Рак шейки матки (РШМ) в структуре онкологической заболеваемости женского населения занимает четвертое место (6,2%) после рака молочной железы (РМЖ) – 16,1%, меланомы кожи – 10,1%, злокачественных новообразований трахеи, бронхов, легкого – 9,0%. Прирост заболеваемости РШМ в Российской Федерации с 2007 г. по 2017 г. составил около 25%, при этом отмечается увеличение числа местнораспространенных форм среди впервые выявленных случаев РШМ [1]. Местнораспространенный РШМ характеризуется неблагоприятным прогнозом, связанным с низкой 5-летней выживаемостью [2, 3]. В социально активной группе женщин, в возрасте от 35 до 55 лет, неуклонный рост заболеваемости распространенными формами РШМ диктует необходимость разработки новых алгоритмов диагностики и оптимизации подходов к лечению [4].
Как известно, стандартным подходом в лечении местнораспространенного РШМ является метод лучевой терапии. Однако тенденция к персонификации при выборе лечебной стратегии, зависящая от местной распространенности опухолевого процесса, формы роста опухоли шейки матки (ШМ) и гистологического его типа, приводит к разработке новых методологических подходов в лечении этой нозологии.
Сложности выбора метода лечения у пациенток с местнораспространенным РШМ в ряде случаев продиктованы кровотечением из распадающейся опухоли, что не позволяет начать лучевую терапию. С другой стороны, существующие методики эмболизации ветвей маточных артерий, с целью остановки кровотечений, негативно отражаются на результатах последующей лучевой терапии, эффективность которой зависит от степени оксигенации облучаемых тканей. И наконец, выбор хирургической тактики не всегда оправдан, так как в большинстве случаев не позволяет выполнить радикальный объем операции.
Так, применение неоадъювантной химиотерапии (ХТ) при местнораспространенном РШМ с последующим хирургическим вмешательством позволяет повысить показатели общей и безрецидивной выживаемости [5–7]. В последние годы были опубликованы исследования, посвященные суперселективной внутриартериальной ХТ [8–10]. По данным литературы, эффект селективной ХТ в подавляющем большинстве случаев (до 88,4%) классифицируется по RECIST 1.1 как стабилизация заболевания, однако прогнозы у пациентов в этой группе могут существенно отличаться [11]. Кроме того, было показано, что оценка эффективности селективной ХТ традиционными методами визуализации, базирующимися на изменении размеров очагов поражения, не обладает достаточной чувствительностью и прогностической ценностью в отношении продолжительности жизни до прогрессирования. Уменьшение размеров опухолевого очага является поздним критерием оценки эффективности лечения из-за относительно медленного удаления макромолекулярных остатков опухолевой клетки из межклеточного пространства после ее гибели [12]. В связи с этим требуется внедрение современного диагностического комплекса, позволяющего неинвазивно проводить количественный и/или полуколичественный анализ изменений, происходящих в тканях под воздействием проводимого лечения.
Современный взгляд на проблему оценки эффективности консервативных методов лечения требует определения метаболических изменений в опухолевой ткани, помимо измерений размеров опухоли и оценки их структуры в процессе и после терапевтического воздействия. По существующим рекомендациям, основными методами неинвазивной диагностики и оценки эффективности лечения РШМ в настоящее время являются: ультразвуковое исследование (УЗИ), магнитно-резонансная томография (МРТ) и позитронная эмиссионная томография, совмещенная с рентгеновской компьютерной томографией (ПЭТ/КТ). Реже в диагностике новообразований ШМ применяется компьютерная томография (КТ). При УЗИ, МРТ и КТ оценка эффективности лечения основана преимущественно на измерении размеров либо объема опухоли до и после терапии. Для КТ и МРТ в рамках данной задачи применяется система RECIST 1.1.
Современные методики МРТ и КТ, ПЭТ-КТ позволяют изучать функциональные особенности и метаболическую активность тканей [13]. Диффузионная и динамическая МРТ с возможностью анализа перфузии в тканях позволяют проследить их функциональное состояние под воздействием химического или радиационного влечения.
КТ-перфузия позволяет оценивать микроциркуляторные изменения в тканях. Термин «перфузия» означает транспорт крови в единице объема ткани за единицу времени и обычно отражает транспорт крови на капиллярном уровне. Теоретические основы перфузионной КТ были описаны Axel L. в 1979 г., однако, использование методики в клинической практике стало возможным лишь в 1990-е гг. с появлением современных мультиспиральных компьютерных томографов (МСКТ) [14–17]. Применение КТ-перфузии дает возможность количественно оценивать состояние ткани с помощью математических моделей и специального программного обеспечения на основании динамического изменения плотности, что отражает транспорт кислорода и питательных веществ на уровне микроциркуляторного русла. В основе использования КТ-перфузии в онкологии лежит факт того, что для злокачественных новообразований свойственно наличие патологического ангиогенеза, характеризующегося экспрессией трансформированными клетками ангиогенных факторов для удовлетворения повышенных потребностей опухолевых клеток в оксигенации [18]. Результаты применения КТ-перфузии для оценки эффективности лечения различных опухолевых заболеваний описаны в многочисленных работах, по данным которых параметры перфузии могут служить индикаторами степени злокачественности и прогноза заболевания [19–22]. Довольно часто уменьшения размеров опухоли не происходит, тем не менее наблюдается положительный эффект в виде снижения функциональной активности, отражаемой перфузионными показателями. Таким образом, такие перфузионные параметры, как скорость (BF – англ. Blood Flow) и объем кровотока (BV – англ. Blood Volume), могут использоваться как вспомогательные объективные параметры в оценке функционального состояния опухолевого очага. Кроме того, параметр проницаемости сосудистой стенки (PS – англ. Permeability Surface) позволяет определить инициальную проницаемость опухолевых сосудов для ХТ-агентов, что может быть использовано при планировании современных методов персонализированного лечения [23].
Первоначальные работы, касающиеся оценки параметров КТ-перфузии при местнораспространенном РШМ, были выполнены на фоне проведения лучевой терапии. Shibuya K. и соавт. сделали вывод на основании анализа данных 40 пациенток, что изменение показателя BF опухоли при РШМ до и после лучевой терапии можно контролировать путем проведения перфузионной КТ [24]. Liu J. и соавт. [25] был изучен другой параметр перфузии – PS у 196 пациентов с распространенным плоскоклеточным РШМ в рамках оценки ответа на химиолучевую терапию (ХЛТ). Перед ХЛТ показатели BV и PS были значительно выше в группе с положительным ответом в сравнении с резистентной группой (р<0,05). Чувствительность и специфичность BV и PS в прогнозировании ответа на терапию составили 65,3 и 83,2% и 75,2 и 72,6% соответственно. В работу Li X. и соавт. по изучению КТ-перфузии в прогнозировании краткосрочного ответа на комбинированную радиохимиотерапию (РХТ) при РШМ были включены 93 пациента не менее чем с IIB стадией по классификации FIGO. Эти пациенты были разделены на две группы в зависимости от наличия или отсутствия краткосрочного ответа. Авторы сравнивали исходные параметры перфузии в обеих группах с помощью многомерного мультирегрессионного анализа, который включал, помимо параметров перфузии, размеры опухоли. В группе пациентов с наличием краткосрочного ответа отмечались более высокие исходные значения BV и PS (р<0,05). Статистическая разница в исходном среднем времени транзита (МТТ – англ. Mean Transit Time) и BF между двумя группами отсутствовала (р>0,05). Было установлено, что перфузионная КТ дает дополнительную информацию для прогнозирования краткосрочного эффекта. Комбинированная РХТ может быть более эффективной при плоскоклеточном РШМ с более высоким исходным уровнем PS и BV [26].
В соответствии с рекомендациями Национальной Комплексной Онкологической сети от 2019 г., проведение ПЭТ/КТ с 2-фтор-2-дезокси-D-глюкозой, меченной 18F (18F-ФДГ), рекомендуется через 3–6 месяцев после ХЛТ местнораспространенного РШМ для выявления прогрессирования с бессимптомным течением. Основным радиофармпрепаратом (РФП) для ПЭТ/КТ, используемым в онкогинекологии, является 18F-ФДГ. Интенсивное накопление 18F-ФДГ в опухолевых клетках обусловлено высоким уровнем экспрессии GLUT-1 на их мембранах и активности гексокиназы и низким уровнем активности глюкозо-6-фосфатазы.
Применение ПЭТ/КТ с 18F-ФДГ в рамках стадирования по Т-критерию не является оправданным ввиду низкого пространственного разрешения метода. В рамках стадирования по N-критерию ПЭТ/КТ с 18F-ФДГ наиболее применима при местнораспространенном РШМ и более эффективна, чем МРТ или КТ [27, 28]. При выявлении отдаленных метастазов ПЭТ/КТ с 18F-ФДГ при РШМ является наиболее информативным из применяемых методов [29].
Высокая точность метода также определена как для выявления локорегионарных рецидивов, так и для обнаружения отдаленных метастазов после проведенного лечения, что крайне важно для планирования спасительного лечения [30–33].
Доказана роль ПЭТ/КТ с 18F-ФДГ как надежного метода для оценки ответа на лечение РШМ и прогноза. Применение ПЭТ-КТ с 18F-ФДГ для ранней оценки эффективности лечения может быть предпочтительнее анатомической визуализации (КТ, МРТ) ввиду того, что метаболические изменения предшествуют анатомическим [34]. Однако несоблюдение временных интервалов может приводить как к ложноположительным, так и к ложноотрицательным результатам [35]. Оптимальные сроки для оценки эффективности проведенного лечения составляют не менее 6 недель после хирургического вмешательства и не менее 12 недель после ХЛТ [36]. На настоящий момент общепринятыми системами оценки ответа на терапию при многих злокачественных новообразованиях с применением ПЭТ/КТ с 18F-ФДГ являются критерии EORTC и PERCIST версии 1.0 [37, 38].
Отрицательным прогностическим фактором 5-летней выживаемости у пациенток с РШМ выступает наличие резидуальной метаболически активной опухолевой ткани при ПЭТ/КТ с 18F-ФДГ после завершения ХЛТ. Так, в серии работ показано, что 5-летняя выживаемость составила 92% при отсутствии гиперметаболических очагов на контрольном скане, 46% – при сохранении резидуальной патологической метаболической активности и 0% – при появлении новых очагов гиперфиксации 18F-ФДГ со средней продолжительностью жизни 3 месяца [39–41]. Высокие значения SUVmax (англ. Standardized Uptake Value – стандартизованный показатель захвата РФП, применяемый для полуколичественной оценки его накопления) при ПЭТ/КТ в опухолевой ткани до начала лечения напрямую коррелируют со степенью риска резидуальной активности, а также локорегионарного рецидива. Banks T.I. и соавт. провели сравнительное исследование возможностей ПЭТ/КТ с 18F-ФДГ и КТ-перфузии при местнораспространенном РШМ до и во время ХЛТ, где использовались метаболические и перфузионные параметры. Суммарная статистика по этим параметрам и их изменениям рассчитывалась в пределах метаболического объема опухоли (MTV – англ. Metabolic Tumor Volume). Корреляцию между показателями оценивали с помощью коэффициента Пирсона (р). Увеличение средних значений BV во время лечения достоверно коррелировало с полным метаболическим ответом при ПЭТ/КТ после лечения. Связь между относительным изменением среднего значения BV и полнотой метаболического ответа предполагает потенциальную роль КТ-перфузии в оценке эффективности ХЛТ местнораспространенного РШМ [42].
С учетом анализа литературы, по данным ряда авторов, КТ-перфузионные параметры могут служить индикаторами прогностической оценки течения РШМ, позволяют оценить эффективность неоадъювантной ХТ у пациенток с распространенными формами. Однако интерпретация данных перфузионной КТ при РШМ неоднозначна, имеет противоречивые данные у разных исследователей, а также, в большинстве случаев, не сопоставлена с результатами референсных методов, к которым в настоящий момент можно отнести ПЭТ-КТ с 18F-ФДГ.
Работ по изучению оценки эффективности регионарной химиотерапии при местнораспространенном РШМ с применением КТ-перфузии в сопоставлении с данными ПЭТ-КТ в настоящий момент в электронных базах данных (PubMed) не найдено.
Учитывая собственный опыт и обнадеживающие результаты, в МНИОИ им. П.А. Герцена – филиал ФГБУ «НМИЦ радиологии» Минздрава России было предпринято пилотное исследование, целью которого являлось определение возможностей КТ-перфузии в оценке местной эффективности селективной ХТ при местнораспространенном РШМ в сопоставлении с данными ПЭТ/КТ с 18F-ФДГ.
Материалы и методы
В исследование были включены 15 пациенток (возрастной интервал 38–58 лет, средний возраст составил 43 года) с IB1—IIIВ стадиями по FIGO, из которых все случаи были представлены плоскоклеточным РШМ, из них 7 – с низкой, 4 – с умеренной и 4 – с высокой степенью дифференцировки.
Всем пациенткам выполняли УЗИ, МРТ органов малого таза, КТ-перфузию ШМ, ПЭТ/КТ всего тела с 18F-ФДГ на инициальном этапе с целью стадирования и оценки распространенности процесса и после проведения первого этапа лечения – 2 курсов регионарной ХТ. Сроки проведения контрольных обследований составляли 2 недели после окончания первого этапа лечения.
КТ-перфузию выполняли на мультиспиральном 64-срезовом компьютерном томографе Optima CT660 (GE Healthcare) со следующими параметрами сканирования: напряжение на трубке – 100 кВ, сила тока – 70–160 мА, матрица – 512×512 пикселей, время одного оборота трубки – 2 с. Для получения перфузионных изображений внутривенно вводилось фиксированное количество неионного водорастворимого контрастного препарата (60 мл) с концентрацией йода 370 мг/мл со скоростью 5 мл/с с последующим введением 30 мл физиологического раствора с той же скоростью. Постпроцессинговая обработка данных осуществлялась на рабочей станции AW v4.6 (GE Healthcare) деконволюционным методом, проводился анализ показателей перфузии опухолевой ткани до и после селективной химиотерапии.
Были изучены 4 параметра КТ-перфузии:
- BF (мл/100 г/мин) – скорость прохождения определенного объема крови через заданный объем ткани за единицу времени;
- BV (мл/100 г) – общий объем крови, проходящий через капилляры и более крупные сосуды в выбранном участке ткани;
- PS (мл/100 г/мин) – проницаемость сосудистой стенки, отражающая общую диффузию через капилляры;
- MTT (с) – среднее время, за которое кровь проходит по сосудистому руслу выбранной зоны интереса.
На полученных параметрических перфузионных картах была выбрана область интереса (ROI – англ. Region Of Interest), включающая первичное опухолевое образование, и получены значения того или иного перфузионного параметра в цифровом значении.
ПЭТ/КТ с 18F-ФДГ выполняли по стандартному протоколу, предложенному производителем, от остеомеатальной линии до середины бедер на комбинированных системах ПЭТ/КТ Biograph mCT 40 (Siemens Healthineers) и Discovery PET/CT 610 (GE Healthcare). Для постпроцессинговой обработки данных использовали рабочую станцию AW v4.6 (GE Healthcare). Полуколичественная оценка накопления РФП производилась при обработке ПЭТ с коррекцией аттенуации с использованием показателя SUVmax, нормализованного на массу тела. Объем интереса (VOI – англ. Volume Of Interest) устанавливался на зону первичного очага с формированием изоконтура 42% от SUVmax. Оценка степени метаболического ответа при ПЭТ/КТ производилась по критериям EORTC.
Результаты
При анализе полученных результатов ПЭТ/КТ местный полный метаболический ответ был зарегистрирован у 1 (6,7%) пациентки, частичный – у 10 (66,7%), стабилизация – у 4 (26,6%) пациенток. Прогрессирование вне зоны первичного очага (легкие) было выявлено у 1 (6,7%) пациентки, у которой местный процесс расценивался как стабилизация.
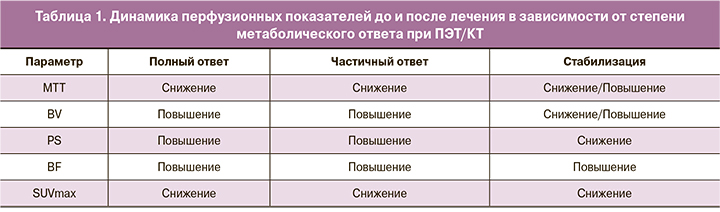
При качественной оценке динамики перфузионных показателей до и после проведенного лечения в группах с различной степенью местного метаболического ответа определялось (табл. 1):
- повышение BV, BF и PS и снижение МТТ в группе полного и частичного метаболического ответа;
- как повышение, так и снижение МТТ и BV, снижение PS и повышение BF в группе метаболической стабилизации.
При количественной оценке динамики перфузионных показателей до и после проведения селективной ХТ в группе с полным метаболическим ответом определялось повышение BV, BF и PS на 73,6, 15,2 и 63,5% соответственно и снижение МТТ на 98,2% (табл. 2).
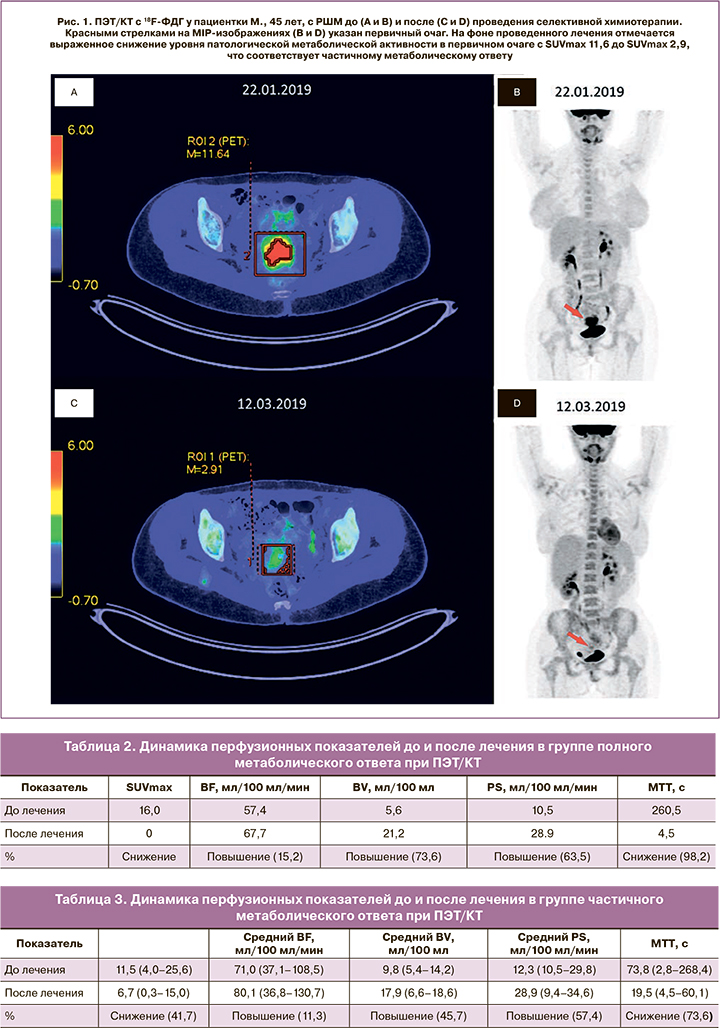
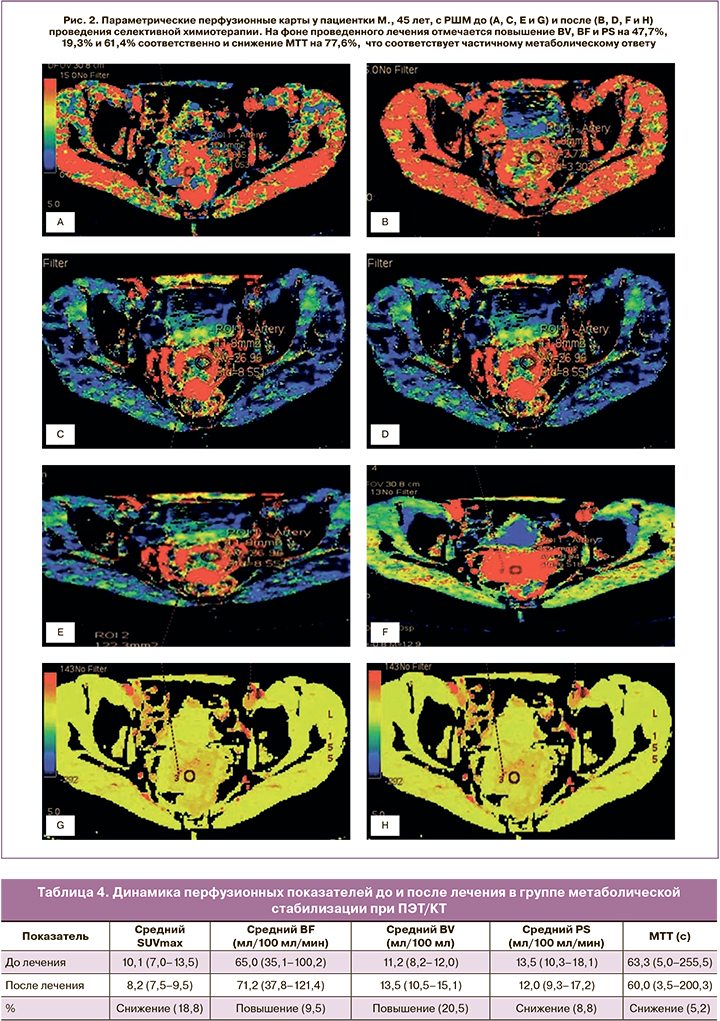
При количественной оценке динамики перфузионных показателей до и после проведения селективной ХТ в группе с частичным метаболическим ответом определялось повышение BV, BF и PS на 45,7, 11,3 и 57,4% соответственно и снижение МТТ на 73,6% (табл. 3, рис. 1, 2).
При количественной оценке динамики перфузионных показателей до и после проведения селективной ХТ в группе метаболической стабилизации определялось повышение BV, BF на 20,5%, 9,5% и снижение PS, МТТ на 8,8%, 5,2% соответственно (табл. 4).
При анализе полученных данных, динамическое изменение всех перфузионных показателей, кроме BF, было более выражено в группах полного и частичного метаболического ответа. Разница таких показателей, как BV и MTT, значительно отличалась во всех исследуемых группах (с возрастанием соответственно полноте метаболического ответа), в то время как разница PS в группах полного и частичного метаболического ответа существенно не различалась, но была значительно больше, чем в группе метаболической стабилизации.
Заключение
Таким образом, на основании полученных данных можно высказаться о том, что перфузионные показатели BV и MTT до и после лечения являются наиболее информативными в оценке эффективности селективной ХТ местнораспространенного РШМ. Получена корреляционная связь динамики таких перфузионных показателей, как BV, PS, MTT и SUVmax, во всех группах, в сопоставлении с метаболическим ответом при ПЭТ/КТ. В связи с этим данная работа является перспективной и требует продолжения с разработкой перфузионных критериев эффективности селективной ХТ при РШМ на большем количестве наблюдений.



