Преимплантационная генетическая диагностика и скрининг (ПГД/ПГС) являются методами, применение которых в сфере вспомогательных репродуктивных технологий (ВРТ) неуклонно растет [1]. Конечной целью данных методов является определение генетической полноценности эмбриона, что в свою очередь снижает риски переноса эмбрионов с патологией и уменьшает шансы рождения детей с пороками развития.
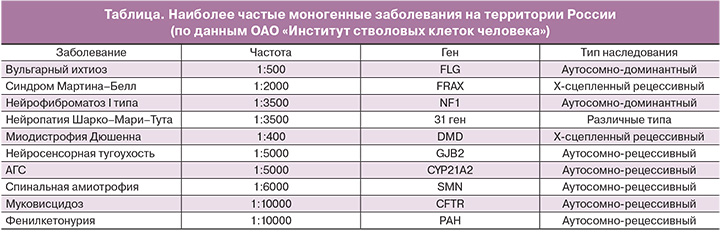
ПГД изначально была разработана для диагностики нарушений у потомства пар с моногенными заболеваниями, такими как муковисцидоз, или заболеваниями, сцепленными с полом, такими как мышечная дистрофия (таблица).
Вскоре эта методика стала применяться для диагностики различных видов анеуплоидии у эмбрионов, которые, как известно, повышают риск невынашивания беременности, мертворождений или рождения детей с хромосомными нарушениями.
Стремительному развитию ПГД в настоящее время способствует внедрение в эмбриологическую практику современных молекулярных методов анализа ДНК: сравнительной геномной гибридизации (CGH), секвенирования, полимеразной цепной реакции (ПЦР) с гибридизационно-флюоресцентной детекцией. Использование данных технологий расширяет возможности исследования генетического статуса эмбрионов человека, полученных in vitro. Независимо от того, какой метод молекулярного анализа используют для ПГД, исследование проводят на материале ДНК одной или нескольких эмбриональных клеток. Первые работы, в которых была продемонстрирована биопсия клеток у эмбрионов человека в целях генетической диагностики, выполнены на 3-и сутки раннего развития, когда эмбрион состоит из 6–8 клеток (стадия дробления). Биопсия бластомеров на восьмиклеточной стадии и сейчас является наиболее распространенной и широко практикуемой во многих лабораториях мира. Тем не менее, в настоящее время уже стали очевидными преимущества биопсии клеток трофэктодермы бластоцист (5-е сутки развития) по сравнению с биопсией бластомеров на стадиях дробления. В первую очередь, при биопсии клеток у бластоцист возможно получение большего объема клеточного материала для исследования, чем при биопсии на стадиях дробления, когда количество изъятого материала ограничено 1–2 клетками. Увеличение числа эмбриональных клеток, полученных в результате биопсии, упрощает проведение генетического анализа, повышает достоверность результата. Кроме того, на данной стадии эмбрионы обладают наиболее высоким потенциалом к имплантации. В ряде современных работ было показано, что частота выявленных анеуплоидий у бластоцист достоверно ниже, чем у дробящихся эмбрионов. Несмотря на очевидные преимущества биопсии трофэктодермы бластоцисты, этот подход имеет существенный недостаток: время для осуществления диагностики ограничено сроками проведения переноса эмбрионов и составляет интервал от нескольких часов до одних суток. Данное обстоятельство может являться причиной отмены переноса эмбрионов в текущем цикле ЭКО, криоконсервации бластоцист.
Хотя ПГД/ПГС являются действенным способом достижения беременности и уменьшения ее неблагоприятных исходов, важно не нанести значимого ущерба эмбриону во время проведения биопсии в целях сохранения его жизнеспособности и репродуктивных возможностей [2].
В рамках программы генетической диагностики наибольший удельный вес занимает ПГС анеуплоидий. Информация, полученная в результате исследования, является важным аргументом селекционного процесса, основанным не только на морфологических, но и на генетических характеристиках эмбриона [3, 4]. Генетический скрининг анеуплоидий позволяет определить хромосомный статус эмбрионов и выбрать эуплоидные эмбрионы на перенос, что дает возможность снизить уровень спонтанных абортов, уменьшить риск рождения детей с генетическими отклонениями, тем самым увеличить вероятность рождения здорового ребенка [4, 5]. Неудачи в предыдущих программах ЭКО/ИКСИ, замершие беременности, самопроизвольные выкидыши и аборты с выявленными хромосомными нарушениями в результате кариотипирования абортивного материала, а также наличие у плода генетических аномалий (синдром Дауна, Патау, Эдвардса и др.) – все это показания для проведения генетического тестирования эмбрионов.
Одним из наиболее важных показаний для проведения ПГД/ПГС является высокий генетический риск передачи наследственной патологии потомству. Такую диагностику проводят для носителей генных и хромосомных аномалий. Это могут быть моногенные болезни (аутосомно-рецессивные, аутосомно-доминантные, сцепленные с Х- или Y-хромосомой), а также хромосомные аномалии (числовые и структурные аберрации хромосом). Врачу-генетику, задачей которого является оценка риска рождения нездорового ребенка и помощь семье в предотвращении данной проблемы, важно помнить, что среди пациентов, нуждающихся в такой диагностике, могут быть пациенты с гонадным мозаицизмом. Генетический анализ образца крови пациента не покажет наличия у него мутации. Предположить же гонадный мозаицизм у пациента можно по данным анамнестического анализа его семьи – исследования имеющихся в семье больных детей, материала абортусов от предыдущих беременностей и эмбрионов, полученных в циклах ЭКО.
Также показанием к проведению ПГД/ПГС является высокий процент различной генетической патологии у мужчин в паре. Изучение хромосом в сперматозоидах, полученных от мужчин с олиго-, астено-, тератозооспермией, включенных в программу ЭКО, выявило повышенный уровень анеуплоидии по сравнению с мужчинами без отклонений в показателях спермограммы. Использование сперматозоидов с неправильным хромосомным набором для оплодотворения приведет к формированию эмбриона с генетической патологией, а это в свою очередь – к неразвивающейся беременности или рождению больного ребенка. Было показано, что до 20% хромосомных нарушений, например, трисомия при болезни Дауна, являются следствием оплодотворения нормальной яйцеклетки патологическим сперматозоидом даже при нормальных показателях спермограммы.
Методы ПГД
Для генетического исследования большинство клиник применяют молекулярные (ПЦР) и молекулярно-цитогенетические методы (FISH, аCGH и NGS).
FISH (флуоресцентная гибридизация in situ) в последнее время применяется очень редко в связи с отсутствием возможности проанализировать сразу все хромосомы
Метод сравнительной геномной гибридизации (Comparative Genomic Hybridization — aCGH) позволяет осуществить исследование одновременно всех хромосом в эмбрионе (все 24 хромосомы).
Применяя технику полимеразной цепной реакции (ПЦР), можно диагностировать заболевания, связанные с доминантными и рецессивными мутациями в уникальных генах (гемофилия, муковисцидоз и др.), определять антигены системы HLA, резус-принадлежность эмбрионов, хромосомные нарушения (транслокации).
Самым инновационным в области ПГД является метод NGS (next generation sequencing, высокопроизводительное секвенирование). Принцип метода NGS принципиально отличается от других методов ПГС. Данный метод основан на определении последовательности ДНК, что открывает новые возможности в диагностике генетической патологии. NGS более четко указывает на наличие мозаицизма у преимплантационных эмбрионов, что позволяет выбрать генетически здоровые эмбрионы для переноса и повысить уровень наступления беременности. Эта технология в последнее время все больше вытесняет метод CGH и находит более широкое применение в клиниках ВРТ за рубежом [6].
Наличие высокого риска образования анеуплоидных гамет при нормальном соматическом кариотипе также является показанием для проведения ПГД/ПГС. В этом случае анализируют число определенных хромосом у эмбрионов, полученных в циклах ЭКО. В настоящее время методом FISH можно рутинно определить число 9–12 хромосом. Тестируемые хромосомы выбирают исходя из их значимости для пренатального и постнатального развития. Целесообразно тестирование числа хромосом – 8, 9, 13, 14, 15, 16, 17, 18, 21, 22, Х, Y [7–9]. Известно, что определение числа пяти хромосом позволяет выявить 40%, а двенадцати хромосом – около 90% всех хромосомных аномалий у эмбрионов, достигших стадии бластоцисты [10].
Метод a-CGH позволяет определять число всех хромосом человека и исследовать несбалансированность по отдельным хромосомным сегментам [11].
Перспективы a-CGH для ПГС очевидны, но пока наряду с высокой себестоимостью метод имеет существенные недостатки, такие как невозможность определения плоидности клетки и невозможность сравнительного кариотипирования со сбалансированной структурной перестройкой хромосом.
На ПГС приходится около 60% всех проводимых в мире ПГД, однако о значимости доимплантационного скрининга анеуплоидий уже пять лет ведутся споры среди специалистов в области ЭКО. Проводить ПГС начали в начале 90-х годов [12] с целью улучшения результатов ЭКО за счет переноса в полость матки эмбрионов без анеуплоидий, наиболее значимых для пре- и постнатального развития, поскольку многочисленными исследованиями было показано, что 50–60% спонтанных прерываний беременностей раннего срока связаны с хромосомной патологией у плода [5]. Исходя из этого понятно, что при переносе эмбрионов после ПГС частота спонтанных потерь беременности будет меньше и, следовательно, результат ЭКО лучше. Кроме того, при переносе эмбрионов без частых хромосомных аномалий предполагалось увеличение частоты имплантации.
Показанием для ПГС также может являться носительство генных мутаций, которые могут вызывать болезни с поздней манифестацией, проявляющиеся в зрелом или пожилом возрасте, в том числе мутаций, предрасполагающих к развитию онкологических заболеваний. Такое носительство не являлось стандартным показанием для проведения преимплантационной диагностики, однако в некоторых случаях, например при мутации гена RB1, предрасполагающей к возникновению ретинобластомы, вероятность развития болезни у потомков в случае передачи мутантной аллели – около 90%. В настоящее время разработаны схемы проведения ПГД для таких онкологических заболеваний, как синдром Ли-Фраумени (p53), ретинобластома (RB1), семейный полипоз (APC), нейрофиброматоз I (NF1) и II (NF2), семейный рак молочной железы и яичников (BRCA1, BRCA2), атаксия-телеангиоэктазия (ATM) и др. [13]. Опубликованы результаты успешной ПГД при рано проявляющейся семейной форме болезни Альцгеймера (мутация гена APP) [14].
Еще одним показанием для проведения ПГД/ПГС в циклах ЭКО может являться носительство генетических болезней, вызванных мутациями в митохондриальных ДНК (мтДНК). Так как зигота получает все митохондрии из яйцеклетки, эти болезни наследуются по материнской линии. Митохондриальные болезни, вызванные мутациями ядерной ДНК, передаются по менделевским законам, следовательно, схема проведения ПГД должна быть такой же, как для моногенных заболеваний. Наследование митохондриальных болезней, вызванных мутациями мтДНК, усложнено гетероплазмией (генетической гетерогенностью популяции митохондрий), различным уровнем гетероплазмии в разных ооцитах, случайным распределением молекул мтДНК между клетками бластоцисты и тем, что доля мутантных молекул мтДНК может по-разному изменяться в тканях в процессе развития и жизни организма. Проявление болезней, вызванных мутациями мтДНК, очень вариабельно, симптомы некоторых болезней различаются как между семьями, так и внутри одной семьи. Даже при наличии зависимости степени тяжести болезни от определенной мутации мтДНК и числа мутантных молекул существуют многочисленные исключения. Сложность прогнозирования течения многих митохондриальных болезней и невозможность при проведении ПГД отобрать эмбрионы, полностью свободные от мутантной мтДНК, создают основные проблемы в проведении такого рода ПГД [15]. В настоящее время рекомендуют развивать ПГД для мтДНК мутаций в пределах научно-исследовательского протокола и информировать потенциальных родителей, что первый цикл ПГД может быть выполнен лишь для сбора информации относительно надежности метода [16]. На сегодняшний день в литературе имеется немного данных о клинических циклах ПГД при мутациях мтДНК [16, 17].
На этапе планирования семьи с целью половой идентификации эмбрионов при проведении ЭКО с применением ПГД/ПГС обращается немало супружеских пар. В европейских странах и США такую диагностику называют «выбор пола по социальным показаниям – social sexing». Согласно данным консорциума ПГД ESHRE (Европейское cообщество репродуктологов и эмбриологов человека), во всем мире происходит снижение числа таких диагностик. Проведение ПГД с целью планирования семьи нельзя считать однозначно оправданным. Однако, если сравнивать этот метод с пренатальной диагностикой, где в большинстве случаев при выявлении плода «ненужного» пола предполагается искусственное прерывание беременности, ПГД кажется более гуманной. С ноября 2011 года в Российской Федерации выбор пола доимплантационных эмбрионов для переноса без медицинских показаний запрещен законом [18].
ЭКО с применением ПГС у женщин старшего репродуктивного возраста
ЭКО имеет относительно низкий успех среди женщин старшего репродуктивного возраста. Было доказано, что спад фертильности с возрастом у женщин взаимосвязан с повышением зависимой от возраста анеуплоидии [19] и также может зависеть от прочих зависимых от возраста процессов. Женщины старшего возраста имеют высокий риск воспроизведения анеуплоидных эмбрионов, что приводит к неудавшейся имплантации, высокому риску выкидыша или рождению ребенка с хромосомными аномалиями.
В 2015 году группой ученых Нью-Йорка было проведено крупное ретроспективное исследование сравнительной оценки показателей имплантации эмбрионов, самопроизвольных абортов и живорождения с применением ПГС женщинам в возрасте от 40 до 43 лет и без такового [20]. Сравнивались исходы беременностей при традиционном ЭКО с переносом свежих бластоцист, ЭКО с переносом замороженных бластоцист без ПГС и ЭКО с переносом замороженных эуплоидных эмбрионов после ПГС. Частота клинических беременностей на количество переносов составила 36,6% (126/344) в группе без ПГС с переносом свежих эмбрионов и 55% (27/49) в группе с ПГС и переносом замороженных эмбрионов. Частота живорождения на число переносов составила 22,7% (78/344) в группе без ПГС и переносом свежих эмбрионов и 51% (24/49) в группе с ПГС и переносом замороженных эмбрионов. Показатели имплантации составили 16,3% (150/919) в группе без ПГС и переносом свежих эмбрионов и 51% (28/55) в группе ПГС с переносом замороженных эмбрионов. Частота невынашивания составила 38,1% (48/126) в группе без ПГС и переносом свежих эмбрионов и 10,7% (3/27) в группе с ПГС. Частота многоплодных беременностей составила 26,2% (33/126) в группе без ПГС и переносом свежих эмбрионов и 3,7% (1/27) в группе с ПГС и переносом замороженных эмбрионов. Отмечалась значительная разница в частоте живорождения на число перенесенных эмбрионов в трех группах (показатель живорождения): 15,8% (48/303) в группе без ПГС с переносом свежих эмбрионов, 19% (12/63) в группе без ПГС с переносом замороженных эмбрионов и 45,5% (25/55) в группе с ПГС с переносом замороженных эмбрионов (вероятность Х2=64,3 с 2° степени свободы, р<0,001). Частота показателей живорождения не сильно различалась при сравнении группы без ПГС с переносом свежих эмбрионов и группы без ПГС с переносом замороженных эмбрионов. Стоит отметить, что показатель живорождения значительно отличался в группе без ПГС с переносом замороженных эмбрионов и группе с ПГС с переносом замороженных эмбрионов (вероятность Х2=9,5, с 1° степени свободы, 0,01<р<0,0025).
Считается, что увеличение частоты анеуплоидии является основной причиной репродуктивных потерь при ЭКО [19–21]. Было отмечено, что около 50–80% всех невынашиваний беременности в первом триместре являются результатом хромосомных аномалий у развивающегося плода [22–26].
Использование изолированной морфологической оценки эмбриона перед переносом его в полость матки не предсказывает потенциальные хромосомные аномалии у эмбрионов. Многочисленные исследования показали значительное количество анеуплоидных эмбрионов, способных получить высшую морфологическую оценку [19, 27–32]. Недавно проведенные исследования продемонстрировали, что показатель анеуплоидии в случае удовлетворительных морфологических показателей составил 44,9% [33]. Позже, при проведении покадровой микроскопии (морфокинетики) для определения эуплоидности эмбрионов было установлено отсутствие значимости морфологических трансформаций [34]. Отсюда следует, что независимый морфологический анализ является неэффективным методом отбора лучших эмбрионов для переноса в полость матки.
Многочисленные исследования указывают на благоприятный эффект ПГС для определения анеуплоидии на клинические результаты у женщин старшего репродуктивного возраста [22, 35–37]. Последние рандомизированные контролируемые испытания доказали, что проведение скрининга 24 хромосом на анеуплоидию для селективного переноса здоровых эмбрионов значительно улучшает результаты за счет предотвращения переноса анеуплоидных эмбрионов. В свою очередь это повышает шансы на имплантацию и уменьшает вероятность выкидыша, связанного с анеуплоидией [33, 38]. Возможность отбора эуплоидных эмбрионов с более высоким потенциалом к имплантации позволяет ограничить количество перенесенных за 1 цикл эмбрионов и, таким образом, снизить вероятность многоплодных беременностей при одновременном повышении общего процента наступления беременности [39]. Другие исследования также показали, что, несмотря на возраст матери, при переносе эуплоидных эмбрионов показатели имплантации остаются неизменными после проведения ПГС [22, 40, 41].
Применение ПГС у супружеских пар с привычным невынашиванием беременности
Привычное невынашивание беременности является серьезной клинической проблемой, которая охватывает 15% всех поставленных клинически беременностей. В классическом определении это 3 и более перенесенных выкидыша на сроках до 20 недели гестации [42]. Большинство невынашиваний беременности являются следствием хромосомных или генетических нарушений. Как в популяции, так и после программы ВРТ основное количество (75–80%) прерываний беременности приходится на I триместр.
Одной из основных генетических причин, вовлеченных в патогенез привычного невынашивания беременности, считается хромосомная перестройка у одного или обоих партнеров [43]. У 4–8% пар с данной проблемой как минимум у одного из партнеров имеются хромосомные нарушения, количественные или структурные [44]. Среди структурных перестроек наиболее распространены реципрокная и Робертсоновская транслокации [45]. Люди, страдающие данной патологией, могут воспроизводить гаметы с неустойчивым набором хромосом, который может быть передан плоду. Стандартным методом обнаружения данных аберраций у пар является кариотипирование [46]. Для хромосомно неустойчивых эмбрионов выкидыш является механизмом естественного отбора. Таким образом, риск вынашивания ребенка с отклонениями у пар с аномальным кариотипом очень низкий при естественном зачатии [47]. Первое успешное клиническое применение ПГД для предотвращения болезней, связанных с Х-хромосомой, было исполнено в 1990 г. С тех пор этот метод применяется для обнаружения многих аутосомальных доминантных и рецессивных моногенных дефектов, структурных хромосомных нарушений и анеуплоидий [48] у пар с привычным невынашиванием беременности [49]. Генетическая неустойчивость у эмбрионов может быть первичной анеуплоидией, вероятность которой, как известно, повышается с возрастом матери и взаимосвязана с повторной неудачной имплантацией и привычным невынашиванием беременности [50]; отсюда следует, что ПГД может быть применена для скрининга специфичных хромосом, которые чаще всего приводят к анеуплоидии [40].
Заключение
ПГД/ПГС являются эффективным методом профилактики репродуктивных потерь и врожденной патологии у потомства и могут служить тактикой выбора при низкой вероятности наступления естественной беременности здоровым плодом (повышенный риск анеуплоидии гамет) и неприемлемости прерывания беременности плодом с «нежелательным» генотипом (болезни с поздней манифестацией, HLA и другие). На сегодняшний день не показано отрицательного влияния ПГД и ПГС на клиническую эффективность программ ЭКО и здоровье новорожденных, рожденных после ЭКО с ПГД и ЭКО с ПГС. Информация, полученная в результате исследования, является важным аргументом селекционного процесса, основанным не только на морфологических, но и на генетических характеристиках эмбриона [3, 4], и способствует рождению здорового потомства.



