Possible predictors of recurrence risk of deep endometriosis
Objective: To evaluate individual risk factors for recurrence of deep endometriosis (DE) on the basis of the obtained clinical, anamnestic and molecular biological data and to develop a formula for the individual recurrence risk of DE. Materials and methods: This was a study of the clinical and anamnestic data of 200 patients with DE who underwent surgical treatment at the Department of General Surgery of the National Medical Research Centre for Obstetrics, Gynecology and Perinatology, Moscow during the period from 2020 to 2021. Group I consisted of 80 patients with recurrent DE, and group II included 120 patients with newly diagnosed DE. All patients underwent surgical treatment followed by immunohistochemical (IHC) assessment. The most significant risk factors for the recurrence of DE were selected using binary logistic regression, and the computer model of a possible recurrence of DE was developed. Results: The detailed study of clinical, anamnestic and IHC data of the patients revealed five risk factors for recurrence of DE, namely: the age of the patients at the time of surgery, the duration of the disease, EphA1 expression in the ectopic endometrium, protein to the VEGF receptor in the eutopic and ectopic endometrium. A mathematical model with a sensitivity of 97.3% and a specificity of 85.7% was developed on the basis of the identified risk factors; the accuracy of the mathematical model was 93.1%. The duration of the recurrence interval averaged 48 (24;72) months. The recurrence interval was significantly longer in patients with a more radical extent of surgery (3 (2;11.5) years versus 1 (1;3) years, respectively (p<0.05)). The recurrence interval was almost three times less in patients after bowel shaving compared to women after intestinal resection (2 (1;3) years versus 3 (2;11.5) years, respectively, p=0.001)). The excision of all visible endometrioid foci reduces the risk of recurrence of endometriosis. Conclusion: The mathematical model with high sensitivity and specificity was developed on the basis of the obtained data using binary logistic regression. In order to determine the possible risks of endometriosis recurrence, it is necessary to validate the presented model which can be useful in the work of obstetrician-gynecologists. Authors’ contributions: Senina D.N. – collecting and processing material, writing the text; Chuprynin V.D. – editing the text; Asaturova A.V. – collecting and processing material, editing the text; Chursin V.V. – analysis of the national and foreign literature, editing the text; Buralkina N.A. – developing the concept and design of the study, editing the text. Conflicts of interest:. The authors declare that there are no conflicts of interest. Funding: The study was carried out within the framework of the State Programme “Integrated approach to the diagnosis and choice of surgical treatment in patients of reproductive age with deep infiltrative endometriosis”. Ethical Approval: The study was approved by the Ethical Review Board of Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Patient Consent for Publication: The patients provided an informed consent for the publication of their data. Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator. For citation: Senina D.N., Chuprynin V.D., Asaturova A.V., Chursin V.V., Buralkina N.A. Possible predictors of recurrence risk of deep endometriosis. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (7): 81-89 (in Russian) https://dx.doi.org/10.18565/aig.2023.122Senina D.N., Chuprynin V.D., Asaturova A.V., Chursin V.V., Buralkina N.A.
Keywords
Deep endometriosis (DE) is the most severe form of the disease which affects approximately 5–12% of women with endometriosis. The only effective method of treating patients with DE is a surgical removal of endometrioid infiltrates as it facilitates the relief of pain syndrome and improves the quality of life of such patients. However, according to various authors, recurrence of endometriosis reaches 60% after the complex treatment which includes surgery and hormonal suppression [1–5]. Given the fact that recurrence of the disease is the main problem, it is necessary to identify early markers of DE and possible risk factors for recurrence of endometriosis.
Currently, risk factors for recurrence of endometriosis are actively searched for. Thus, Busacca M. et al. (2006) and Biacchiardi C.P. et al. (2011) identified the following main factors: the young age of the patient at the time of surgical treatment, body mass index, lack of radical surgery, infertility. The young age of patients as a risk factor for recurrence of endometriosis is most likely associated with a high concentration of estradiol in the blood, since estradiol affects the growth of endometrioid implants [6, 7]. Other authors argue that the recurrence rate is due to the aggressiveness of endometriosis rather than radical surgical intervention [8]. Kozachenko A.V. et al. (2018) suggest that there are the following risk factors for recurrence of endometriosis: intense acyclic pelvic pain, a history of surgical interventions for extragenital endometriosis, infertility, ovarian stimulation with hormones during assisted reproduction treatment, stage III–IV endometriosis, severe adhesions in the pelvis, DE, large endometrioid cysts and/or bilateral ovarian lesions [9].
To date, the main cause of advanced stages of endometriosis is a delay in diagnosis due to the absence of specific symptoms and molecular biological markers of diagnosis.
The analysis of the endometrium is considered to be a promising method of early and minimally invasive diagnosis of endometriosis including its deep forms; however, none of the appropriate analyses of the uterine mucosa can be recommended for implementation in clinical practice nowadays [10].
Since there are some similarities in the development of DE and malignant tumors, many markers used today as biomarkers of malignant cells can be produced in endometriosis as well. Ephrin receptors (Eph) which belong to the largest family of receptor tyrosine kinases are of particular interest in this regard. A number of studies were initiated due to the discovery of a new Eph/ephrin system [11] which regulates both physiological and pathological processes. According to these studies, most Eph receptors regulate the processes of tumor progression and can be considered as markers of prognosis for survival, as well as a target for pathogenetic therapy [12, 13]. These receptors are activated by binding to the appropriate ephrin ligands attached to the membrane [14].
For example, EphA1 and EphA2 receptors are expressed in malignant cells; and a correlation between the prognosis for survival and the level of expression of EphA1 and EphA2 receptors has been revealed [15].
Muftaidinova Sh.K. et al. (2021) studied the activity of EphA1, EphA2 and EphA3 receptors in ectopic foci of the endometrium in various phases of the menstrual cycle. Abnormally increased expression of ephrin receptors was revealed in patients with various forms of endometriosis especially during the secretory phase of the menstrual cycle. The expression of EphA1 in the eutopic and ectopic endometrium in women with superficial endometriosis does not differ from the expression in the normal endometrium. And on the contrary, EphA1 is expressed in the eutopic and ectopic endometrium in all phases of the menstrual cycle in DE; the expression index does not have a statistically significant difference from that in adenocarcinoma [16].
Despite the results obtained after fundamental studies, there is still no clear understanding of pathogenesis and regulation mechanisms of endometriosis; therefore, the effectiveness of both pharmaceutical and surgical treatment of DE remains low [17]. The new methods of diagnosis and treatment of endometriosis on the basis of studying the molecular aspects of endometriosis pathogenesis are searched for [18].
Eph receptors may become the main markers of infiltrative forms of endometriosis if they are studied further; thus, the research into this issue is relevant. Endometriosis as a chronic inflammatory disease is usually characterized by a complex system of activation and inhibition of numerous factors leading to impaired regulation of apoptosis, immune system, angiogenesis, cell proliferation [19]. In this connection, the study of markers of these processes, namely, Bcl-2, Caspase3, VEGF, is also of particular interest.
The aim of the study is to evaluate individual risk factors for recurrence of DE on the basis of the obtained clinical, anamnestic and molecular biological data and to develop a formula for the individual recurrence risk of DE.
Materials and methods
The analysis of the clinical and anamnestic data of 200 patients with DE was performed. The patients underwent surgical treatment at the Department of General Surgery of the National Medical Research Centre for Obstetrics, Gynecology and Perinatology, Moscow during the period from 2020 to 2021. Group I consisted of 80 patients with recurrence of DE, and group II included 120 patients with new-onset DE. The comparison group included 40 women with tubal and peritoneal infertility factor. The average age of the patients was 35.6±5.64 years and it did not differ significantly among the study groups. DE was diagnosed on the basis of therapeutic and diagnostic laparoscopy and confirmed by histological examination. A thorough analysis of the clinical and anamnestic data of patients (age, duration of the disease, surgical history, nature of menstrual function, etc.) was carried out. Special attention was paid to group I: an in-depth analysis of previously performed surgical interventions for endometriosis was performed; the extent of the operation, the duration of postoperative hormone therapy and the interval between recurrences were analyzed.
During surgical treatment, samples of eutopic and ectopic endometrium (colorectal infiltrate) were obtained; subsequently, the samples underwent immunohistochemical (IHC) study for EphA1, EphA4 and EHB4 receptors, as well as VEGF, Bcl-2, and Caspase3 proteins.
A histological examination was performed at the first stage of the IHC study. There was an assessment of a macropreparation which was fixed in a 10% formalin solution for 24 hours, then paraffin blocks were prepared and sections 4–5 microns thick were made with the help of a microtome. Then the preparation was examined under the light microscope at magnification from 50 to 400. IHC examination of the endometrium and endometrioid foci in the intestinal wall was carried out using tissue matrices with the help of the Tissue-Tek Quick-Ray tissue microarray system. The sections were then stained to assess the expression of EphA1, EphA4, EphB4 receptors, VEGF, Bcl-2, and Caspase3 proteins.
In order to get the proper IHC reaction, positive and negative controls were set; slice samples were used as negative controls which underwent the standard IHC reaction procedure, but without the addition of primary antibodies. After the IHC reaction, the sections were additionally contrasted with hematoxylin and located in a synthetic Shandon-Mount medium. All samples were then evaluated using the QuickScore scale (Q-score). The result of staining was assessed by multiplying the percentage of positively stained cells (P) by the intensity of staining (I) (Q=P×I), maximum 300 [20].
Statistical analysis
Statistical processing and analysis of patients’ data were performed out using MS Office Excel and GraphPad Prism 9.0.0.121 (USA). A test for normal distribution of data was carried out at the first stage. Taking into account that the groups in our study had a distribution different from normal, we used methods of nonparametric statistics; data analysis was carried out using the Mann–Whitney U test, the description of quantitative data was presented in the form of a median (Me) with an interquartile range (Q1; Q3). The differences among the groups were considered statistically significant at p<0.05. A multivariate logistic regression analysis was subsequently performed; possible predictors of recurrence of endometriosis were identified from a combination of clinical and IHC data in a step-by-step mode; binary regression analysis was performed at the second stage. The final formula for assessing the risk for recurrence of DE was developed as a result of a multifactorial analysis. In the process of creating a mathematical model, the regression equation was used:
y=a+b1*x1+ b2*x2+…+ bn*xn,
where y is a dependent variable that has two values: 0 refers to the absence of a recurrence of DE, 1 refers to a recurrence of DE; a is a constant; bn are regression coefficients; xn are independent variables.
The probability of diagnosing a recurrence of endometriosis was calculated using the binary regression formula:
p=1/1+e-у
where p is the predictive probability, e is the exponent, the approximate value of which is 2.72.
Logistic regression will make it possible to predict the probability of recurrence of DE depending on the values of the available predictors (factors).
Results
A detailed analysis of the clinical and anamnestic data of the patients included in the study was carried out at the first stage of the study; its results were added to the MS Office Excel spreadsheet.
The average age of women with recurrence of DE was 35 (32; 39) years, and it was 36 (32;40) years in women without recurrence; the age of women did not significantly differ between the study groups (p=0.09). When the first operation was performed, the patients with recurrence of DE were significantly younger in comparison with the women of the second group, namely, 28 (25.75; 32) years versus 36 (32;40) years, respectively, p<0.0001), and it may be an important factor contributing to recurrence of endometriosis.
After studying the literature data [21, 22] on weight loss in patients with endometriosis in comparison with women without endometriosis, we analyzed the parameters of weight and height in the patients of the study groups. The analysis did not reveal significant differences in the average parameters of height, weight and body mass index in patients with colorectal endometriosis in comparison with women without endometriosis (p>0.05). The detailed study showed that patients with recurrence of DE were significantly more likely to have a body weight deficit in comparison with women with new-onset disease (16.3% versus 3.3%, respectively, p=0.0032).
According to the study design, menstrual function was compared. It was revealed that dysmenorrhea was significantly more common in patients of group I compared to ones of group II (91% versus 69%, respectively, p=0.0002). No other significant differences were found.
Amenorrhea is known to be a favorable factor for the course of endometriosis. This issue is under intense research nowadays [23, 24]. Our study did not reveal significant differences in the parity of women between the study groups. Infertility rate had almost the same percentage in the study groups and there were no significant statistical differences (46.2% versus 58.3%, p=0.093). However, the duration of infertility was significantly more frequent in patients with recurrence of DE than in the group with new-onset DE (5 (3;7) years versus 3 (2;4) years, respectively, p=0.0001) (Table 1).
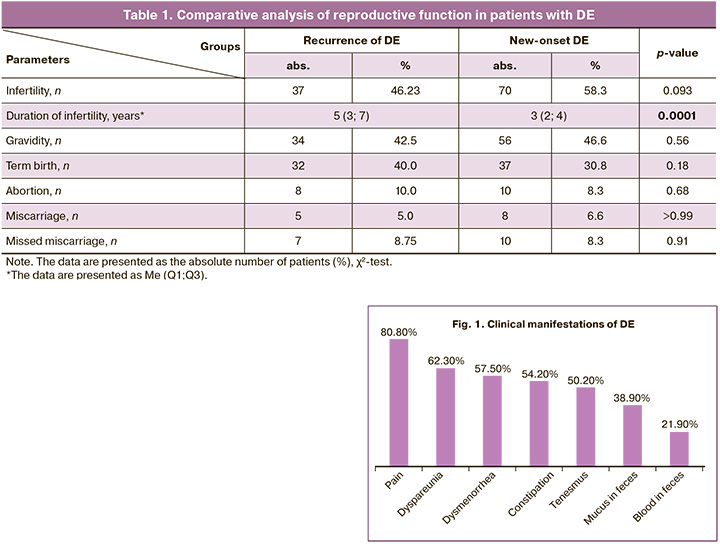
The patients who were admitted for surgical treatment for recurrence of the disease had a history of different previous surgical interventions for various forms of DE. The detailed study revealed that most patients had a history of one surgical intervention, and every third patient had two operations or more. Most women (71.2%) had excision of foci of endometriosis of the pelvic peritoneum, and all of them (100.0%) had ovarian resection. Endometriosis was more aggressive in a third of the patients, and therefore they underwent “shaving” (16.3%) or segmental resection of the intestine (12.5%).
The duration of the disease was longer in the group with recurrence of endometriosis in comparison with the group of patients with new-onset DE (median of patients with recurrence of DE 6 (4;9) versus median of patients with new-onset DE 2 (1;3), p<0.05).
Repeated surgical intervention (recurrence interval) was performed in patients after an average of 6 (4;10) years. Recurrence interval was significantly longer in patients with a more radical extent of surgery (3 (2;11.5) years versus 1 (1;3) years, respectively, p<0.05). The patients who underwent bowel shaving had a recurrence interval almost three times less in comparison with women who underwent bowel resection (2 (1;3) years versus 3 (2;11.5) years, respectively, p=0.001). After surgical treatment, 81.3% of patients received anti–relapse hormone therapy with various groups of medications: dienogest 2 mg – 57.5%; gonadotropin–releasing hormone agonists – 17.5%; combined oral contraceptives – 20%; intrauterine systems with levonorgestrel – 2.5%. On average, the duration of anti-relapse therapy was 9 (6;12) months. The analysis of the effect of hormone therapy and the duration of the relapse-free interval revealed no significant differences among the groups of medications and the duration of the relapse-free interval. Therefore, it can be concluded that radical surgical intervention leads to a long interval between recurrences.
The main clinical manifestations of colorectal endometriosis were abdominal pain (80.8%), dyspareunia (62.3%), dysmenorrhea (57.5%), intestinal symptoms (mucus in feces (38.9%), blood in feces (21.9%), tenesmus (50.2%), constipation (54.2%)) before or during the first menstruation days (Fig. 1). The time from the appearance of clinical manifestations to surgery (duration of the disease) averaged 4 (2;7) years and was significantly longer in patients with recurrence of the disease (6 (4;10) years and 3 (2;5) years, respectively, p<0.05).
After a comprehensive follow-up examination, the patients underwent surgical treatment. According to the results of ultrasound examination of the pelvic organs and magnetic resonance imaging, colorectal endometriosis was diagnosed only in 20.3% of patients; after colonoscopy it was diagnosed in 33% of patients in the study groups. Thus, it should be noted that additional diagnostic methods are not informative enough in the diagnosis of DE.
During the surgical intervention, the optimal extent of the operation was performed with the excision of foci of retrocervical endometriosis (98%); adhesiolysis was performed in 83.8% due to the massive scar and adhesive process of the pelvis; salpingectomy was performed in some cases (22.5%), and resection or bowel shaving was performed in 100% of cases. It was noted that intestinal resection was most frequently performed in the group with recurrence of DE in comparison with the second group (75% versus 43.3%, respectively, p<0.0001). Also, ureterolysis of the lower urinary tract was performed significantly more often in patients with recurrence of the disease (43.8 and 15.8%, respectively, p<0.0001).
During the surgical intervention, samples of the eutopic and ectopic endometrium were obtained from all patients. IHC examination of the samples was carried out; as a result, expression in ephrin receptors (EphA1, EphA4, EphB4), VEGF, Bcl-2, Caspase3 receptors in the eutopic and ectopic endometrium was studied. Among the entire cohort of patients with endometriosis, 23 samples with recurrence of DE and 29 samples with new-onset DE were selected for the IHC study; an additional group of patients (n=40) without endometriosis (with tubal and peritoneal infertility) was introduced as a comparison group.
After the IHC study, a significantly high expression of EphA4, EHB4, VEGF receptors was revealed in the eutopic endometrium of women with DE compared to women without endometriosis (comparison group). There was also a significantly high expression of EphA1, EphA4, EHB4, VEGF receptors in the colorectal infiltrate in patients with DE in comparison with the eutopic endometrium of the control group.
The results of our study revealed significant differences between the group with recurrence of DE and patients with new-onset DE in the expression of ephrin receptor EphA1 in the ectopic endometrium, as well as the receptor for VEGF protein in the ectopic and eutopic endometrium (Fig. 2).
Thus, we decided to use EphA1 ephrin receptors in the ectopic endometrium, VEGF receptors in the eutopic and ectopic endometrium in a possible mathematical model. The statistical analysis of the findings of the study groups is shown in Figure 2.
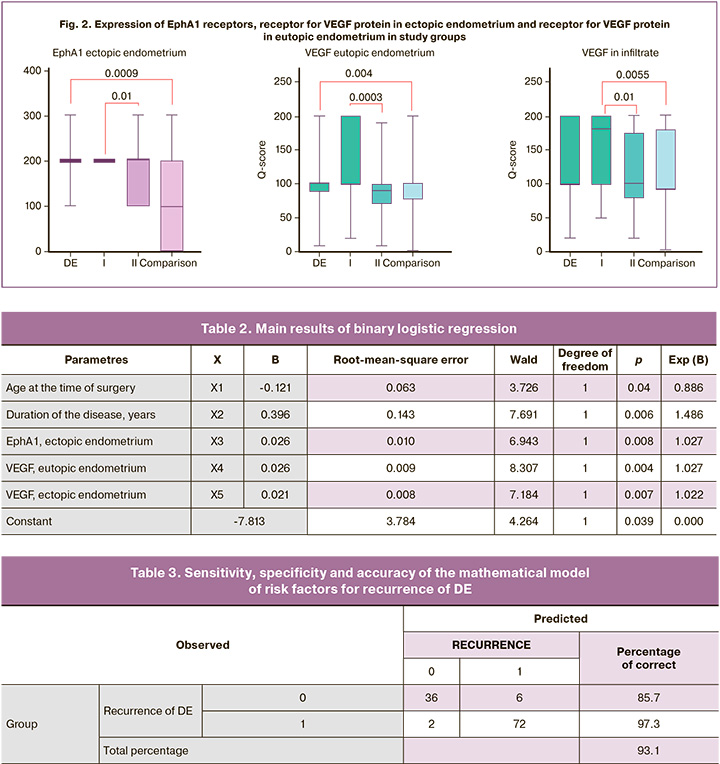
After the analysis, five statistically significant risk factors for the recurrence of DE were identified which may affect the development of recurrence. These are the patient’s age at the time of the first operation, the duration of the disease, expression in the EphA1 receptor in the ectopic endometrium, expression in the receptor for VEGF protein in the ectopic and ectopic endometrium. The results of binary logistic regression are presented in Table 2.
As a result of the study, the mathematical model of the risk factors for recurrence of DE has a sensitivity of 97.3% and a specificity of 85.7%, the accuracy of the mathematical model is 93.1% (Table 3).
The prognostic probability of diagnosing recurrence of DE was estimated on the basis of the obtained calculated regression coefficients according to the equation described below:
p=1/(1+2.72-(-7.81+(-0.121)*x1+0.396*x2+0.026*х3+0.026*x4+0.021*x5))*100%;
where p is a probability of recurrence of DE, е is the mathematical constant equal to 2.72. The accuracy of the mathematical model was 93.1%.
The results obtained after the development of the mathematical model of the risks for recurrence of DE were subjected to ROC analysis; the ROC curve was constructed on its basis (Fig. 3).
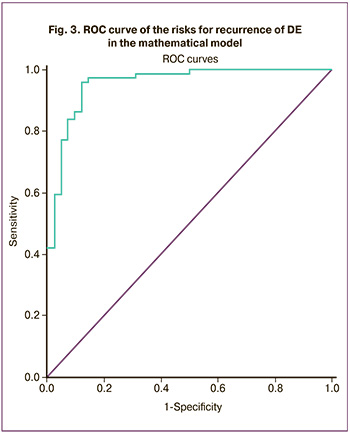
In the predictive model, the area under the ROC curve (AUC) was 0.956 (95% CI: 0.918-0.994) which is indicative of the high predictive ability of the developed model.
Discussion
The results of some studies show several risk factors for recurrence of endometriosis: the young age of patients at the time of surgery, the age of menarche, parity, body mass index, severity of the disease, the extent of the performed operation [25, 26]. The risk for recurrence of endometriosis increases if the lesions are not completely removed during surgery, and recurrence occurs at the same place where surgical treatment was previously performed [27]. In our study, we found that the young age of the patient at the time of surgery is the main risk factor for recurrence of endometriosis. One retrospective study involving 56 women of early reproductive age (the average age was 19.0±1.1 years) revealed a high percentage of recurrence of endometriosis in 32 patients (56%, 95% confidence interval 43–68%) during a five-year follow-up. The researchers noted that the frequency of recurrence of the disease is higher in young women in comparison with women of older reproductive age; they also found that the frequency of recurrence steadily increased over time after the first operation. The authors did not identify the correlation between the frequency of recurrence and symptoms, the stage of the disease, and postoperative treatment [28]. In our study we identified a predictor of recurrence, namely, the duration of the disease; we also revealed the correlation between the extent of the performed operation and the duration of the recurrence interval: more radical surgical intervention led to lower probability of recurrence.
Molecular predictors play a leading role in forecasting the recurrence of DE. Thus, ephrin receptors are able to participate in various processes of homeostasis regulation, such as proliferation, apoptosis, differentiation, cell adhesion and cell migration [29]. The EphA1 receptor plays an important role in the regulation of folliculogenesis, blastocyst transport, as well as embryo implantation and placenta formation; this receptor ensures invasion of the trophoblast into the thickness of the decidualized endometrium [30, 31]. The expression of the EphA1 receptor was detected in some forms of cancer of the reproductive system; for example, a significant expression of the EphA1 receptor was detected in ovarian cancer and cervical cancer [32]. Unfortunately, ephrin receptors have not been previously studied in a model with endometriosis. However, the mechanisms of regulation of the metabolism of DE cells are similar to malignant neoplasms whose regulation involves ephrin receptors. Therefore, we suggested that ephrin receptors can regulate the mechanisms of angiogenesis, apoptosis, migration, proliferation in ectopic endometrial cells. In our study we revealed a significant expression in ephrin receptors (EphA1, EphA4, EHB4) and receptor for VEGF protein which confirms our hypothesis about the similarity of pathological mechanisms of endometriosis and cancer [33]. We did not find any publications in the foreign literature on the relationship between the recurrence of DE and ephrin receptors. However, in our study we obtained overexpression in the EphA1 receptor in the ectopic endometrium in the group of patients with recurrence of DE in comparison with the group of patients with new-onset DE. Thus, EphA1 can be used as a marker of early diagnosis of a recurrence of DE.
The optimal method of treating endometriosis is surgical treatment. In our study the duration of recurrence interval averaged 48 (24;72) months. The recurrence interval was significantly longer in patients with a more radical extent of surgery (3 (2;11.5) years versus 1 (1;3) years, respectively, p<0.05). The recurrence interval was almost 3 times less in patients after bowel shaving in comparison with women after bowel resection (2 (1;3) years versus 3 (2;11.5) years, respectively, p=0.001). Thus, excision of all visible endometrioid foci reduces the risk of recurrence of endometriosis.
Conclusion
The mathematical model with high sensitivity and specificity was developed on the basis of the obtained data using binary logistic regression. In order to determine the possible risks of endometriosis recurrence, it is necessary to validate the presented model which can be useful in the work of obstetrician-gynecologists.
References
- Abo C., Moatassim S., Marty N., Saint Ghislain M., Huet E., Bridoux V. et al. Postoperative complications after bowel endometriosis surgery by shaving, disc excision, or segmental resection: a three-arm comparative analysis of 364 consecutive cases. Fertil. Steril. 2018; 109(1): 172-8.e1.https://dx.doi.org/10.1016/j.fertnstert.2017.10.001.
- Singh S.S., Gude K., Perdeaux E., Gattrell W.T., Becker C.M. Surgical Outcomes in patients with endometriosis: a systematic review. J. Obstet. Gynaecol. Can. 2020; 42(7): 881-8.e11. https://dx.doi.org/10.1016/j.jogc.2019.08.004.
- Zakhari A., Edwards D., Ryu M., Matelski J.J., Bougie O., Murji A. Dienogest and the risk of endometriosis recurrence following surgery: a systematic review and meta-analysis. J. Minim. Invasive Gynecol. 2020; 27(7): 1503-10.https://dx.doi.org/10.1016/j.jmig.2020.05.007.
- Zakhari A., Delpero E., McKeown S., Tomlinson G., Bougie O., Murji A. Endometriosis recurrence following post-operative hormonal suppression: a systematic review and meta-analysis. Hum. Reprod. Update. 2021; 27(1): 96-107. https://dx.doi.org/10.1093/humupd/dmaa033.
- Bozdag G. Recurrence of endometriosis: risk factors, mechanisms and biomarkers. Womens Health (London). 2015; 11(5): 693-9.https://dx.doi.org/10.2217/whe.15.56.
- Busacca M., Chiaffarino F., Candiani M., Vignali M., Bertulessi C., Oggioni G., Parazzini F. Determinants of long-term clinically detected recurrence rates of deep, ovarian, and pelvic endometriosis. Am. J. Obstet. Gynecol. 2006; 195(2): 426-32. https://dx.doi.org/10.1016/j.ajog.2006.01.078.
- Biacchiardi C.P., Piane L.D., Camanni M., Deltetto F., Delpiano E.M. et al. Laparoscopic stripping of endometriomas negatively affects ovarian follicular reserve even if performed by experienced surgeons. Reprod. Biomed. Online. 2011; 23(6): 740-6. https://dx.doi.org/10.1016/j.rbmo.2011.07.014.
- Джобава Э.М., Мандрыкина Ж.А., Логинова К.Б., Доброхотова Ю.Э. Дисменорея. Этиопатогенез, дифференциальная диагностика и терапия в практике современного акушера-гинеколога. РМЖ. Мать и дитя. 2012; 1: 28-34. [Dzhobava E.M., Mandrykina Zh.A., Loginova K.B., Dobrokhotova Yu.E. Dysmenorrhea. Etiopathogenesis, differential diagnosis and therapy in the practice of a modern obstetrician-gynecologist. Russian Journal of Woman and Child Health. 2012; (1): 28-34. (in Russian)].
- Борисова А.В., Козаченко А.В., Франкевич В.Е., Чаговец В.В., Кононихин А.С., Стародубцева Н.Л., Коган Е.А., Адамян Л.В. Факторы риска развития рецидива наружного генитального эндометриоза после оперативного лечения: проспективное когортное исследование. Медицинский совет. 2018; 7: 32-8. [ Borisova A.V., Kozachenko A.V., Frankevich V.E., Chagovets V.V., Kononokhin A.S., Starodubtseva N.L., Kogan E.A., Adamyan L.V. Risk factors for recurrence of external genital endometriosis after surgical treatment: prospective cohortant study. Medical Council. 2018; (7): 32-8. (in Russian)]. https://dx.doi.org/10.21518/2079-701X-2018-7-32-38.
- Makieva S., Giacomini E., Ottolina J., Sanchez A.M., Papaleo E., Viganò P. Inside the endometrial cell signaling subway: Mind the Gap(s). Int. J. Mol. Sci. 2018; 19(9): 2477. https://dx.doi.org/10.3390/ijms19092477.
- Hirai H., Maru Y., Hagiwara K., Nishida J., Takaku F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science. 1987; 238(4834): 1717-20. https://dx.doi.org/10.1126/science.2825356.
- Wada H., Yamamoto H., Kim C., Uemura M., Akita H., Tomimaru Y. et al. Association between ephrin-A1 mRNA expression and poor prognosis after hepatectomy to treat hepatocellular carcinoma. Int. J. Oncol. 2014; 45(3): 1051-8. https://dx.doi.org/10.3892/ijo.2014.2519.
- Yamamoto H., Tei M., Uemura M., Takemasa I., Uemura Y., Murata K. et al. Ephrin-A1 mRNA is associated with poor prognosis of colorectal cancer. In.t J. Oncol. 2013; 42(2): 549-55. https://dx.doi.org/10.3892/ijo.2012.1750.
- Davis S., Gale N.W., Aldrich T.H., Maisonpierre P.C., Lhotak V., Pawson T. et al. Ligands for EPH-related receptor tyrosinekinases that require membrane or clustering for activity. Science. 1994; 266(5186): 816-9.https://dx.doi.org/10.1126/science.7973638.
- Pasquale E.B. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat. Rev. Cancer. 2010; 10(3): 165-80. https://dx.doi.org/10.1038/nrc2806.
- Муфтайдинова Ш.К., Чупринин В.Д., Файзуллина Н.М., Буралкина Н.А.,Щеголев А.И., Файзуллин Л.З., Оводенко Д.Л., Асатурова А.В., Серов В.Н. Экспрессия рецептора тирозинкиназы (epha2) в эндометрии у пациенток с глубоким инфильтративным эндометриозом. Акушерство и гинекология. 2020; 3: 110-4. [Muftaidinova Sh.K., Chuprynin V.D., Faizullina N.M., Buralkina N.A., Shchegolev A.I., Faizullin L.Z., Ovodenko D.L., Asaturova A.V., Serov V.N. Endometrial expression of tyrosine kinase receptor (EphA2) in patients with deep infiltrating endometriosis. Obstetrics and Gynecology. 2020; (3): 110-4. (in Russian)].https://dx.doi.org/10.18565/aig.2020.3.110-114.
- Кулаков В.И., Григорян К.В., Гаспаров А.С., Камилова Д.П., Стыгар Д.А. Восстановление репродуктивной функции у пациенток с эндометриоз-ассоциированным бесплодием после комплексного лечения. Проблемы репродукции. 1999; 2: 59-61. [Kulakov V.I., Grigoryan K.V., Gasparov A.S., Kamilova D.P., Stygar D.A. Recovery of reproductive function in patients with endometriosis-associated infertility after complex treatment. Problems of Reproduction. 1999; (2): 59-61. (in Russian)].
- Цицкарева Д.З., Ярмолинская М.И., Сельков С.А. Эффективность цитокинотерапии в комбинированном лечении больных с глубоким инфильтративным эндометриозом. Журнал акушерства и женских болезней. 2016; 65(спецвыпуск): 66-8. [Tsitskareva D.Z., Yarmolinskaya M.I., Selkov S.A. Efficacy of cytokine therapy in the combined treatment of patients with deep infiltrative endometriosis. Journal of Obstetrics and Women's Diseases. 2016; 65(Special issue): 66-8. (in Russian)].
- Somigliana E., Vigano' P., Parazzini F., Stoppelli S., Giambattista E.,Vercellini P. Association between endometriosis and cancer: a comprehensive review and a critical analysis of clinical and epidemiological evidence. Gynecol. Oncol. 2006; 101(2): 331-41. https://dx.doi.org/10.1016/j.ygyno.2005.11.033.
- Charafe-Jauffret E., Tarpin C., Bardou V.J., Bertucci F., Ginestier C.,Braud A.C. et al. Immunophenotypic analysis of inflammatory breast cancers: identification of an 'inflammatory signature'. J. Pathol. 2004; 202(3): 265-73.https://dx.doi.org/10.1002/path.1515.
- Goetz T.G., Mamillapalli R., Taylor H.S. Low body mass index in endometriosis is promoted by hepatic metabolic gene dysregulation in mice. Biol. Reprod. 2016; 95(6): 115. https://dx.doi.org/10.1095/biolreprod.116.142877.
- Rowlands I.J., Hockey R., Abbott J.A., Montgomery G.W., Mishra G.D. Body mass index and the diagnosis of endometriosis: findings from a national data linkage cohort study. Obes. Res. Clin. Pract. 2022; 16(3): 235-41.https://dx.doi.org/10.1016/j.orcp.2022.04.002.
- Vannuccini S., Clemenza S., Rossi M., Petraglia F. Hormonal treatments for endometriosis: the endocrine background. Rev. Endocr. Metab. Disord. 2022; 23(3): 333-5. https://dx.doi.org/10.1007/s11154-021-09666-w.
- Porta R.P., Sangiuliano C., Cavalli A., Marques Pereira L.C.H., Masciullo L., Piacenti I. et al. Effects of breastfeeding on endometriosis-related pain: a prospective observational study. Int. J. Environ. Res. Public Health. 2021; 18(20): 10602. https://dx.doi.org/10.3390/ijerph182010602.
- Liu X., Yuan L., Shen F., Zhu Z., Jiang H., Guo S.W. Patterns of and risk factors for recurrence in women with ovarian endometriomas. Obstet. Gynecol. 2007; 109(6): 1411-20. https://dx.doi.org/10.1097/01.AOG.0000265215.87717.8b.
- Fedele L., Bianchi S., Zanconato G., Bergamini V., Berlanda N., Carmignani L. Long-term follow-up after conservative surgery for bladder endometriosis. Fertil. Steril. 2005; 83(6): 1729-33. https://dx.doi.org/10.1016/j.fertnstert.2004.12.047.
- Carmona F., Martínez-Zamora M.A., Rabanal A., Martinez-Roman S., Balasch J. Ovarian cystectomy versus laser vaporization in the treatment of ovarian endometriomas: a randomized clinical trial with a five-year follow-up. Fertil. Steril. 2011; 96(1): 251-4. https://dx.doi.org/10.1016/j.fertnstert.2011.04.068.
- Tandoi I., Somigliana E., Riparini J., Ronzoni S., Vigano' P., Candiani M. High rate of endometriosis recurrence in young women. J. Pediatr. Adolesc. Gynecol. 2011; 24(6): 376-9. https://dx.doi.org/10.1016/j.jpag.2011.06.012.
- Gucciardo E., Sugiyama N., Lehti K. Eph - and ephrin - dependent mechanisms in tumor and stem cell dynamics. Cell. Mol. Life Sci. 2014; 71(19): 3685-710. https://dx.doi.org/10.1007/s00018-014-1633-0.
- Wu Y., Du Z., Mou J., Qiu X., Chen J., Cai S. et al. The functions of EphA1 receptor tyrosine kinase in several tumors. Curr. Med. Chem. 2022; 30(20): 2340-53. https://dx.doi.org/10.2174/0929867329666220820125638.
- Fujii H., Tatsumi K., Kosaka K., Yoshioka S., Fujiwara H., Fujii S. Eph–ephrin a system regulates murine blastocyst attachment and spreading. Dev. Dyn. 2006; 235(12): 3250-8. https://dx.doi.org/10.1002/dvdy.20977.
- Ieguchi K., Maru Y. Roles of EphA1/A2 and ephrin-A1 in cancer. Cancer Sci. 2019; 110(3): 841-8. https://dx.doi.org/10.1111/cas.13942.
- Kajiyama H., Suzuki S., Yoshihara M., Tamauchi S., Yoshikawa N., Niimi K. et al. Endometriosis and cancer. Free Radic. Biol. Med. 2019; 133: 186-92.https://dx.doi.org/10.1016/j.freeradbiomed.2018.12.015.
Received 17.05.2023
Accepted 21.07.2023
About the Authors
Daria N. Senina, graduate student of the Surgical Department, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia, 119992, Russia, Moscow, Trubetskaya str., 8-2; Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia,117997, Russia, Moscow, Ac. Oparin str., 4, +7(904)189-30-63, seninadasha1995@gmail.com
Vladimir D. Chuprynin, PhD, Head of the Surgical Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, v_chuprynin@oparina4.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.
Alexandra V. Asaturova, Dr. Med. Sci., Head of the 1st Pathology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(926)994-43-14, a_asaturova@oparina4.ru, 117997, Russia, Moscow, Oparin str., 4.
Vyacheslav V. Chursin, doctor at the surgical department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,
Ministry of Health of Russia, v_chursin@oparina4.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.
Natalya A. Buralkina, Dr. Med. Sci., Senior Researcher at Surgical Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, natalyaburalkina@yandex.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.



