the villous tree showed that in the study group in 23 (65.7%) cases there was the predominance of branching terminal villi, the increased number of capillaries and syncytio-capillary membranes (the prevalence of angiogenesis with vascular branching) at moderate and severe degree (32-33 points) [8]. It was significantly more often than one in the comparison group – 11 (40.7%) cases, p = 0.051 (Fig. 2 A-E).
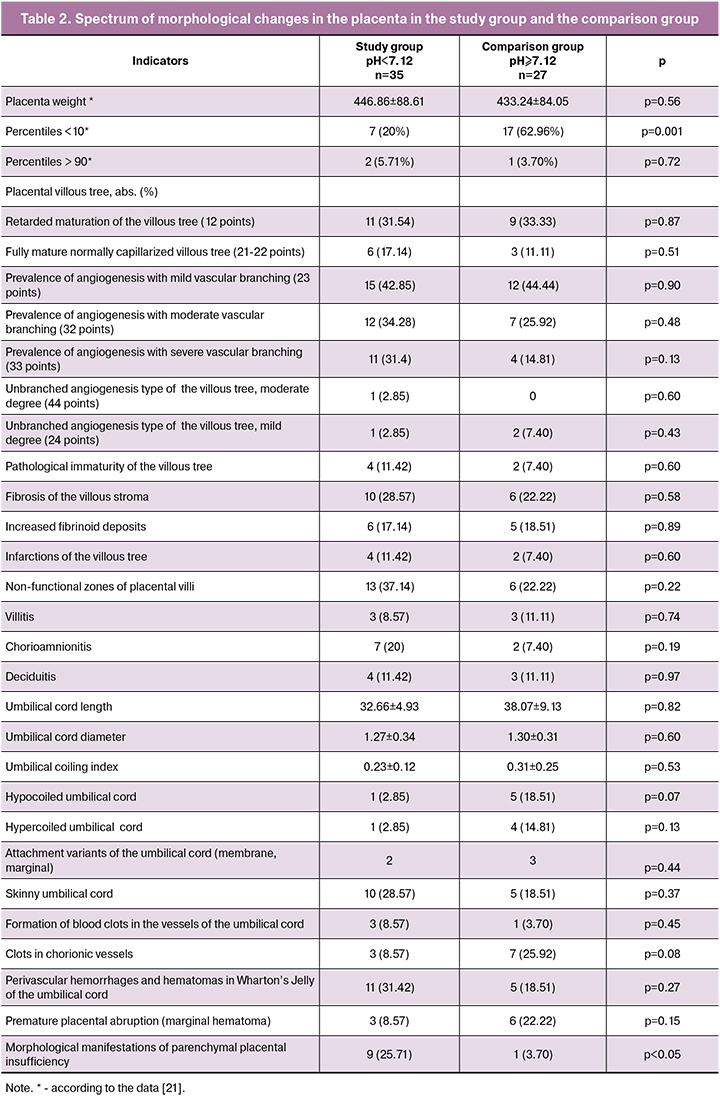
The study group more frequently demonstrated a morphological complex characteristic mainly of parenchymal placental insufficiency, namely а decrease in the number of vessels in the villous stroma, impaired angiogenesis, the presence of blood clots, that is impaired vascularization of the villi, villous infarcts of different time periods (Fig. 2J), hematomas and intervillous thrombi, non-functional zones of the villous tree (Fig. 2F), a marked increase in the number of syncytial knots (Fig. 2F), foci of sclerotic villi (p = 0.05). However, the placenta weight <10 percentiles was 2.4 times less common in the study group than in the comparison group.
In addition, significant differences were revealed in placental lesions of the study group (Fig. 1) such as hypercapillarization of terminal villi (moderate and severe degrees) and the pathological immaturity of the villous tree leading to placental insufficiency.
Discussion
Fetal hypoxia during childbirth is likely to develop in cases of the mother-placenta-fetus system disturbances. It is believed that more than 75% of cases of perinatal mortality are associated with fetal hypoxia, neonatal asphyxia, and hypoxia-related brain damage due to the pathology of the placenta and umbilical cord [9]. However, isolated pathological changes in the umbilical cord or villous tree with a moderate decrease in fetoplacental blood flow are usually compensated and do not have a pronounced effect on the fetus. Meanwhile, even slight decrease in blood flow accompanied by reduced compensatory capabilities in the mother-placenta-fetus system causes fetal hypoxia.
In our study, more than 30 parameters of the umbilical cord and placenta were evaluated.
It is considered that the normal umbilical cord length is about 45-60 cm, and its diameter is 1-2 cm [11, 12, 13]. The normal number of coils (2-2.5 coils per 10 cm), absorbs the impact on the umbilical cord [13, 14, 15]. The relative (due to the entanglement of the parts of the fetal body) and the absolute shortness of the umbilical cord (less than 35 cm) limits the movement of the fetus and contributes to the mechanical damage to its vessels. An excessively long umbilical cord (more than 70 cm) is often associated with the formation of true umbilical cord knots, as well as entanglement around the neck and body of the fetus and, therefore, its relative shortening. The umbilical cord vessels are surrounded by a jelly-like substance (Wharton’s Jelly), which does not only fix them, but also protects them from injuries and compression, therefore, a decrease in the number of Wharton’s jelly is a risk factor. Umbilical cord can be considered skinny if its thickness is less than 1 cm [11].
Other umbilical factors that can mediate the development of fetal hypoxia are abnormal attachment of the umbilical cord (marginal, membrane attachment of the umbilical cord, vasa praevia (vasa praevia is a condition in which fetal blood vessels cross or run near the internal opening of the uterus), insertio furcata (the cord vessels lose their protective coating of Warton’s Jelly prior to inserting into the body of the fetus), the presence of torsion and compression of the umbilical cord by bands, e.g. fibrous tissue, aneurysm of the umbilical cord, false knots (associated with the formation of knots and loops of the umbilical vessels) and the true umbilical cord knots, umbilical cord hematoma (Fig. 2G), malformations, including a single umbilical artery.
One of the important causes of blood flow disturbances in the umbilical cord is fetal thrombotic vasculopathy that is the formation of blood clots and sludge in the vessels of the umbilical cord (Fig. 2H) and chorion [16, 17, 18]. In addition to mechanical factors, the formation of blood clots is caused by impairment of the blood rheological properties, with a slowing of blood flow in the vessels, as well as disturbances in the blood coagulation system. The latter occurs due to the mutations in the genes responsible for thrombophilia development [8, 17]. However, fetal thrombotic vasculopathy with the formation of parietal thrombi as a cause of impaired blood flow through the umbilical cord is less common than one associated with structural changes or mechanical damage [11].
Despite the fact that most of the above factors were revealed in both groups, we were not able to identify a statistical relationship with the development of fetal hypoxia. This result indicates that the features of the umbilical cord, which may predispose to the development of fetal hypoxia, are quite common. However, the development of oxygen deficiency depends on the severity of pathological changes in the umbilical cord and is largely compensated by the protective mechanisms of the placenta (high rate of oxygen exchange and restoration of blood flow) and the fetus (hemodynamic remodeling, high level of fetal hemoglobin).
At full-term pregnancy the placenta weight averages 470 g after separating it from the membranes and umbilical cord. The size of the placental disc averages 22x22x1.5-2.5 cm [7, 13]. According to our study, placental mass did not correlate with fetal status and pH of fetal blood. On the contrary, in the comparison group, placenta weight less than 10 percentiles was found 2.4 times more often. Despite the revealed differences in the weight of the placenta, morphological changes that may be regarded as placental insufficiency were significantly more often detected in the group with acute fetal hypoxia during childbirth. Our results indicate that the placental weight is not in direct proportion to the degree of oxygen supply. It is possible that the higher placenta weight in the group with fetal hypoxia reflects placental compensatory changes in response to partial morphofunctional inferiority, which is compensated by an increase in the placenta weight.
When estimating the maturity of the villous tree, its normal condition is equal to 22 points, that is terminal villi (through which the gas exchange is carried out predominantly) prevail over mature intermediate villi [13]. Compensatory mechanisms with morphological manifestations include terminal villi hypercapillarization (branched angiogenesis), the increased number of syncytio-capillary membranes and syncytial knots (Tenney-Parker changes) [6, 13]. Hypercapillarization of terminal villi is the compensatory mechanism in hypoxia [6], and it is related to the high level of angiogenic factors, including VEGF promoting the vascular growth [19].
The restructuring of angiogenesis leads to significant hypercapillarization of terminal villi (32-33 points on the scale) [13], the diameter of the capillary diminishes [20], causing a slowdown in microcirculation and fetoplacental blood flow. Therefore, platelet aggregation increases in the lumen of the capillaries resulting in the formation of microclots. The intensification of these processes leads to focal fibrosis of the villous stroma. The changes that were originally aimed at compensating the negative effect of hypoxia contribute to a gradual transition to the decompensation stage. In our study, severe and moderate branched angiogenesis was much more common in the study group, than in the comparison group. Moreover, the pathological immaturity of the placental villous tree, obviously, does not provide the necessary level of compensation of hypoxia impact.
The above mentioned morphological features of the placenta are primarily associated with the restructuring of angiogenesis, as well as with the pathological immaturity of the villous tree. These features are indicative of the development of acute fetal hypoxia during childbirth in the presence of predisposing factors in mother-placenta-fetus system such as disturbances or depletion of compensatory mechanisms. As a result, the villous tree was rebuilt and subclinical placental insufficiency developed. The impact of additional trigger factors in labor (uterine contractile activity, compression of the umbilical cord and others) caused decompensation with the development of symptoms of acute fetal hypoxia.
Thus, in uncomplicated pregnancy, the compensatory placental capabilities contribute to the high resistance of the fetus to acute oxygen deficiency. Morphofunctional disorders of the placenta, which may not have clinical manifestations in the antenatal period, in a stressful situation of childbirth are a factor determining decompensation with the development of clinically significant fetal hypoxia.
References
- Литвицкий П.Ф. Гипоксия. Вопросы современной педиатрии. 2016; 15 (1): 45–58. [Litvitsky P.F. Hypoxia. Voprosy sovremennoi pediatrii — Current Pediatrics. 2016; 15 (1): 45–58. (in Russian)] doi: 10.15690/vsp.v15i1.1499
- Приходько А.М., Романов А.Ю., Евграфова А.В., Шуклина Д.А. Определение уровня pH и лактата крови из предлежащей части плода для оценки его состояния в родах. Вопросы гинекологии, акушерства и перинатологии. 2017; 16 (6): 96–9. [Prikhodko A.M., Romanov A.Yu., Evgrafova A.V., Shuklina D.A. Determination of the pH level and blood lactate from the adjacent part of the fetus to assess its condition in childbirth. Voprosy gynecologii, akusherstva I perinatologii/Questions of gynecology, obstetrics and perinatology. 2017; 16(6): 96–9 (in Russian)].
- Приходько А.М., Романов А.Ю., Баев О.Р. Ультразвуковое исследование в родах: техника и практическое применение. Акушерство и гинекология. 2019; 6: 151–54. https://dx.doi.org/10.18565/aig.2019.6.151–154. [Prikhodko A.M., Romanov A.YU., Baev O.R. Ultrasound during labor: techniques and practical application. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (6): 151–4. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.6.151-154.
- Приходько А.М., Романов А.Ю., Шуклина Д.А., Баев О.Р. Показатели кислотно-основного равновесия и газовый состав артериальной и венозной пуповинной крови в норме и при гипоксии плода. Акушерство и гинекология. 2019; 2: 93–7. [Prikhodko A.M., Romanov A.Yu., Shuklina D.A., Baev O.R. The indicators of acid-base balance and the gas composition of umbilical cord arterial and venous blood in health and fetal hypoxia. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (2): 93–7. (in Russian)]. doi:10.18565/aig.2019.2.93-97
- Приходько А.М., Баев О.Р. Определение кислотно-основного состояния пуповинной крови. Показания и техника. Акушерство и гинекология. 2018; 5: 127–31. [Prikhodko A.M., Baev O.R. Determination of umbilical cord blood acid-base status. Indications and techniques. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (5): 127–31. (in Russian)]. doi:10.18565/aig.2018.5.127-131.
- Милованов А.П. Патология системы мать–плацента–плод: руководство для врачей. М.: Медицина, 1999. 448 c. [Milovanov A.P. Patoligia sistemy mat'-placenta-plod: A guide for doctors. 448. М.: Medicine, 1999. 448 s. (in Russian)].
- Низяева Н.В., Волкова Ю.С., Муллабаева С.М., Щеголев А.И. Методические основы изучения ткани плаценты и оптимизация режимов преподготовки материала. Акушерство и гинекология. 2014; 8: 10–18. [Nizyaeva N.V., Volkova Yu.S., Mullabaeva S.M., Shchegolev A.I. The methodical bases for placental tissue examination and the optimization of material pre-preparation regimens. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2014; 8: 10–18. (in Russian)].
- Benirschke K., Burton G.J. Baergen R.N. Pathology of the human placenta. N.Y.: Springer, 2006; 498 р.
- Щеголев А.И., Туманова У.Н., Ляпин В.М. Нарушения структуры и васкуляризации ворсин плаценты при задержке роста плода. Современные проблемы науки и образования. 2018; 4. 12–18. [Schegolev A.I., Tumanova U.N., Lyapin V.M. Disturbances of the structure and vascularization of placental villi with fetal growth retardation. Sovremennye problemy obrazovaniya/Modern problems of science and education. 2018; 4. 12–18. (in Russian)]. doi: 10.26442/2079-5696_2018.4.12-18
- Савельева Г.М. Пути снижения перинатальной заболеваемости и смертности Вестник Российской ассоциации акушеров-гинекологов, 1998, 2: 101-104. [Savelyeva G.M. Ways to reduce perinatal morbidity and mortality. Vestnik Rossiyskoi assotsyatsyi akusherov i ginekologov/Bulletin of the Russian Association of Obstetricians and Gynecologists. 1998; 2: 101–104(in Russian)].
- Redline R.W. Clinical and pathological umbilical cord abnormalities in fetal thrombotic vasculopathy. Hum Pathol. 2004; 35(12):1494–8.
- Redline R.W., Pappin A. Fetal thrombotic vasculopathy: the clinical significance of extensive avascular villi. Hum Pathol. 1995; 26 (1): 80–5. DOI: 10.1016/0046-8177(95)90118-3
- Benirschke K., Burton G.J. Baergen R.N. Pathology of the human placenta. N.Y.: Springer, 2012; 939 р. doi:10.1007/978-3-642-23941-0
- de Laat M.W., Franx A., Bots M.L., Visser G.H., Nikkels P.G. Umbilical coiling index in normal and complicated pregnancies. Obstet Gynecol. 2006; 107 (5): 1049–55. doi:10.1097/01.AOG.0000209197.84185.15
- Siva Sree Ranga, Vasantha Mallika. Morphological variations of umbilical cord in human placenta. Int J Anat Res. 2019; 7(3.1): 6786–9. ISSN 2321-4287. https://dx.doi.org/10.16965/ijar. 2019.226.
- Issa A.H., Jaber B.M.S. Evaluation of predictive ability for the indicator of natal cord coils of the pregnant women. World J Pharm Res. 2019; 8 (5): 197–208. ISSN 2277– 7105.
- Кирющенков П.А., Ходжаева З.С., Тетруашвили Н.К., Донников А.Е. Значение полиморфизма гена ингибитора активатора плазминогена I типа (SERPINE1: 5G/4G) при отслойках хориона и плаценты на ранних сроках беременности. Акушерство и гинекология. 2012; (5): 34–7. [Kiryushchenkov P.A., Khodzhayeva Z.S., Tetruashvili N.K., Donnikov A.E. Significance of plasminogen activator inhibitor type 1 gene (SERPINE1: 5G>4G) polymorphism in chorionic detachment and placental abruption in early pregnancy. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2012; 5: 34–7. (in Russian)].
- Machin G.A., Ackerman J., Gilbert-Barness E. Abnormal umbilical cord coiling is associated with adverse perinatal outcomes. Pediatr Dev Pathol. 2000; 3(5): 462–71. doi: 10.1007/s100240010103
- Burton G.J., Charnock-Jones D.S., Jauniaux E. Regulation of vascular growth and function in the human placenta. Reproduction. 2009; 138(6): 895–902. doi:10.1530/REP-09-0092.
- Shchyogolev A.I., Dubova E.A., Pavlov K.A., Lyapin V.M., Kulikova G.V., Shmakov R.G. Morphometric characteristics of terminal villi of the placenta in pre-eclampsia. Bulletin of Experimental Biology and Medicine. 2013; 154 (1): 92–95.
- Pinar H., Sung C.J. Oyer C.E., Singer D.B. Reference values for singleton and twin placental weights. Pediatr Pathol Lab Med. 1996;16(6): 901–7. doi: 10.1080/15513819609168713
Received 02.10.2019
Accepted 04.10.2019
About the Authors
Natalia V. Nizyaeva, PhD., MD, Senior Researcher, Pathology department, National Research Medical Center for Obstetrics, Gynecology, and Perinatology,Ministry of Healthcare of Russia; Ac. Oparin street, 4Moscow, Russian Federation, 117997;
Mob: 8 (926) 248-28-93. E-mail: niziaeva@gmail.com ORCID: orcid.org/0000-0001-5592-5690.
Andrey M. Prikhodko, PhD physician of the first Maternity Department Federal State Budget Institution «National medical research center of obstetrics, gynecology and Perinatology» of the Ministry of health of Russia. Phone 84954381188, e-mail a_prikhodko@oparina4.ru. ORCID: orcid.org/ 0000-0002-66-15-2360
Alexandra V. Evgrafova second year postgraduate student of the maternity departments, «National medical research center of obstetrics, gynecology and Perinatology»
of the Ministry of health of Russia. Phone 84954381188, e-mail a_evgrafova@oparina4.ru. ORCID: orcid.org/0000-0002-9429-3208
Oleg V. Tysyachnyy, PhD, scientific researcher of Department of innovative technologies of the Institute of obstetrics Federal State Budget Institution «National medical research center of obstetrics, gynecology and Perinatology» of the Ministry of health of Russia. Phone 84954381188. e-mail olti23@mail.ru. ORCID: orcid.org/0000-0001-9282-9817.
Oleg R. Baev, MD, Phd, professor, the head of the first Maternity Department Federal State Budget Institution «National medical research center of obstetrics, gynecology and Perinatology» of the Ministry of health of Russia. Phone 84954381188, e-mail o_baev@oparina4.ru; FGAOU VO “First Moscow state medical University. I. M. Sechenov” Ministry Of Health Of Russia (Sechenovskiy University). Department of obstetrics, gynecology, perinatology and reproduction. ORCID: orcid.org/0000-0001-8572-1971.
For citation: Nizyaeva N.V., Prikhodko A.M., Evgrafova A.V., Tysyachnyi O.V., Baev O.R. Clinical and morphological features of the placenta in acute intrauterine hypoxia during childbirth.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2019; 12: 96-104.(In Russian).
https://dx.doi.org/10.18565/aig.2019.12.96-104



